Introduction/Background:
Recombinant coagulation factor Xa (FXa), inactivated Zh-zo, also known as andexanet alfa (AA), is a modified version of human FXa that has been developed as an antidote to neutralize the bleeding effects of oral FXa inhibitors such as, Apixaban and Rivaroxaban. The relative biological effect of these drugs have been investigated using various clot based and amidolytic methods for FXa inhibition. This Factor Xa inhibitory activity acts as a surrogate marker for the circulating level of these agents. We have recently reported that the FXa activity of these Anti-Xa agents does not fully reflect their biologic spectrum (JCath 25,1-11,2019). Whole blood assays such as thromboelastographic analysis represent a global assay which takes into account both the plasmatic and cellular components of blood and provides a more physiologic endpoint to study the anticoagulant effects of these drugs. The purpose of this study was to investigate the anticoagulant effects of currently available oral Anti-Xa agents such as Apixaban, Betrixaban, Edoxaban, and Rivaroxaban and their relative neutralization by AA in terms of such thromboelastographic parameters as R, K, Angle and MA.
Materials and Methods:
Analysis was carried out in whole blood using thromboelastography (TEG) using the TEG 5000 Hemostasis System (Haemonetics Corp, Massachusetts). Blood was drawn from healthy donors in individual groups (n=5-10) into 3.2% citrated tubes. In the TEG cup for testing, 0.2 M CaCl2, saline (with a filler and control), each of the individual FXa Inhibitors at a final concentration(FC) of 1 ug/mL, AA at FC of both 100 ug/mL and 50 ug/mL were tested for the relative neutralization of the anticoagulant effects. TEG parameters such as R-time, K-time, angle and MA were measured. All results were compiled individually for the saline control, 100 ug/mL AA and 50 ug/mL AA supplemented systems. Statistical analysis was carried out via an F test for equality of variances followed by the appropriate t test for equal or unequal variances.
Results:
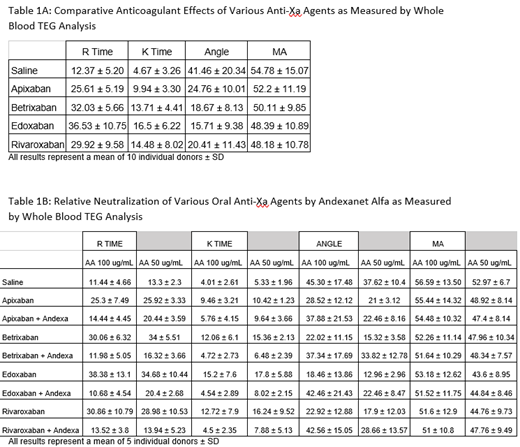
When comparing the anticoagulants directly to one another, it was observed that Edoxaban shows the strongest anticoagulant effects in both R and K time followed by Betrixaban, then Rivaroxaban which were very similar in their anticoagulative effects, with Apixaban showing the weakest anticoagulant effect as shown in Table 1A. In the reversal studies as shown in Table 1B, as measured by various TEG parameters, R-Time, AA (FC=100 ug/mL) showed full neutralization effects in Apixaban (p=.027), Betrixaban(p=<.01), Edoxaban(p<.01), and Rivaroxaban(p<.01). In K-time, Betrixaban and Edoxaban were fully neutralized (respectfully p=.049 and p=.035) with partial neutralizations of Apixaban and Rivaroxaban. No significant neutralization was noted in the Angle and MA. AA at 50 ug/mL showed full neutralizations, in R-time, in Betrixaban and Rivaroxaban (Betrixaban[p<.01] and Rivaroxaban[p=.0287]), AA at this concentration showed partial neutralizations of Apixaban and Edoxaban. In K-time, AA showed full neutralization of Betrixaban and Edoxaban (Betrixaban[p=<.01] and Edoxaban[p<.01]). Apixaban and Rivaroxaban saw no neutralization effect by AA at FC=50 ug/mL in K-time. AA did not exhibit any significant neutralization effects in the Angle or MA parameters.
Summary and Conclusion:
All of the 4 Agents produced measurable anticoagulative activities at 1 ug/mL as measured by the TEG parameters. Edoxaban exhibited the strongest anticoagulative effect followed by Betrixaban and Rivaroxaban whereas Apixaban showed much weaker anticoagulant effects. AA FC=100 ug/mL showed much stronger, consistent, and complete neutralization effects of all of the 4 FXa Inhibitors when compared to AA at FC=50 ug/mL. These results strongly suggest that regardless of the variable anticoagulative effect exhibited by the FXa Inhibitors, AA at FC=100ug/mL fully neutralized the anticoagulant effects of this agent as measured by the TEG parameters. AA is shown to be the most effective in neutralizing Betrixaban in R-Time and K-Time at both concentrations of AA. AA was seen to neutralize Apixaban the least. It can be concluded that the effect of AA as a neutralizing agent is both drug and donor dependent and therefore dosage adjustment may be needed for the optimal clinical outcome with this antidote.
Tafur:Recovery Force: Consultancy; Janssen: Other: Educational Grants, Research Funding; BMS: Research Funding; Idorsia: Research Funding; Daichi Sanyo: Research Funding; Stago: Research Funding; Doasense: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal