Introduction: Platelet recruitment to generate a thrombus is key to bleeding cessation. That recruitment is dependent on a series of platelet activation processes that include adhesion to the exposed vessel matrix, platelet-platelet adhesion and platelet granule release. How platelet activation is patterned to generate a thrombus has previously been studied by intravital light microscopy, two-photon microscopy and scanning electron microscopy at resolutions insufficient to infer platelet activation at the level of the individual platelet. Here, we present a collaborative effort to stratify spatially the extent of platelet activation at the cellular level in a mouse jugular vein puncture model. We used wide-area transmission electron microscopy (WA-TEM) and serial block face scanning electron microscopy (SBF-SEM) at a resolution of 3 to 100 nm across whole thrombi to determine activation state of individual platelets. Our results, indicate a pattern of platelet stratification within the puncture wound that varies in a time-dependent manner with distinct structural stages in the formation of the thrombus.
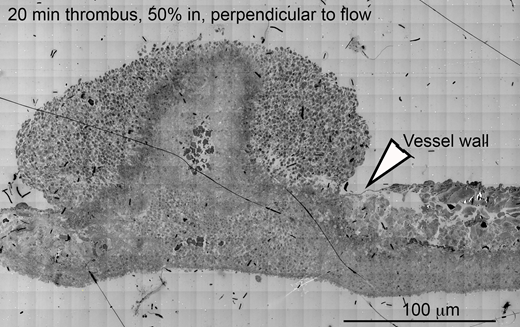
Methods: Jugular vein thrombi from C57BL/6 mice were collected 1, 5, or 20 min after a 300 µm needle puncture and prepared for EM imaging. For WA-TEM, hundreds of overlapping 3 nm resolution images were acquired using a gondimeter stage. The images sets were aligned using NIH Fiji software to create a single high-res, thrombus-wide image. Individual platelets were stratified into morphologically defined activation states (1: no activation, 2: decreased granule number, 3: no visible granules left, 4: hollow inside). The spatial distribution of platelet stratification was analyzed using iVision software. For SBF-SEM, 100-nm XY-resolution SEM images were collected every 200 nm and 20 nm XY-resolution images every 20 µm. Semi-automated stratification of platelet activation state in individual slices of the thrombus were combined into a 3-D representation using Amira software. Volumetric distributions of platelets with respect to the puncture hole and the vascular wall were quantified.
Results:Thrombus Formation Stage 1 (anchor and extend) -- One min post puncture, platelets were anchored in clumps along the exposed vessel wall. Near the damaged vessel wall was a peripheral layer of activated or degranulated platelets (states 3 and 4) covered by additional layers of less-activated platelets (state 1 and 2). Short cylindrical ingrowths extended into the 300 µm hole. Unexpectedly, large aggregates of platelets with a mixture of activation states (states 1 - 4) were found extending from these anchor points into the hole and vertically into the intravascular space. Aggregate surface layers were composed mostly of degranulated platelets (states 3 and 4). Less than 40% of neighboring platelets were of the same activation state as their neighbor. Surprisingly, <2% of platelet-occupied volume within the puncture hole contained largely degranulated platelets (aggregates of only states 3 and 4).
Stage 2(cap and erect) -- At 5 min after injury, the puncture hole was capped. ~70% of platelets neighboring degranulated platelets (3 and 4) formed visible aggregates within the thrombus. These aggregates were found along the exposed vessel wall and encasing vertical platelet aggregate towers containing a mixture of platelets in different states (1 - 4). Towers were typically separated by large cavities. SBF-SEM images, a machine-based, unbiased sampling of the underlying platelet distribution, revealed that ~10% of the platelet volume in the puncture hole of the thrombus and the intravascular towers contained largely degranulated platelets, similar to the data from WA-TEM.
Stage 3(infill and remodeling) - At 20 min post-puncture, the thrombus was filled with a mixture of platelets of varying activation states, which surrounded central, vertical aggregates (towers) of degranulated platelets seen at 5 min. Only minor cavity space was apparent. The intravascular surface of the thrombus was covered with an ~10 platelet-thick layer of loosely packed, variably activated platelets (states 1 - 4).
Conclusions: Our results demonstrate dynamic spatial patterns of platelet activation within a forming puncture-wound thrombus. Such patterns yield insights into thrombus formation and suggest the need to reference platelets defects and anti-thrombotics drugs against new models.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal