Despite advances in understanding of the biology of acute myeloid leukemia (AML), cure remains elusive for the majority of patients. ABT-199 (Venetoclax) is a small-molecule BH3 mimetic that selectively inhibits BCL-2 causing cell death. First generation BET inhibitor ABBV-075 and Venetoclax were recently shown to be synergistic in AML cell lines (Bui MH,Cancer Res 2017). ABBV-744 is a highly selective inhibitor for the BDII of BET family proteins, exhibiting greater than 300-fold more potent binding affinity to the BDII bromodomain of BRD4 relative to BDI (Warren Kati AACR 2018; Xiaoyu Lin AACR 2018). In this study, we evaluated the anti-leukemia efficacy of the concomitant BCL-2 blockade by venetoclax and of BDII inhibition with ABBV-744 in primary AML samples.
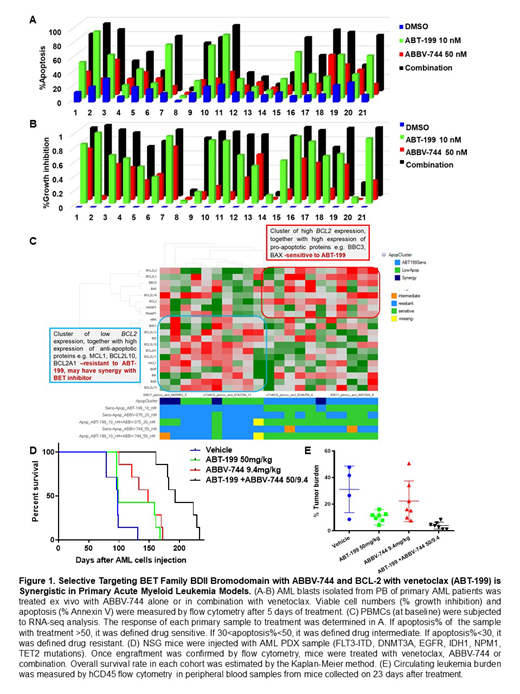
Anti-leukemia activity of venetoclax and ABBV-744 was examined in 21 primary AML samples with diverse genomic alterations. The combination significantly enhanced cell death (57.0 ± 6.3%) compared to the single agent treatment (43.9 ± 5.7% in ABT-199 10 nM group, p<0.001 and 23.8 ± 2.9% in ABBV-744 20nM group, p<0.001, Fig.1A). ABBV-744 reduced viable cell numbers in the majority of AML cases (31.7 ± 5.2%) and the cell growth suppression was more profound in the combination group (77.2 ± 6.3 %, p<0.001, Fig.1B).
In two AML primary samples tested, combination treatment of ABBV-744 and ABT-199 induced apoptosis with caspase-3 activation and PARP cleavage through regulation of the key proteins regulating survival and proliferation pathways (e.g. (BCL-2, BCL-XL, MCL-1, c-Myc)).
To identify biomarkers of response to therapy, we performed the baseline transcriptome analysis of AML cells used for in vitro response assessment (n=25) by RNA-sequencing (RNA-seq), and correlated baseline gene expression levels with in vitro response to therapy. Based on response to venetoclax or combination, samples were divided into 3 groups: sensitive to venetoclax (n=10), samples with no response to single agent or combination ("low apoptosis", n=7) and samples resistant to venetoclax as a single agent but responsive to venetoclax/ ABBV-744 combination ("synergy", n=4). AML samples sensitive to venetoclax and venetoclax/ABBV-744 combination were characterized by high level of BCL2 and lower levels of MCL1 and BCL2L1 transcripts, consistent with known inability of venetoclax to inhibit MCL-1 and BCL2L1 (Fig.1C).The resistant samples additionally expressed higher levels of anti-apoptotic genes such as GADD45, BCL2L10, PMAIP1. AML cells that showed synergy between venetoclax/ABBV-744 expressed low levels of AR, IL1R1 genes and had high CCND1 expression. The gene expression analysis indicated that the genes differentially expressed in the synergy vs. low apoptosis samples overlap with the genes inhibited by dual BCL-2/BCL-XL inhibitor ABT-737.
To test the efficacy of this regimen in vivo, we established a patient-derived xenograft (PDX) from an AML patient with FLT3-ITD, DNMT3A, EGFR, IDH1, NPM1, TET2 mutations in NSG mice. Upon engraftment, mice were randomized to receive vehicle; single agent venetoclax at 50 mg/kg; ABBV-744 at 9.4 mg/kg; or venetoclax plus ABBV-744 for 21 days. After 21 days of therapy, flow cytometry data demonstrated significantly reduced leukemia burden in venetoclax treated group (9.5% ± 1.7%) but not in ABBV-744 group (22.3% ± 5.8%) compared to controls (30.8% ± 3.9%), with lowest tumor burden in the combination group (5.0% ± 0.8%, p<0.01) (Fig. 1E). Combination of ABBV-744 and venetoclax treatment delayed AML progression and extended the survival compared to the untreated mice (median survival, 193 days vs 99 days, p<0.001) (Fig. 1D). No significant impact on mice' weight was noted, and no clinical signs of toxicity recorded over the course of therapy.
In summary, combinatorial blockade of BDII bromodomain and of BCL-2 anti-apoptotic pathway facilitates apoptotic cell death, suppresses proliferation in the majority of primary AML cells and produces anti-AML activity in AML PDX models in vivo at tolerable doses of both agents. This combination is currently undergoing testing in a Phase I clinical trial in AML (NCT03360006).
Kuruvilla:The University of Texas M.D.Anderson Cancer Center: Employment. Lin:AbbVie: Employment. Uziel:AbbVie: Employment, Other: stock or other options. Lu:AbbVie: Employment. Zhang:AbbVie: Employment. Huang:AbbVie: Employment. Zhang:The University of Texas M.D.Anderson Cancer Center: Employment. Shen:AbbVie: Employment. Konopleva:Astra Zeneca: Research Funding; Ablynx: Research Funding; Eli Lilly: Research Funding; Kisoji: Consultancy, Honoraria; Ascentage: Research Funding; Agios: Research Funding; Reata Pharmaceuticals: Equity Ownership, Patents & Royalties; Amgen: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria, Research Funding; Cellectis: Research Funding; Genentech: Honoraria, Research Funding; Stemline Therapeutics: Consultancy, Honoraria, Research Funding; Calithera: Research Funding; F. Hoffman La-Roche: Consultancy, Honoraria, Research Funding; Forty-Seven: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal