Background: The majority of adult patients (pts) with B-ALL achieve complete remission (CR) with contemporary multi-agent chemotherapy regimens. Persistence or recurrence of measurable residual disease (MRD+) in CR is associated with an increased risk of relapse and lower overall survival (OS) rates. Blinatumomab (blina) is a CD19-CD3 bispecific T-cell engager (BiTE) monoclonal antibody effective in the treatment of relapsed/refractory B-ALL and for the eradication of MRD+. In the BLAST trial, 78% of patients achieved complete MRD response (MRD-) with blina and responders had an improved 4-year OS of 52%. Uncertainty remain whether pts MRD- after blina should undergo allogeneic hematopoietic stem cell transplant (HSCT). In pts with Philadelphia-chromosome positive (Ph+) B-ALL, blina in combination with tyrosine kinase inhibitors (TKI) has not been evaluated for eradication of MRD. The objective of this trial was to evaluate the safety and efficacy of blina in pts with B-ALL and MRD+.
Methods: This is an open-label, single-arm, Phase II trial including pts with B-ALL in CR with persistent or recurrent MRD+. Pts in first CR (CR1) or in second CR or beyond (CR2+) were eligible. MRD+ was defined as ≥ 0.01% B-ALL cells by 6-color multiparameter flow cytometry for Ph- B-ALL pts and BCR-ABL1 to ABL1 transcripts ratio of ≥ 0.01% International Scale (IS) by RT-qPCR for pts with Ph+ ALL. Pts received continuous IV infusion of blina 28 µg/day over 4 weeks followed by a 2-week treatment-free interval. For the first cycle, blina was initiated at 9 µg/day for 1 week and then escalated to 28 µg/day, if tolerated. A TKI of treating physician's choice was added for pts with Ph+ ALL. Responders could receive up to 4 additional consolidation cycles (for a total of 5), and pts could proceed to HSCT at any time. The primary endpoint was relapse-free survival (RFS). Secondary endpoints included the MRD negativity rate, OS, and the safety profile.
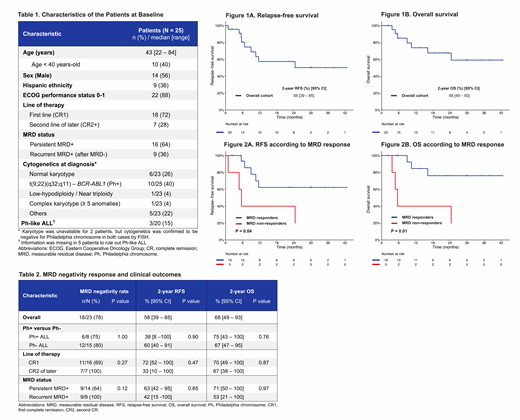
Results: Between December 2015 and June 2019, 25 patients with B-ALL in CR with MRD+ were enrolled. Baseline characteristics of the patients are shown in Table 1. Ten (40%) pts had Ph+ B-ALL. Eighteen (72%) pts were in CR1 and 7 (28%) were in CR2+. Sixteen (64%) pts had persistent MRD+, and 9 (36%) had MRD+ recurrence after being negative for a median or 20 months (range, 2-36).
Pts received a median of 2 cycles (range, 1-5) of blina. For pts with Ph+ B-ALL, blina was combined with ponatinib in 8 pts, bosutinib in 1 pt and dasatinib in 1 pt. The rate of MRD- with blina was 78% (18/23 pts). MRD- was achieved in 6/8 (75%) pts with Ph+ ALL and 12/15 (80%) pts with Ph- ALL (p = 1.00); 11/16 (69%) pts in CR1 vs 7/7 (100%) pts in CR2+ (p = 0.27); and 9/14 (64%) pts with persistent MRD+ vs 9/9 (100%) with recurrent MRD+ (p = 0.12) (Table 2). The median time to MRD- was 1.3 month (range, 1.0 - 5.6); 16/18 (89%) responders achieved MRD- after one cycle. One Ph- ALL pt achieved MRD- after 2 cycles and one Ph+ ALL pt achieved MRD- after 4 cycles. Six of 18 (33%) responding pts proceeded to HSCT after blina (3/11 in CR1 and 3/7 in CR2+), with a median time from treatment start to HSCT of 3.1 months (range, 2.1 - 6.4). Post-HSCT, 1 pt died of transplant-related complications and 5 remain alive in CR. Among 12 responding pts who did not proceed to HSCT, 4 relapsed and 8 are alive in remission.
With a median follow-up of 25 months (range, 2 - 43), the median RFS and OS have not been reached. The 2-year RFS and OS were 58% [95% CI, 39 - 85%] and 68% [95% CI, 49 - 93%], respectively (Figure 1A-1B). There was no difference in RFS and OS according of Ph+ status or between pts in CR1 and in CR2+ (Table 2). The 2-year RFS and OS rates for complete MRD responders were 62% [95% CI, 41 - 95%] and 76% [95% CI, 56 - 100%], versus 40% [95% CI, 14 - 100%] and 40% [95% CI, 56 - 100%) for non-responders, respectively (Figure 2A-2B). The 2-year RFS and OS for pts who proceeded to HSCT were both 78% [95% CI, 55 - 100%], versus 37% [95% CI, 15 - 88%] and 56% [95% CI, 31 - 100%) for pts who were not transplanted, respectively.
Blinatumomab-related adverse events of any grade were observed in 8/25 pts (32%). Cytokine release syndrome was reported in 3 pts (12%; grade 2, n=2; grade 3, n=1) and neurological adverse events in 4 pts (16%; grade 2; n=3, grade 3, n=1). All events resolved with supportive management and blina could be restarted.
Conclusion: Blinatumomab is highly effective for eradication of MRD+ in pts with B-ALL. Pts who achieve MRD- with blina have an excellent outcome. The study continues to accrue patients.
Kantarjian:Pfizer: Honoraria, Research Funding; Jazz Pharma: Research Funding; Novartis: Research Funding; Daiichi-Sankyo: Research Funding; Astex: Research Funding; AbbVie: Honoraria, Research Funding; Agios: Honoraria, Research Funding; Immunogen: Research Funding; Cyclacel: Research Funding; Takeda: Honoraria; Amgen: Honoraria, Research Funding; Ariad: Research Funding; Actinium: Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Research Funding. Khoury:Angle: Research Funding; Stemline Therapeutics: Research Funding; Kiromic: Research Funding. Ravandi:Cyclacel LTD: Research Funding; Menarini Ricerche: Research Funding; Macrogenix: Consultancy, Research Funding; Selvita: Research Funding; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Xencor: Consultancy, Research Funding. Short:AstraZeneca: Consultancy; Amgen: Honoraria; Takeda Oncology: Consultancy, Research Funding. Takahashi:Symbio Pharmaceuticals: Consultancy. Borthakur:AbbVie: Research Funding; PTC Therapeutics: Consultancy; GSK: Research Funding; Incyte: Research Funding; Bayer Healthcare AG: Research Funding; Eisai: Research Funding; Tetralogic Pharmaceuticals: Research Funding; Merck: Research Funding; Argenx: Membership on an entity's Board of Directors or advisory committees; Arvinas: Research Funding; Cantargia AB: Research Funding; Xbiotech USA: Research Funding; Agensys: Research Funding; BioTheryX: Membership on an entity's Board of Directors or advisory committees; FTC Therapeutics: Membership on an entity's Board of Directors or advisory committees; Strategia Therapeutics: Research Funding; AstraZeneca: Research Funding; Janssen: Research Funding; BioLine Rx: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Research Funding; Eli Lilly and Co.: Research Funding; BMS: Research Funding; Cyclacel: Research Funding; Oncoceutics: Research Funding; Oncoceutics, Inc.: Research Funding; NKarta: Consultancy; Polaris: Research Funding. Konopleva:Amgen: Consultancy, Honoraria; F. Hoffman La-Roche: Consultancy, Honoraria, Research Funding; Genentech: Honoraria, Research Funding; Ascentage: Research Funding; Kisoji: Consultancy, Honoraria; Reata Pharmaceuticals: Equity Ownership, Patents & Royalties; Ablynx: Research Funding; Astra Zeneca: Research Funding; Agios: Research Funding; Cellectis: Research Funding; AbbVie: Consultancy, Honoraria, Research Funding; Eli Lilly: Research Funding; Forty-Seven: Consultancy, Honoraria; Stemline Therapeutics: Consultancy, Honoraria, Research Funding; Calithera: Research Funding. Alvarado:Jazz Pharmaceuticals: Research Funding; Abbott: Honoraria. Jain:Adaptive Biotechnologies: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Verastem: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Precision Biosciences: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Cellectis: Research Funding; Servier: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; AstraZeneca: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Incyte: Research Funding; ADC Therapeutics: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Research Funding; Genentech: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen Pharmaceuticals, Inc.: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pharmacyclics, an AbbVie company: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Kadia:Amgen: Membership on an entity's Board of Directors or advisory committees, Research Funding; AbbVie: Consultancy, Research Funding; Pfizer: Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Research Funding; Celgene: Research Funding; Genentech: Membership on an entity's Board of Directors or advisory committees; Pharmacyclics: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; Bioline RX: Research Funding; Jazz: Membership on an entity's Board of Directors or advisory committees, Research Funding. Garcia-Manero:Amphivena: Consultancy, Research Funding; Helsinn: Research Funding; Novartis: Research Funding; AbbVie: Research Funding; Celgene: Consultancy, Research Funding; Astex: Consultancy, Research Funding; Onconova: Research Funding; H3 Biomedicine: Research Funding; Merck: Research Funding. Jabbour:Amgen: Consultancy, Research Funding; AbbVie: Consultancy, Research Funding; Cyclacel LTD: Research Funding; Pfizer: Consultancy, Research Funding; Takeda: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Adaptive: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal