Pediatric acute myeloid leukemia (AML) continues to have a cure rate of only 50% despite the use of highly intensive cytotoxic chemotherapy. Transcriptome sequencing of several AML samples by the NCI/COG TARGET AML Initiative identified mesothelin (MSLN) to be highly overexpressed in about one-third of pediatric AML (Tarlock et al., Blood, 128:2873, 2016). Because MSLN is not expressed in normal bone marrow samples (Fan et al., Blood, 130:3792, 2017) and only to a low level in other human organs and tissues, MSLN is an attractive therapeutic target for pediatric AML (Kaeding et al., Blood, 130:2641, 2017). The anti-MSLN antibody-drug conjugate (ADC) anetumab ravtansine (BAY 94-9343) generated by conjugating MSLN-antibody with tubulin inhibitor DM4 (Meso-ADC), and isotype control antibody conjugated with the same drug (Iso-ADC) were used to evaluate the efficacy of MSLN targeting in vivo.
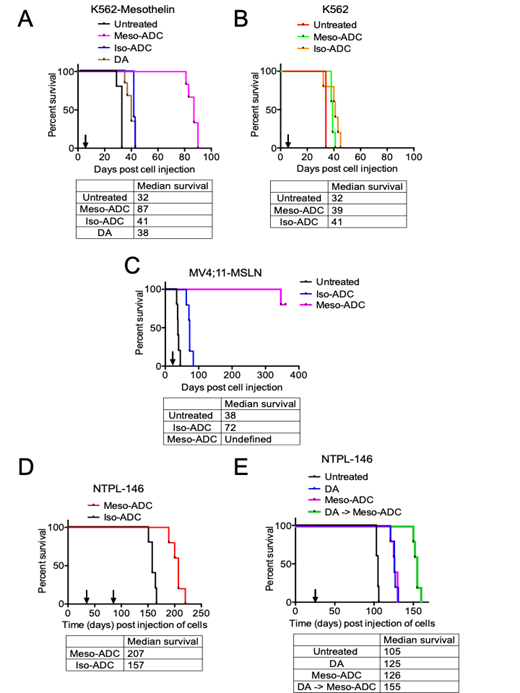
MSLN-overexpressing K562 (K562-MSLN) CML cells and MV4;11 (MV4;11-MSLN) AML cells were generated by lentiviral transduction of MSLN cDNA. Cell line-derived xenografts (CDX) were created by injecting the MSLN-transduced or parental (MSLN-) cells into NSG-SGM3 mice via the tail vein. Mice were randomly assigned to treatment groups when the median percentage of human cells in mouse peripheral blood was greater than 0.5%. K562-MSLN CDX mice treated with Meso-ADC (5 mg/Kg Q3dx3, i.v.) survived a median of 46 days longer than those treated with Iso-ADC (P=0.0011) and significantly longer than comparison groups, including K562-MSLN CDX mice treated with daunorubicin and Ara-C (DA, P=0.0008) or untreated (P=0.0018) (Fig. 1A). Median survival of K562 CDX mice treated with Meso-ADC, Iso-ADC, or untreated was similar (Fig. 1B). MV4;11-MSLN CDX mice treated with Meso-ADC exhibited complete remission and remained disease-free at 1 year post cell injection, with AML cell burden remaining <0.1% throughout the study period (Fig. 1C). In contrast, MV;11-MSLN CDX mice treated with Iso-ADC or untreated succumbed to disease at 72 and 38 days, respectively. Taken together, these results indicate that Meso-ADC was efficacious in reducing leukemia burden, and this effect required MSLN expression in target cells.
We have generated a panel of patient-derived xenograft (PDX) lines by transplanting and serially propagating primary pediatric AML samples into NSG-SGM3 mice. The efficacy of Meso-ADC was also evaluated in a systemic PDX model using a MSLN+ PDX line (NTPL-146). NTPL-146 PDX mice treated with Meso-ADC (5 mg/Kg, Q3dx3 -x2 cycles) survived a median of 50 days longer than those treated with Iso-ADC (P=0.0018, Fig. 1D, arrows indicate time when each treatment cycle was initiated). In an independent experiment with NTPL-146 PDX mice, a survival benefit of Meso-ADC treatment over no treatment was observed after 1 cycle of Meso-ADC treatment (5 mg/Kg, Q3dx3, P=0.0019, Fig. 1E). Additionally, a combination therapy strategy with daunorubicin and Ara-C followed by Meso-ADC (DA -> Meso-ADC) resulted in improved median survival over Meso-ADC (P=0.0027) or DA treatment alone (P=0.0018) (Fig. 1E). The disseminated MSLN+ leukemia mouse models described herein support MSLN-targeted antibody-drug conjugate as a potential treatment strategy in MSLN+ AML. Furthermore, we provide the first in vivo demonstration of synergy between MSLN-targeted therapy and conventional chemotherapy in MSLN+ AML, warranting additional investigation to validate and optimize novel strategies for combination therapy.
Kaeding:Celgene: Employment. Schatz:Bayer AG: Employment. Sommer:Bayer AG: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal