
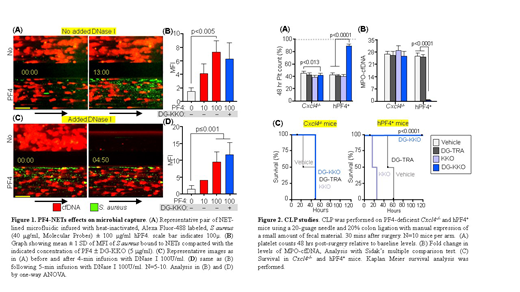
Sepsis is a dysregulated response to infection that leads to life-threatening organ damage. This process induces the release of neutrophil extracellular traps (NETs), webs of negatively-charged cell-free DNA (cfDNA) complexed with positively-charged histones that capture pathogens but also damage host tissue. We believe that upon presentation, most patients with sepsis have already released a large amount of NETs so that preventing NET release (NETosis), or accelerating their lysis with DNase, may be ineffective or harmful. Murine studies of NET lysis in sepsis have met with mixed results raising the concern that this strategy may lead to the release of captured bacteria and harmful NET degradation products (NDPs) such as cell free (cf) DNA and histones. We propose NET stabilization as an alternative therapy in sepsis. In a microfluidic system, we have shown that NETs bound the platelet chemokine platelet factor 4 (PF4, CXCL4), a highly-positively charged protein that aggregates polyanionic molecules including DNA. As a result of this aggregating effect, PF4 physically compacts NETs without inducing histone release. These compact PF4-NET complexes are resistant to DNase lysis. Using a microfluidic system, in which channels coated with NETs were infused with labeled Staphylococcus aureus particles, we now show that PF4 compaction of NETs markedly enhances their ability to capture bacteria. Likely, PF4, which is known to bind to the bacterial cell wall, cross aggregates pathogens to NET DNA. Thus, without added PF4, little bacteria is entrapped in the microfluidic chamber (Fig. 1A & 1B) and those captured are readily released by the infusion of DNase I (Fig. 1C & 1D). In contrast, when PF4 is added, microbial entrapment is markedly enhanced (Fig. 1A & 1B) and persists even with the infusion of DNase (Fig. 1C & 1D). When human (h) PF4 binds to polyanions it changes conformation, revealing HIT antigenic sites that bind the HIT-like monoclonal antibody KKO. Bound KKO further enhances PF4-NET complex resistance to DNase I in the microfluidic chamber, preventing the release of NDPs and entrapped bacteria upon exposure to DNases. These in vitro observations led us to hypothesize that infused PF4 and/or KKO may serve as a targeted therapeutic in sepsis. However, unmodified KKO stimulates leukocytes and induces a prothrombotic state. Indeed, infused KKO accelerates mortality in murine models of sepsis in mice that express hPF4 (hPF4+) (Fig. 2C). We, therefore, modified KKO using an IgG-specific endoglycosidase to create deglycosylated KKO (DG-KKO) that retains the ability to bind to PF4-NET complexes, but has little capacity to interact with hematopoietic cell Fc receptors or activate complement. DG-KKO binding also protects hPF4-NET complexes from DNase lysis and does not interfere with bacterial capture (Fig. 1B). In a murine lipopolysaccharide (LPS) endotoxemia model, DG-KKO infusion prevented thrombocytopenia, decreased the release of NDPs, and enhanced survival exclusively in hPF4+ mice (not shown). Although treatment with LPS recapitulates many aspects of sepsis, it relies on treatment with a bacterial toxin rather than live pathogens. Therefore, to determine if hPF4 and/or DG-KKO mitigate bacterial-induced sepsis, we repeated our studies using cecal ligation and puncture (CLP) to induce polymicrobial sepsis. Infused hPF4, but more so, infused DG-KKO decreased the severity of thrombocytopenia, reduced NDP release, and improved survival (Fig. 2A-2C). These murine studies demonstrate that PF4 released from activated platelets stabilizes NETs, making them resistant to DNase lysis and enhancing their ability to capture bacteria. Infused PF4 and DG-KKO further enhance NET stability, decrease the release of NDPs, and enhance bacterial entrapment, leading to better outcomes. These studies provide mechanistic insights into how NETs contribute to end-organ damage in sepsis and offer a targeted and novel therapeutic that minimizes their harmful effects, while enhancing their protective properties.
Arepally:Biokit: Patents & Royalties; Apotex Pharmaceuticals: Consultancy; Veralox Therapeutics: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal