Background: The incidence of venous thromboembolism (VTE) in children has risen significantly. (Raffini, Huang et al. 2009) There are currently four direct oral anticoagulants (DOACs) - apixaban, dabigatran, edoxaban, and rivaroxaban - approved for the acute treatment and prevention of VTE in adults. Advantages of these medications over the traditionally used anticoagulants, enoxaparin and warfarin, include fixed dosing, no need for routine laboratory monitoring, few drug interactions and no dietary restrictions. Despite lack of information on the safety and efficacy of these agents in children, pediatric hematologists across the United States are using DOACs in their patients based on extrapolated data from adult studies.
The American Thrombosis and Hemostasis Network (ATHN) is a nonprofit network of over 140 federally funded Hemophilia Treatment Centers (HTCs) which provides the infrastructure for clinical and surveillance-based research. ATHN maintains the ATHNdataset (ADS), a "limited dataset" free of protected health information, with data collected on patients with bleeding and clotting disorders at participating HTCs within the Human Resources and Services Administration (HRSA)-supported regional hemophilia networks across the US. The authors acknowledge ATHN, the ATHN-affiliated U. S. Hemophilia Treatment Centers and their 39,000+ patients who have contributed their demographic, clinical, and genetic information to the ATHNdataset.
Methods: The objective of this study was to describe the characteristics of pediatric patients diagnosed with VTE in the ADS, focusing on those patients who received a DOAC. Data were abstracted for patients in the ADS who had acute VTE at age <21 years from January 2010 to March 2019. Data extraction included basic demographics and information about VTE and treatment.
Results: A total of 1,094 pediatric VTE cases were captured in the ADS. 577 (52.7%) were male. Caucasians were the most prevalent racial group (n = 809; 74%), followed by African-Americans (n = 203; 18.6%).14.9% (n = 163) were Hispanic.
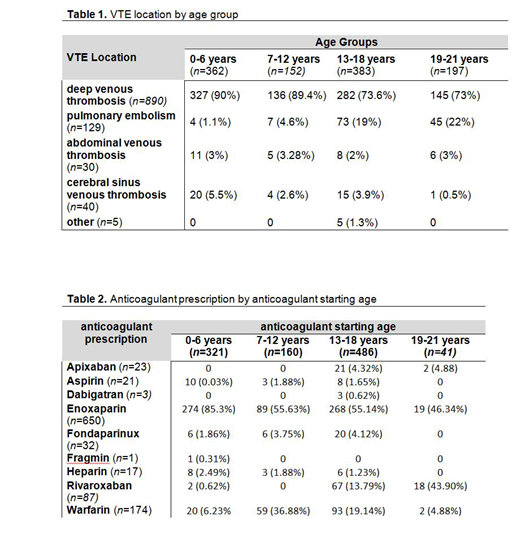
Deep venous thrombosis (DVT) was the most prevalent pediatric VTE reported in the ADS (n=889, 81.3%), followed by pulmonary embolism and cerebral venous thrombosis (n=130, 11.9% and n=40, 3.7% respectively). VTE location by age group is listed in Table 1. The most common DVT location was the lower extremities or pelvis, comprising 37.5% (n = 333) of all reported DVTs. Upper extremities or upper thorax DVT occurred less often (n = 211; 23.8 %). 345 (38.8 %) cases were reported only as "DVT" without a specific thrombus location.
We reviewed 1,051 anticoagulant prescriptions for 650 VTE patients (mean 1.6 prescriptions per person). Enoxaparin was the most commonly prescribed anticoagulant (n = 676 prescriptions; 64.3%) followed by warfarin (n = 178 prescriptions, 16.9%). Interestingly, 116 (11%) patients, from 21 HTCs, had a DOAC prescribed as their anticoagulant regimen. Anticoagulant prescription by anticoagulant starting age is shown in Table 2.
Further analysis of the DOAC subgroup showed that rivaroxaban was the most prescribed DOAC with 77.6% (n = 90/116) of the patients using this agent. Apixaban and dabigatran use was also reported (n= 23, 19.8% and n= 3, 2.6% respectively). The majority of DOACs were prescribed for patients older than 13 years of (111/116, 95.7 %). In children between 3 to 6 years of age (n = 3), rivaroxaban was the only DOAC prescribed. DOACs were primarily used to treat DVT of the extremities (84/116 patients). Other scenarios in which DOACs were also prescribed were PE and abdominal venous thrombosis patients (26, and 4 patients, respectively). Anticoagulant prescription by anticoagulant starting age is shown in Table 2.
Conclusion: DVT of the lower extremities and pelvis is the most prevalent pediatric VTE in the ADS. Enoxaparin and warfarin remain the main anticoagulant agents used for pediatric VTE treatment. Despite lack of an FDA-approved pediatric indication, hematologists in US-based HTCs are already using DOACs in pediatric patients with VTE. As further characterization of DOAC use in children is needed, the authors, in collaboration with ATHN, are currently building a multi-institutional retrospective and prospective registry, ATHN 15. This registry will serve as a resource for pediatric hematologists to collect real-world use of DOACs in children, as we await the results of prospective clinical trials.
Davila:Octapharma: Other: Grant to attend VWD meeting ; Genentech: Other: Advisory board; Spire Learning: Speakers Bureau. Raffini:Bayer: Other: Advisory Board; CSL Behring: Other: Advisory Board; Roche: Other: Advisory Board. Thornburg:Sanofi Genzyme: Research Funding; Bluebird bio: Other: Data Safety Monitoring Board; Genentech: Speakers Bureau; NovoNordisk: Research Funding; Ironwood: Other: Data Safety Monitoring Board; Sanofi Genzyme: Other: Data Safety Monitoring Board. Corrales-Medina:Octapharma: Membership on an entity's Board of Directors or advisory committees; Kedrion: Membership on an entity's Board of Directors or advisory committees; Bayer: Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda-Shire: Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal