Key Points
Daratumumab plus carfilzomib/dexamethasone induced deep durable responses, regardless of prior treatment with lenalidomide.
Splitting the first dose of daratumumab was feasible, and the regimen was well tolerated.
Abstract
Patients with relapsed or refractory multiple myeloma (RRMM) have limited treatment options and poor survival outcomes. The increasing adoption of lenalidomide-based therapy for frontline treatment of multiple myeloma has resulted in a need for effective regimens for lenalidomide-refractory patients. This phase 1b study evaluated daratumumab plus carfilzomib and dexamethasone (D-Kd) in patients with RRMM after 1 to 3 prior lines of therapy, including bortezomib and an immunomodulatory drug; lenalidomide-refractory patients were eligible. Carfilzomib- and daratumumab-naïve patients (n = 85) received carfilzomib weekly on days 1, 8, and 15 of each 28-day cycle (20 mg/m2 initial dose, escalated to 70 mg/m2 thereafter) and dexamethasone (40 mg/wk). Of these, 10 patients received the first daratumumab dose as a single infusion (16 mg/kg, day 1 cycle 1), and 75 patients received a split first dose (8 mg/kg, days 1-2 cycle 1). Subsequent dosing was per the approved schedule for daratumumab. Patients received a median of 2 (range, 1-4) prior lines of therapy; 60% were lenalidomide refractory. The most common grade 3/4 treatment-emergent adverse events were thrombocytopenia (31%), lymphopenia (24%), anemia (21%), and neutropenia (21%). Infusion-related reactions were observed in 60% and 43% of single and split first-dose patients, respectively. Overall response rate was 84% (79% in lenalidomide-refractory patients). Median progression-free survival (PFS) was not reached; 12-month PFS rates were 74% for all treated patients and 65% for lenalidomide-refractory patients. D-Kd was well tolerated with low neutropenia rates, and it demonstrated deep responses and encouraging PFS, including in patients refractory to lenalidomide. The trial was registered at www.clinicaltrials.gov as #NCT01998971.
Introduction
Over the past decade, the introduction of novel agents has improved clinical outcomes for patients with multiple myeloma (MM); however, nearly all relapse, requiring subsequent therapy.1 Patients with successive relapses or who are refractory to treatment have poor survival, highlighting that novel therapies and treatment combinations are urgently needed in these patients with relapsed or refractory multiple myeloma (RRMM).2 In particular, increasing the adoption of lenalidomide earlier in the myeloma treatment paradigm as maintenance therapy post high-dose melphalan and autologous stem cell transplantation (ASCT), or as a first-line therapy for elderly patients, has resulted in an increasing need for effective treatments for lenalidomide-refractory RRMM.3,4 Efficacy results from phase 3 studies of novel combination therapies in lenalidomide-refractory patients remain unsatisfactory, and recent studies of lenalidomide-based combination therapies in RRMM exclude lenalidomide-refractory patients.5-10
Daratumumab is a human immunoglobulin Gκ (IgGκ) monoclonal antibody targeting CD38 with a direct on-tumor11-14 and immunomodulatory mechanism of action.15-17 Daratumumab is approved in many countries as a monotherapy and in combination with standard-of-care regimens in RRMM and in nontransplant newly diagnosed multiple myeloma (NDMM).18 Phase 3 clinical trials have demonstrated that daratumumab-based combinations significantly reduce the risk of progression or death by ≥50% and induce rapid, deep, and durable responses in RRMM and NDMM, including the absence of minimal residual disease (MRD).19-21 Analyses from the phase 3 CASTOR trial of daratumumab plus bortezomib and dexamethasone (D-Vd) in a subgroup of patients who were lenalidomide refractory at last prior line of therapy21 and data from the daratumumab plus pomalidomide and dexamethasone arm of the phase 1b MMY1001 trial (89% lenalidomide refractory)6 suggest that the addition of daratumumab to standard-of-care regimens is effective in lenalidomide-refractory RRMM. In clinical studies, the median duration of the first daratumumab IV infusion was 7.0 hours, because the first infusion requires a larger infusion volume (1,000 mL) and a slower initial infusion rate (50 mL/h) compared with the second infusion (500 mL at 50 mL/h, median duration 4.3 hours) and subsequent infusions (500 mL at 100 mL/h, median duration 3.4 hours).18 Splitting the first daratumumab dose over 2 days may improve patient convenience and ease daratumumab administration in outpatient settings by reducing infusion duration.
Carfilzomib, a proteasome inhibitor (PI), is approved as a monotherapy for patients who have received ≥1 line of therapy, and carfilzomib plus dexamethasone (Kd) or carfilzomib plus lenalidomide and dexamethasone (KRd) is approved for patients who have received 1 to 3 lines of therapy.22 Carfilzomib is approved for twice-weekly administration using 20/27 mg/m2 and 20/56 mg/m2 dosing schedules, and it was recently approved in combination with dexamethasone for once-weekly dosing using a 20/70 mg/m2 dosing schedule based on results from the phase 3 A.R.R.O.W. trial.22,23 Subgroup analyses from the phase 3 ENDEAVOR study demonstrated that carfilzomib (20/56 mg/m2 dosing schedule) plus 20 mg dexamethasone demonstrated encouraging activity in lenalidomide-refractory RRMM patients.7,8
The favorable tolerability of triplet or quadruplet daratumumab-based regimens observed across studies in MM6,19-21,24,25 provided the rationale for evaluating the combination of daratumumab and weekly carfilzomib in the multiarm phase 1b study MMY1001. Here, we report the safety, pharmacokinetics, and preliminary efficacy of daratumumab plus carfilzomib and dexamethasone (D-Kd) in patients with RRMM, including lenalidomide-refractory patients. The feasibility of splitting the first dose of daratumumab over 2 days was also investigated.
Methods
Eligibility criteria
Patients were ≥18 years of age and had documented myeloma, defined as ≥10% monoclonal plasma cells in the bone marrow or a biopsy-proven plasmacytoma per International Myeloma Working Group (IMWG) criteria,26 and had an Eastern Cooperative Oncology Group (ECOG) performance status ≤2. Patients had received 1 to 3 prior lines of antimyeloma therapy, including bortezomib and an immunomodulatory drug (IMiD), achieved at least a partial response (PR) to 1 prior line of therapy, and had disease progression after their last line of therapy. Lenalidomide-refractory patients (disease progression while on or within 60 days of completion of any dose of lenalidomide) were eligible. Patients who had progressed while on or within 6 months of bortezomib therapy were eligible unless bortezomib was given in the last line of therapy. Eligible patients had measurable disease if serum M protein level was ≥1.0 g/dL for patients with IgG disease or ≥0.5 g/dL for patients with IgA, IgD, or IgE disease or if urine M protein level was ≥200 mg/24 h (per IMWG criteria). Patients with light chain MM were required to have involved serum Ig free light chain ≥10 mg/dL and an abnormal serum Igκ:λ free light chain ratio. Patients were required to have hemoglobin ≥8 g/dL, absolute neutrophil count ≥1.0 × 109/L, aspartate aminotransferase and alanine aminotransferase ≤2.5 times the upper limit of normal, total bilirubin ≤2.0 mg/dL, calculated creatinine clearance ≥20 mL/min/1.73 m2, corrected serum calcium <14 mg/dL or free ionized calcium <6.5 mg/dL, platelet count ≥75 × 109/L in patients for whom <50% of bone marrow nucleated cells were plasma cells (>50 × 109/L, otherwise), and left ventricular ejection fraction (LVEF) ≥40%.
Patients were excluded if they had received previous treatment with daratumumab or carfilzomib, had undergone ASCT within 12 weeks before treatment start, or had received antimyeloma therapy within 2 weeks before treatment start. Patients with a diagnosis of monoclonal gammopathy of undetermined significance, smoldering MM, amyloidosis, or Waldenström disease were excluded. The study excluded patients with meningeal involvement of myeloma; chronic obstructive pulmonary disease (with a forced expiratory volume in 1 second <50% of predicted normal); moderate, severe, or uncontrolled asthma; or significant heart disease.
Study design
This was an open-label nonrandomized multicenter multiarm phase 1b study of daratumumab in combination with a variety of backbone regimens in patients with NDMM and RRMM. Results from the D-Kd treatment arm, which only included patients with prior therapy, are discussed here.
All patients were treated in 28-day cycles until disease progression (cycle 1 was 29 days). Daratumumab (16 mg/kg IV) was administered weekly (days 1, 8, 15, and 22) during cycles 1 and 2, every 2 weeks (days 1 and 15) during cycles 3 to 6, and every 4 weeks thereafter. Ten patients received a single first daratumumab dose (16 mg/kg) on day 1 cycle 1. The remaining patients received the first dose of daratumumab split over 2 days (8 mg/kg on days 1 and 2 of cycle 1) to collect safety and pharmacokinetic data for split dosing. Infusion-related reactions (IRRs) were managed by interruption of daratumumab administration to allow for symptom management; infusions were resumed at a reduced rate, which was gradually increased if no further reactions occurred. Carfilzomib was administered weekly on days 1, 8, and 15 of each 28-day cycle as a 30-minute infusion (prior to daratumumab on days when both were administered). Patients received an initial carfilzomib dose of 20 mg/m2 on day 1 cycle 1, which increased to 70 mg/m2 on day 8+ cycle 1, if deemed tolerable. Dexamethasone was administered at a dose of 40 mg/wk (20 mg/wk in patients aged >75 years). During the weeks when patients received daratumumab, dexamethasone (20 mg) was administered before the infusion and the day after the infusion; otherwise, dexamethasone was administered as a single dose.
Preinfusion medications included diphenhydramine, acetaminophen, and montelukast (required before the first dose and optional for subsequent doses). Patients receiving a split first dose of daratumumab also received diphenhydramine and acetaminophen on day 2 cycle 1. If dexamethasone was given prior to daratumumab infusion and the dose was reduced because of toxicity, methylprednisolone could be given postinfusion.
Study end points and analyses
The primary end points were the safety and tolerability of D-Kd. Safety evaluations included adverse event (AE) monitoring, physical examinations, electrocardiogram monitoring, clinical laboratory tests, vital sign measurements, and ECOG performance status. Toxicities were graded using the National Cancer Institute Common Terminology Criteria for Adverse Events Version 4.27
Secondary end points included overall response rate (ORR) and overall survival (OS). Response to treatment and disease progression were evaluated according to the IMWG response criteria at the end of each treatment cycle.28,29 M protein measurements in serum and urine were assessed by a central laboratory. Serum and urine immunofixation electrophoresis (IFE) was performed at screening and when complete response (CR) was suspected. A daratumumab-specific IFE assay was used to confirm CR for patient samples in which daratumumab interference with IFE was suspected.30 Samples for pharmacokinetic analysis were collected predose and postinfusion on day 1 of cycles 1 to 4 (and on day 2 cycle 1 for patients receiving split first dose) and were analyzed as described previously.31
Exploratory end points included progression-free survival (PFS), MRD, and pharmacokinetics. MRD testing was optional in this phase 1 study and was evaluated in bone marrow aspirate samples from patients who achieved a CR or better; it was assessed at the time of suspected CR and at 12 and 18 months following the first treatment dose. Samples were prepared with Ficoll and evaluated using a clonoSEQ Assay (Version 2.0; Adaptive Biotechnologies, Seattle, WA) at sensitivity thresholds of 0.01% (1 cancer cell/10 000 nucleated cells [10−4]), 10−5, and 10−6.
Study oversight
The study was registered at ClinicalTrials.gov (#NCT01998971). The clinical study sites’ institutional review boards or ethics committees approved this study. All patients provided written informed consent. The study design and analyses were devised by the investigators and sponsor. The investigators and their research teams collected the study data. Janssen conducted the final data analysis and verified the accuracy of the data. The investigators were not restricted by confidentiality agreements and had full accessibility to all data. Writing assistance was funded by Janssen Global Services, LLC.
Statistical analyses
Sample size was not determined based on formal hypothesis testing. A sample size of 80 was proposed to provide sufficient safety and preliminary efficacy data to plan a randomized study with D-Kd for patients with relapsed disease. Descriptive statistics for treatment-emergent AEs (TEAEs) were summarized, including AEs of clinical interest: IRRs, infections, and cardiac function. Responses were categorized per IMWG criteria and, for each response category, a 2-sided 95% exact confidence interval (CI) was calculated. ORR was analyzed for all treated patients and defined as the proportion of patients with stringent CR, CR, very good PR (VGPR), or PR. Post hoc subgroup analyses were performed to determine whether clinical characteristics were associated with selected efficacy and safety end points. PFS and OS were estimated using the Kaplan-Meier method.
Results
Patients
A total of 85 patients was enrolled in the study and received ≥1 dose of study treatment. Baseline characteristics and prior therapies are presented in Table 1. Median (range) age was 66 (38-85) years, and median (range) number of prior therapies was 2 (1-4). All patients had received prior treatment with bortezomib (31% were bortezomib refractory), 95% had received prior treatment with lenalidomide, and 73% had undergone ASCT. Fifty-one (60%) patients were refractory to lenalidomide; the demographics of the lenalidomide-refractory cohort were representative of the overall population (Table 1). Lenalidomide-refractory patients had a median (range) of 2 (1-4) prior lines of therapy; 6 (12%) patients had only 1 prior line of therapy. Of the 67 patients with available cytogenetic data (locally assessed via fluorescence in situ hybridization or karyotype testing), 19% had a high-risk cytogenetic anomaly at screening.
Baseline characteristics and prior treatment history
| Characteristic . | All treated (N = 85) . | Lenalidomide refractory (n = 51) . |
|---|---|---|
| Age, median (range), y | 66 (38-85) | 66 (38-85) |
| ECOG performance status | ||
| 0-1 | 78 (92) | 47 (92) |
| 2 | 7 (8) | 4 (8) |
| No. of prior lines | ||
| Median (range) | 2 (1-4) | 2 (1-4) |
| 1 | 20 (24) | 6 (12) |
| 2 | 40 (47) | 26 (51) |
| 3 | 23 (27) | 18 (35) |
| >3 | 2 (2) | 1 (2) |
| Prior ASCT | 62 (73) | 33 (65) |
| Prior bortezomib | 85 (100) | 51 (100) |
| Prior IMiD | 85 (100) | 51 (100) |
| Lenalidomide | 81 (95) | 51 (100) |
| Pomalidomide | 13 (15) | 9 (18) |
| Thalidomide | 21 (25) | 11 (22) |
| Refractory to:* | ||
| Lenalidomide | 51 (60) | 51 (100) |
| Pomalidomide | 11 (13) | 9 (18) |
| Bortezomib | 26 (31) | 21 (41) |
| PI + IMiD | 25 (29) | 22 (43) |
| Characteristic . | All treated (N = 85) . | Lenalidomide refractory (n = 51) . |
|---|---|---|
| Age, median (range), y | 66 (38-85) | 66 (38-85) |
| ECOG performance status | ||
| 0-1 | 78 (92) | 47 (92) |
| 2 | 7 (8) | 4 (8) |
| No. of prior lines | ||
| Median (range) | 2 (1-4) | 2 (1-4) |
| 1 | 20 (24) | 6 (12) |
| 2 | 40 (47) | 26 (51) |
| 3 | 23 (27) | 18 (35) |
| >3 | 2 (2) | 1 (2) |
| Prior ASCT | 62 (73) | 33 (65) |
| Prior bortezomib | 85 (100) | 51 (100) |
| Prior IMiD | 85 (100) | 51 (100) |
| Lenalidomide | 81 (95) | 51 (100) |
| Pomalidomide | 13 (15) | 9 (18) |
| Thalidomide | 21 (25) | 11 (22) |
| Refractory to:* | ||
| Lenalidomide | 51 (60) | 51 (100) |
| Pomalidomide | 11 (13) | 9 (18) |
| Bortezomib | 26 (31) | 21 (41) |
| PI + IMiD | 25 (29) | 22 (43) |
Unless otherwise noted, all data are n (%).
Refractoriness was based on most recent prior medication.
Disposition and drug exposure
Of the 85 patients treated with D-Kd, 45% discontinued treatment: 31% because of progressive disease, 6% because of withdrawal of consent, 5% because of AEs, 2% because of the physician’s decision, and 1% because of death. Patient disposition for the lenalidomide-refractory cohort was consistent with all treated patients. The median (range) number of treatment cycles was 16 (1-30). A total of 83 (98%) patients escalated to carfilzomib 70 mg/m2 within the first 2 cycles.
Ten patients received the first daratumumab dose as a single infusion (day 1 cycle 1), with a median (range) infusion time for the first infusion of 7.1 (6.5-8.9) hours. For the 75 patients who received a split first dose, median (range) infusion time was 4.3 (3.9-10.6) hours on day 1 cycle 1 and 4.2 (3.9-8.6) hours on day 2 cycle 1. Following administration of the first 16 mg/kg dose, pharmacokinetic concentrations were similar for single and split first dosing (Table 2).
Daratumumab serum concentrations with single vs split first dose in the all treated population
| . | Postfirst dose . | C3 D1 preinfusion . | C3 D1 end of infusion . | |
|---|---|---|---|---|
| C1 D1 end of infusion . | C1 D2 end of infusion . | |||
| Single first dose | ||||
| Patients, n | 8 | — | 9 | 9 |
| Mean daratumumab serum concentration, μg/mL (SD) | 321.14 (49.0) | 517.46 (137.2) | 895.61 (169.5) | |
| CV, % | 15.3 | — | 26.5 | 18.9 |
| Geometric mean daratumumab serum concentration, μg/mL | 317.73 | 502.54 | 882.53 | |
| Split first dose | ||||
| Patients, n | — | 69 | 52 | 52 |
| Mean daratumumab serum concentration, μg/mL (SD) | 255.03 (71.87) | 618.53 (256.2) | 951.44 (350.1) | |
| CV, % | — | 28.2 | 41.4 | 36.8 |
| Geometric mean daratumumab serum concentration, μg/mL | 244.90 | 549.57 | 855.74 | |
| . | Postfirst dose . | C3 D1 preinfusion . | C3 D1 end of infusion . | |
|---|---|---|---|---|
| C1 D1 end of infusion . | C1 D2 end of infusion . | |||
| Single first dose | ||||
| Patients, n | 8 | — | 9 | 9 |
| Mean daratumumab serum concentration, μg/mL (SD) | 321.14 (49.0) | 517.46 (137.2) | 895.61 (169.5) | |
| CV, % | 15.3 | — | 26.5 | 18.9 |
| Geometric mean daratumumab serum concentration, μg/mL | 317.73 | 502.54 | 882.53 | |
| Split first dose | ||||
| Patients, n | — | 69 | 52 | 52 |
| Mean daratumumab serum concentration, μg/mL (SD) | 255.03 (71.87) | 618.53 (256.2) | 951.44 (350.1) | |
| CV, % | — | 28.2 | 41.4 | 36.8 |
| Geometric mean daratumumab serum concentration, μg/mL | 244.90 | 549.57 | 855.74 | |
C, cycle; CV, coefficient of variation; D, day; SD, standard deviation.
Safety
The most frequent all-grade hematologic TEAEs were thrombocytopenia (67%) and anemia (51%), and the most frequent nonhematologic TEAEs were nausea (41%), upper respiratory tract infection (41%), asthenia (40%), and vomiting (40%) (Table 3). The most frequent grade 3/4 TEAEs were thrombocytopenia (31%), lymphopenia (24%), anemia (21%), neutropenia (21%), hypertension (18%), and asthenia (12%) (Table 3). Grade 3/4 infections were observed in 16 (19%) patients, with pneumonia being the most common (5%). A similar safety profile was observed in the lenalidomide-refractory cohort.
Most common (>10%) TEAEs
| . | Any grade . | Grade 3/4 . |
|---|---|---|
| Total TEAEs | 85 (100) | 65 (77) |
| Thrombocytopenia | 57 (67) | 26 (31) |
| Anemia | 43 (51) | 18 (21) |
| Nausea | 35 (41) | 1 (1) |
| Upper respiratory tract infection | 35 (41) | 1 (1) |
| Asthenia | 34 (40) | 10 (12) |
| Vomiting | 34 (40) | 1 (1) |
| Pyrexia | 30 (35) | 1 (1) |
| Diarrhea | 29 (34) | 2 (2) |
| Dyspnea | 29 (34) | 3 (4) |
| Insomnia | 27 (32) | 4 (5) |
| Neutropenia | 26 (31) | 18 (21) |
| Hypertension | 24 (28) | 15 (18) |
| Lymphopenia | 23 (27) | 20 (24) |
| Cough | 22 (26) | 0 |
| Headache | 21 (25) | 1 (1) |
| Back pain | 20 (24) | 0 |
| Bronchitis | 14 (17) | 0 |
| Fatigue | 13 (15) | 3 (4) |
| Nasopharyngitis | 13 (15) | 0 |
| Respiratory tract infection | 13 (15) | 0 |
| Constipation | 12 (14) | 0 |
| Gastroenteritis | 12 (14) | 0 |
| Peripheral edema | 12 (14) | 0 |
| Influenza | 11 (13) | 3 (4) |
| Muscle spasms | 11 (13) | 0 |
| Pain in extremity | 10 (12) | 0 |
| Musculoskeletal chest pain | 9 (11) | 0 |
| . | Any grade . | Grade 3/4 . |
|---|---|---|
| Total TEAEs | 85 (100) | 65 (77) |
| Thrombocytopenia | 57 (67) | 26 (31) |
| Anemia | 43 (51) | 18 (21) |
| Nausea | 35 (41) | 1 (1) |
| Upper respiratory tract infection | 35 (41) | 1 (1) |
| Asthenia | 34 (40) | 10 (12) |
| Vomiting | 34 (40) | 1 (1) |
| Pyrexia | 30 (35) | 1 (1) |
| Diarrhea | 29 (34) | 2 (2) |
| Dyspnea | 29 (34) | 3 (4) |
| Insomnia | 27 (32) | 4 (5) |
| Neutropenia | 26 (31) | 18 (21) |
| Hypertension | 24 (28) | 15 (18) |
| Lymphopenia | 23 (27) | 20 (24) |
| Cough | 22 (26) | 0 |
| Headache | 21 (25) | 1 (1) |
| Back pain | 20 (24) | 0 |
| Bronchitis | 14 (17) | 0 |
| Fatigue | 13 (15) | 3 (4) |
| Nasopharyngitis | 13 (15) | 0 |
| Respiratory tract infection | 13 (15) | 0 |
| Constipation | 12 (14) | 0 |
| Gastroenteritis | 12 (14) | 0 |
| Peripheral edema | 12 (14) | 0 |
| Influenza | 11 (13) | 3 (4) |
| Muscle spasms | 11 (13) | 0 |
| Pain in extremity | 10 (12) | 0 |
| Musculoskeletal chest pain | 9 (11) | 0 |
All data are n (%). N = 85.
Serious TEAEs were reported in 38 (45%) patients; 7 (8%) events were considered reasonably related to daratumumab, 15 (18%) events were considered reasonably related to carfilzomib, and 12 (14%) events were considered reasonably related to dexamethasone. The most common (≥3 patients) serious TEAEs were pneumonia (5%), as well as upper respiratory tract infection, general physical health deterioration, and hypercalcemia (4% each). Four (5%) patients discontinued treatment because of TEAEs, including grade 4 thrombocytopenia, grade 3 asthenia, grade 3 prostate cancer, and grade 2 back pain (1 case each). Three deaths due to TEAEs occurred: 2 due to general physical health deterioration (not related to treatment) and 1 due to multiple organ dysfunction (possibly related to treatment).
All-grade cardiac TEAEs occurred in 24 patients, with a median (range) onset time of 206 (1-583) days. One patient had a grade 4 TEAE (left ventricular failure) that resolved; it was not related to daratumumab and was very likely related to carfilzomib. Seven grade 3 cardiac TEAEs occurred that resolved: systolic dysfunction (n = 2) and myocarditis, cardiac failure, myocardial ischemia, atrial fibrillation, and sinus tachycardia (n = 1 each). Two (2%) patients had unresolved grade 3 cardiac TEAEs (congestive cardiomyopathy and cardiac failure) that were not related to daratumumab. Carfilzomib was interrupted (7 patients) or withdrawn (3 patients) for all grade 3/4 cardiac TEAEs, with the exception of 1 case, in which only daratumumab was interrupted (grade 3 sinus tachycardia); cardiac TEAEs improved in grade when carfilzomib was interrupted. Overall, no notable change from baseline over time was observed for median LVEF. Median (range) LVEF was 64% (44-83) at baseline (n = 84), 62% (46-77) at cycle 6 (n = 54), 61% (32-76) at cycle 12 (n = 47), 59% (50-74) at cycle 18 (n = 22), and 63% (53-76) at cycle 24 (n = 10).
IRRs occurred in 6 (60%) of the 10 patients who received the single first-dose infusion and in 32 (43%) of the 75 patients who received the split first-dose infusion; the vast majority took place during the first infusion (Table 4). IRRs were generally mild, with only 3 grade 3/4 IRRs (dyspnea, hypertension, and sinus tachycardia; all in the split dose group). IRRs occurring in >1 patient during all infusions in the split first-dose group were allergic rhinitis and vomiting (6 patients each), throat irritation, dyspnea, nausea, pyrexia, and flushing (3 patients each), and cough, nasal congestion, chills, and hypertension (2 patients each). For patients receiving the single first-dose infusion, IRRs occurring in >1 patient during all infusions included nausea (4 patients) and vomiting and hypertension (2 patients each).
IRRs during all infusions
| . | Single first dose (n = 10) . | Split first dose (n = 75) . | ||||||
|---|---|---|---|---|---|---|---|---|
| First infusion . | Second infusion . | Subsequent infusions . | Total . | First infusion . | Second infusion . | Subsequent infusions . | Total . | |
| Total patients with IRR | 5 (50) | 2 (20) | 1 (10) | 6 (60) | 28 (37) | 1 (1) | 6 (8) | 32 (43) |
| Patients with an IRR | ||||||||
| Allergic rhinitis | 0 | 0 | 0 | 0 | 6 (8) | 0 | 0 | 6 (8) |
| Throat irritation | 1 (10) | 1 (10) | 0 | 1 (10) | 4 (4) | 0 | 1 (1) | 3 (4) |
| Cough | 0 | 0 | 0 | 0 | 2 (3) | 0 | 0 | 2 (3) |
| Dyspnea | 0 | 0 | 0 | 0 | 3 (4) | 0 | 0 | 3 (4) |
| Nasal congestions | 0 | 0 | 0 | 0 | 2 (3) | 2 (3) | 0 | 0 |
| Wheezing | 0 | 0 | 0 | 0 | 1 (1) | 0 | 0 | 1 (3) |
| Vomiting | 2 (20) | 0 | 0 | 2 (20) | 5 (7) | 0 | 1 (1) | 6 (8) |
| Nausea | 3 (30) | 2 (20) | 0 | 4 (40) | 1 (1) | 1 (1) | 1 (1) | 3 (4) |
| Abdominal discomfort | 0 | 0 | 0 | 0 | 1 (1) | 0 | 0 | 1 (1) |
| Pyrexia | 0 | 1 (10) | 0 | 1 (10) | 1 (1) | 2 (3) | 0 | 3 (4) |
| Chills | 0 | 0 | 0 | 0 | 2 (3) | 0 | 0 | 2 (3) |
| Administration site inflammation | 0 | 0 | 0 | 0 | 1 (1) | 0 | 0 | 1 (1) |
| Feeling cold | 0 | 0 | 0 | 0 | 1 (1) | 0 | 0 | 1 (1) |
| Hyperthermia | 0 | 0 | 0 | 0 | 1 (1) | 0 | 0 | 1 (1) |
| Flushing | 0 | 0 | 0 | 0 | 2 (3) | 1 (1) | 0 | 3 (4) |
| Hypertension | 1 (10) | 0 | 0 | 2 (20) | 2 (3) | 0 | 0 | 2 (3) |
| Hot flush | 0 | 0 | 0 | 0 | 2 (3) | 0 | 0 | 2 (3) |
| Hypotension | 0 | 0 | 0 | 0 | 1 (1) | 0 | 0 | 1 (1) |
| Tachycardia | 0 | 0 | 1 (10) | 1 (10) | 0 | 0 | 1 (1) | 1 (1) |
| Sinus tachycardia | 0 | 0 | 0 | 0 | 1 (1) | 0 | 0 | 1 (1) |
| Erythema | 1 (10) | 0 | 1 (10) | 1 (10) | 1 (1) | 0 | 0 | 1 (1) |
| Hyperhidrosis | 0 | 0 | 1 (10) | 1 (10) | 0 | 1 (1) | 0 | 1 (1) |
| Pruritus | 0 | 0 | 0 | 0 | 1 (1) | 0 | 0 | 1 (1) |
| Rash | 0 | 0 | 0 | 0 | 1 (1) | 0 | 0 | 1 (1) |
| Urticaria | 0 | 0 | 0 | 0 | 1 (1) | 0 | 0 | 1 (1) |
| Dizziness | 0 | 0 | 0 | 0 | 1 (1) | 0 | 0 | 1 (1) |
| Back pain | 0 | 0 | 0 | 0 | 1 (1) | 0 | 0 | 1 (1) |
| Eyelid edema | 0 | 0 | 0 | 0 | 1 (1) | 0 | 0 | 1 (1) |
| Nervousness | 1 (10) | 0 | 1 (10) | 1 (10) | 0 | 0 | 0 | 0 |
| . | Single first dose (n = 10) . | Split first dose (n = 75) . | ||||||
|---|---|---|---|---|---|---|---|---|
| First infusion . | Second infusion . | Subsequent infusions . | Total . | First infusion . | Second infusion . | Subsequent infusions . | Total . | |
| Total patients with IRR | 5 (50) | 2 (20) | 1 (10) | 6 (60) | 28 (37) | 1 (1) | 6 (8) | 32 (43) |
| Patients with an IRR | ||||||||
| Allergic rhinitis | 0 | 0 | 0 | 0 | 6 (8) | 0 | 0 | 6 (8) |
| Throat irritation | 1 (10) | 1 (10) | 0 | 1 (10) | 4 (4) | 0 | 1 (1) | 3 (4) |
| Cough | 0 | 0 | 0 | 0 | 2 (3) | 0 | 0 | 2 (3) |
| Dyspnea | 0 | 0 | 0 | 0 | 3 (4) | 0 | 0 | 3 (4) |
| Nasal congestions | 0 | 0 | 0 | 0 | 2 (3) | 2 (3) | 0 | 0 |
| Wheezing | 0 | 0 | 0 | 0 | 1 (1) | 0 | 0 | 1 (3) |
| Vomiting | 2 (20) | 0 | 0 | 2 (20) | 5 (7) | 0 | 1 (1) | 6 (8) |
| Nausea | 3 (30) | 2 (20) | 0 | 4 (40) | 1 (1) | 1 (1) | 1 (1) | 3 (4) |
| Abdominal discomfort | 0 | 0 | 0 | 0 | 1 (1) | 0 | 0 | 1 (1) |
| Pyrexia | 0 | 1 (10) | 0 | 1 (10) | 1 (1) | 2 (3) | 0 | 3 (4) |
| Chills | 0 | 0 | 0 | 0 | 2 (3) | 0 | 0 | 2 (3) |
| Administration site inflammation | 0 | 0 | 0 | 0 | 1 (1) | 0 | 0 | 1 (1) |
| Feeling cold | 0 | 0 | 0 | 0 | 1 (1) | 0 | 0 | 1 (1) |
| Hyperthermia | 0 | 0 | 0 | 0 | 1 (1) | 0 | 0 | 1 (1) |
| Flushing | 0 | 0 | 0 | 0 | 2 (3) | 1 (1) | 0 | 3 (4) |
| Hypertension | 1 (10) | 0 | 0 | 2 (20) | 2 (3) | 0 | 0 | 2 (3) |
| Hot flush | 0 | 0 | 0 | 0 | 2 (3) | 0 | 0 | 2 (3) |
| Hypotension | 0 | 0 | 0 | 0 | 1 (1) | 0 | 0 | 1 (1) |
| Tachycardia | 0 | 0 | 1 (10) | 1 (10) | 0 | 0 | 1 (1) | 1 (1) |
| Sinus tachycardia | 0 | 0 | 0 | 0 | 1 (1) | 0 | 0 | 1 (1) |
| Erythema | 1 (10) | 0 | 1 (10) | 1 (10) | 1 (1) | 0 | 0 | 1 (1) |
| Hyperhidrosis | 0 | 0 | 1 (10) | 1 (10) | 0 | 1 (1) | 0 | 1 (1) |
| Pruritus | 0 | 0 | 0 | 0 | 1 (1) | 0 | 0 | 1 (1) |
| Rash | 0 | 0 | 0 | 0 | 1 (1) | 0 | 0 | 1 (1) |
| Urticaria | 0 | 0 | 0 | 0 | 1 (1) | 0 | 0 | 1 (1) |
| Dizziness | 0 | 0 | 0 | 0 | 1 (1) | 0 | 0 | 1 (1) |
| Back pain | 0 | 0 | 0 | 0 | 1 (1) | 0 | 0 | 1 (1) |
| Eyelid edema | 0 | 0 | 0 | 0 | 1 (1) | 0 | 0 | 1 (1) |
| Nervousness | 1 (10) | 0 | 1 (10) | 1 (10) | 0 | 0 | 0 | 0 |
All data are n (%).
Efficacy
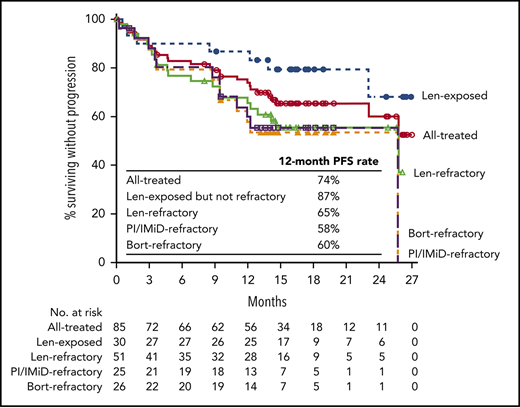
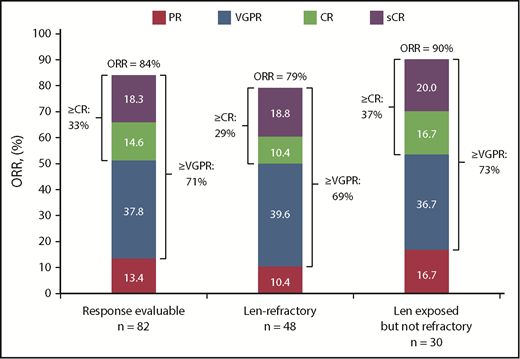
At a clinical cutoff (29 June 2018), median (range) follow-up was 16.6 (0.5-27.4) months overall and 16.4 (0.5-27.2) months for the lenalidomide-refractory cohort. The response evaluable analysis set included 82 patients who received ≥1 dose of any component of study treatment, were treated with >2 cycles or discontinued study treatment, and had ≥1 postbaseline disease assessment. At a median follow-up of 16.6 months, ORR was 84% in all treated patients, with 33% achieving a best response of CR or better and 71% achieving VGPR or better (Figure 1; Table 5). For the lenalidomide-refractory subgroup, ORR was 79%; 29% of patients achieved CR or better, and 69% of patients achieved VGPR or better. For bortezomib-refractory patients, ORR was 84%, including 20% of patients achieving CR or better. In an analysis of patients by baseline cytogenetic risk, patients with high-risk features achieved an ORR of 69% compared with an ORR of 90% for standard-risk patients (Table 5). Of the 11 patients overall with CR or better who underwent MRD testing, the MRD-negative rate (10−5) was 36% (4 patients); MRD assessments in patient subgroups are shown in Table 5.
Response rates in patients treated with daratumumab plus Kd. Data are based on a computerized algorithm. Len, lenalidomide; sCR, stringent complete response.
Response rates in patients treated with daratumumab plus Kd. Data are based on a computerized algorithm. Len, lenalidomide; sCR, stringent complete response.
ORR and MRD based on prior treatment history
| Subgroup . | ORR* . | MRD-negative rate (10−5)† . | ||
|---|---|---|---|---|
| Patients in group, n % Patients in group, n % | ||||
| All response evaluable | 82 | 84 | 11 | 36 |
| Refractory to lenalidomide | 48 | 79 | 5 | 20 |
| Lenalidomide exposed but not refractory | 30 | 90 | 4 | 50 |
| Refractory to bortezomib | 25 | 84 | 26 | 8 |
| Refractory to IMiD | 53 | 77 | 5 | 20 |
| IMiD exposed but not refractory | 29 | 97 | 6 | 50 |
| Refractory to PI and IMiD | 24 | 83 | 2 | 0 |
| Cytogenetic risk‡ | ||||
| High§ | 13 | 69 | 3 | 67 |
| Standard | 52 | 90 | 6 | 33 |
| Subgroup . | ORR* . | MRD-negative rate (10−5)† . | ||
|---|---|---|---|---|
| Patients in group, n % Patients in group, n % | ||||
| All response evaluable | 82 | 84 | 11 | 36 |
| Refractory to lenalidomide | 48 | 79 | 5 | 20 |
| Lenalidomide exposed but not refractory | 30 | 90 | 4 | 50 |
| Refractory to bortezomib | 25 | 84 | 26 | 8 |
| Refractory to IMiD | 53 | 77 | 5 | 20 |
| IMiD exposed but not refractory | 29 | 97 | 6 | 50 |
| Refractory to PI and IMiD | 24 | 83 | 2 | 0 |
| Cytogenetic risk‡ | ||||
| High§ | 13 | 69 | 3 | 67 |
| Standard | 52 | 90 | 6 | 33 |
Data are based on a computerized algorithm.
Of the 27 patients who achieved CR or better, 11 received MRD testing.
Biomarker risk-evaluable population.
Includes patients who have del17p, t(14;16), t(4;14) or a combination of these by fluorescence in situ hybridization or karyotype.
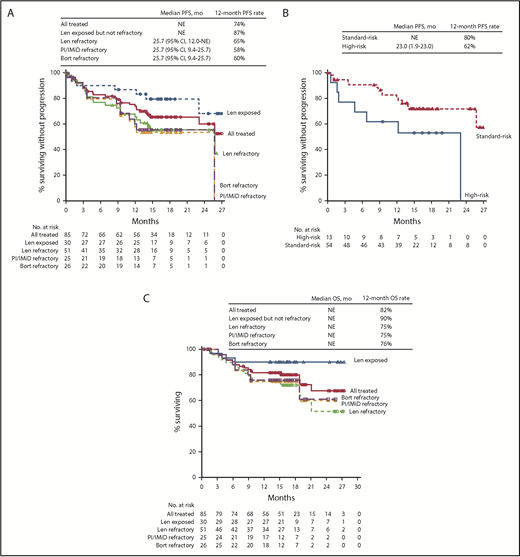
Median PFS was not reached in the all treated population, with 12- and 18-month PFS rates of 74% and 66%, respectively. In the lenalidomide-refractory cohort, the 12-month PFS rate was 65% (18-month PFS, 56%; Figure 2A). For bortezomib-refractory patients, 12-month PFS was 60%, and 18-month PFS was 55%. For patients in the PI/IMiD-refractory subgroup, 12-month PFS was 58%, and 18-month PFS was 53%. Median PFS was not reached for patients who were exposed, but not refractory, to IMiDs, with a 12-month PFS rate of 93% and an 18-month PFS rate of 86% (Figure 2A). In an exploratory analysis of PFS based on cytogenetic risk classification at baseline, the 12-month PFS rate was 80% in patients with standard risk (n = 54) and 62% in those with high-risk status (n = 13; Figure 2B); 18-month PFS rates were 72% and 53%, respectively.
PFS and OS in patients treated with daratumumab plus Kd. Median PFS in the all treated population and across subgroups (A), median PFS by cytogenetic risk group at baseline (B), and median OS in the all treated population and across subgroups (C) at a median follow-up of 16.6 months. Bort, bortezomib; Len, lenalidomide; NE, not evaluable.
PFS and OS in patients treated with daratumumab plus Kd. Median PFS in the all treated population and across subgroups (A), median PFS by cytogenetic risk group at baseline (B), and median OS in the all treated population and across subgroups (C) at a median follow-up of 16.6 months. Bort, bortezomib; Len, lenalidomide; NE, not evaluable.
At a median follow-up of 16.6 months, median OS had not been reached in the all treated population; the 12-month OS rate was 82% (Figure 2C). Median OS also had not been reached for the lenalidomide-refractory and PI/IMiD-refractory subgroups, with 12-month OS rates of 75% for both groups; the 12-month OS rate for bortezomib-refractory patients was 76%. For patients with prior IMiD exposure who were not IMiD refractory, 12-month OS was 97%. In patients classified as having high cytogenetic risk at baseline, 12-month OS was 69% (4 events); 12-month OS for patients with standard cytogenetic risk was 88% (9 events).
Discussion
In this study, the combination of daratumumab and weekly Kd showed promising efficacy with acceptable tolerability in patients with RRMM, including those with lenalidomide-refractory disease. All patients in this study were previously treated with a PI and an IMiD, and their disease had progressed after these standards of care. D-Kd in this study demonstrated a safety profile consistent with previous reports for the individual agents. Carfilzomib treatment has been associated with cardiovascular AEs, including low rates of grade 3/4 cardiac failure, dyspnea, and hypertension.32,33 However, in randomized phase 3 studies, the incidence of treatment discontinuation or death due to these cardiac events is low and comparable between treatment arms, highlighting the importance of control arms in elucidating treatment-related toxicities.33 In this study, median LVEF remained stable, cardiac TEAEs were manageable and typically resolved, and no patient discontinued study treatment or died from cardiac TEAEs.
Splitting the first dose of daratumumab reduced the duration of the first infusion (4.3 vs 7.1 hours), with similar pharmacokinetic concentrations following administration of the first 16 mg/kg dose and similar rates of IRRs. A split first dose of daratumumab is also under evaluation in the daratumumab plus KRd arm of this study in patients with NDMM34 and in the phase 2 LYRA study of daratumumab plus cyclophosphamide, bortezomib, and dexamethasone in patients with NDMM or relapsed MM.35 Similar reductions in the durations of first infusions were observed, suggesting that this may be an option to improve patient convenience and ease of outpatient administration for initial dosing. Based on the data from the present study, the split first daratumumab dosing regimen was recently approved.36,37
Results from this study showed that D-Kd induced deep and durable responses, regardless of prior lenalidomide exposure or refractoriness. At a median follow-up of 16.6 months, ORR was 84%, including 33% of patients with CR or better and 71% with VGPR or better. Deep responses were also achieved in lenalidomide-refractory patients, with an ORR of 79%, CR or better rate of 29%, and VGPR or better rate of 69%. Results from optional MRD testing in patients with CR or better show promising results across subgroups; however, results for MRD negativity in subgroups should be interpreted with caution because of the small patient number. Based on experience with daratumumab plus standard-of-care regimens, we anticipate that these responses may continue to deepen with longer follow-up. Median PFS was not reached with D-Kd for all treated patients, and an 18-month PFS rate of 56% in the lenalidomide-refractory subgroup is highly encouraging. Efficacy benefits were consistent across subgroups, with high response rates and impressive PFS and OS rates for patients who were bortezomib refractory, IMiD exposed but not refractory, IMiD refractory, refractory to both a PI and an IMiD, and those with high cytogenetic risk at baseline.
These findings complement the results of CASTOR, in which D-Vd induced a high ORR and significantly reduced the risk for disease progression or death compared with Vd alone in the overall population (median 2 prior lines of therapy) and in a subset of patients who were lenalidomide refractory at the last prior line of therapy (median PFS 9.3 months with D-Vd vs 4.4 months with Vd; hazard ratio [HR], 0.36; 95% CI, 0.26-0.63; P = .0002).21 ORR was 81% vs 50% (P = .0021) and the MRD-negative rate (10−5) was 9% vs 0% with D-Vd and Vd, respectively. In the present study, addition of daratumumab to another PI, carfilzomib, resulted in deep and durable responses, including MRD negativity, in patients with RRMM, including those with lenalidomide-refractory disease. Although limited by the small sample size, lack of randomization, and absence of information on the prior dose of lenalidomide, these results suggest that carfilzomib may provide a more potent option than bortezomib, when combined with daratumumab, for the treatment of lenalidomide-refractory patients. Daratumumab has also demonstrated efficacy in combination with pomalidomide and dexamethasone in patients with lenalidomide-refractory RRMM, with a median PFS of 9.9 months and ORR of 66% in the all treated population (median 4 prior lines of therapy; 89% were lenalidomide refractory).6
The results from this study compare favorably with studies of regimens not containing daratumumab in patients with lenalidomide-refractory RRMM. In the phase 3 study ELOQUENT-3 of elotuzumab plus pomalidomide and dexamethasone vs pomalidomide and dexamethasone alone, in which 97% of patients were refractory to lenalidomide (median 3 prior lines of therapy), elotuzumab plus pomalidomide and dexamethasone resulted in a median PFS of 10.3 months vs 4.7 months for pomalidomide and dexamethasone alone (HR, 0.34; 95% CI, 0.34-0.86; P = .008).38 A subgroup analysis of the phase 3 OPTIMISMM trial of pomalidomide plus Vd compared with Vd alone demonstrated a median PFS of 9.53 months with pomalidomide plus Vd vs 5.59 months with Vd in lenalidomide-refractory patients with a median of 2 prior lines of therapy (HR, 0.65; 95% CI, 0.50-0.84; P < .001).10 In a subgroup analysis of lenalidomide-refractory disease from the ENDEAVOR study of Kd vs Vd in RRMM (median 2 prior lines of therapy), median PFS was 8.6 vs 6.6 months (HR, 0.80; 95% CI, 0.57-1.11).7,8 In the current study of the addition of daratumumab to Kd for lenalidomide-refractory patients with a median of 2 prior lines of therapy (all previously treated with a PI and IMiD), median PFS was a remarkable 25.7 months. These results highlight the encouraging efficacy of D-Kd, but further studies are required to fully understand the impact of the type of and response to prior therapies for selection of therapy in second and later lines.
The limitations of this study are the small sample size, lack of a control or active-comparator arm, and median follow-up of only 16.6 months. Thus, longer follow-up time and a phase 3 randomized controlled trial are required to confirm the benefits of the D-Kd regimen for RRMM, particularly in lenalidomide-refractory patients. Evaluation of daratumumab plus Kd for lenalidomide-exposed RRMM is underway in the phase 3 CANDOR study; additionally, the phase 3 APOLLO study is investigating daratumumab plus pomalidomide and dexamethasone for lenalidomide-exposed RRMM.
In summary, no new safety signals were observed for D-Kd, and neutropenia rates were low. A split first daratumumab dose was well tolerated and may improve patient convenience for initial dosing by reducing the time required for the first infusion. Weekly carfilzomib dosing was also feasible and well tolerated. D-Kd is well tolerated and induced deep and durable responses in patients with RRMM, regardless of prior lenalidomide exposure or refractoriness. Patients with relapsed MM and prior lenalidomide exposure represent a growing population with a high unmet need, and daratumumab-based regimens are under active investigation in this clinical scenario.
The data-sharing policy of Janssen Pharmaceutical Companies of Johnson & Johnson is available at https://www.janssen.com/clinical-trials/transparency. As noted on this site, requests for access to the study data can be submitted through the Yale Open Data Access (YODA) Project site at http://yoda.yale.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the patients who participated in the MMY1001 study and their families, as well as the study coinvestigators, research nurses, and coordinators at each of the clinical sites.
This work was supported by Janssen Research & Development, LLC. Medical writing and editorial support were provided by Elise Blankenship (MedErgy) and were funded by Janssen Global Services, LLC.
Authorship
Contribution: A.C., S.L., J.U., and J.S. conceived and designed the study, acquired data, and analyzed and interpreted results; L.B., B.A., and P.R.-O. acquired study data; J.M.-L., M.-V.M., J.B., A.O., L.P., A.J., J.W., and P.M. acquired study data and analyzed and interpreted results; and C.d.B. and P.L.C. conceived and designed the study and analyzed and interpreted results. All authors drafted and reviewed the manuscript, approved the final version, decided to publish this report, and vouch for data accuracy and completeness.
Conflict-of-interest disclosures: A.C. has acted as a consultant for Array BioPharma, Celgene, Novartis, Millennium, Amgen, Janssen Oncology, and Bristol-Myers Squibb; received travel expenses from Takeda, Celgene, Novartis, Amgen, Janssen Oncology, and Bristol-Myers Squibb; and received research funding from Array BioPharma, Celgene, Millennium, Novartis, Onyx, Janssen, Pharmacyclics, Acetylon Pharmaceuticals, Biotest, and Bristol-Myers Squibb. J.M.-L. has received research funding from Bristol-Myers Squibb, Janssen, Novartis, Pfizer, and Celgene; received honoraria from/participated in speakers bureaus for Bristol-Myers Squibb, Janssen, Celgene, Novartis, Incyte, and Takeda; and reports ownership interest in Vivia Biotech. M.-V.M. has served on advisory boards for and received honoraria from Janssen, Amgen, Takeda, Celgene, GlaxoSmithKline, EDO, and Pharmamar. J.B. has received honoraria for lectures from Janssen, Amgen, Celgene, Takeda, and Binding Site. L.B. has received honoraria from and acted as a consultant for Celgene, Takeda, Janssen, and Amgen. A.O. has served on advisory boards or sponsored symposia for Amgen, Celgene, Janssen, and Takeda. B.A. has received honoraria from Janssen, Amgen, and Celgene and has served on advisory committees for Amgen. P.R.-O. has served on advisory boards for and received honoraria for lectures from Janssen, Celgene, Takeda, Sanofi, Merck Sharp & Dohme, and Bristol-Myers Squibb. L.P. has participated in speakers bureaus for Alexion Pharmaceuticals and Seattle Genetics. A.J. has served on advisory boards or as a consultant for and has received honoraria from AbbVie, Amgen, Adaptive, Bristol-Myers Squibb, Celgene, Janssen, Karyopharm, Takeda, and SkylineDx. C.d.B., J.W., P.L.C., J.U., and J.S. are employees of Janssen Research & Development. S.L. has acted as a consultant for or served in an advisory role for Amgen, Bristol-Myers Squibb, Celgene, GlaxoSmithKline, Janssen Oncology, Millennium, Novartis, and Onyx. P.M. has served on advisory boards and received honoraria from Janssen, Amgen, Takeda, AbbVie, and Celgene.
Correspondence: Ajai Chari, Tisch Cancer Institute, Mount Sinai School of Medicine, 1 Gustave Levy Place, Box 1185, New York, NY 10029; e-mail: ajai.chari@mountsinai.org.