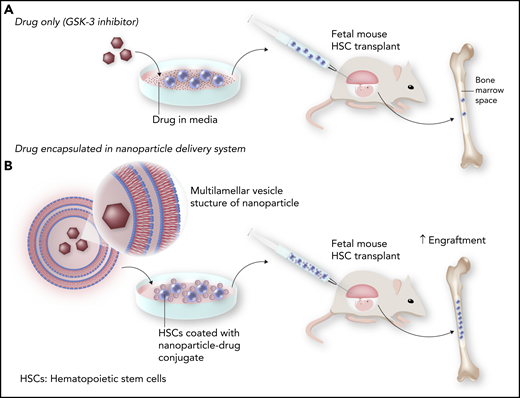
In this issue of Blood, Loukogeorgakis et al1 demonstrate improved fetal engraftment of transplanted hematopoietic stem cells (HSCs) in an in utero mouse model by coating the transplanted cells with functional nanoparticles. These nanoparticles continuously deliver a hydrochloride of CHIR99021, a molecule that inhibits glycogen synthase kinase-3 (GSK-3) and improves the repopulation capacity of the HSCs (see figure). Inhibition of GSK-3 in vivo has been shown to increase HSC engraftment in adult mouse transplantation models.2 However, similar levels of engraftment through inhibition of GSK-3 would be difficult to achieve in fetal mouse models because of limitations in continuous drug delivery modalities when targeting fetal tissues. To date, the most feasible method of continuous delivery of any substance to a fetus has been via the maternal circulation, but this method often results in variable, insufficient, and systemic rather than targeted delivery to the fetus, while simultaneously exposing the mother to the substance.3 To overcome this limitation, Loukogeorgakis and colleagues make use of a nanoparticle drug delivery system that involves a multilayered, lipid-based vesicle encapsulating the drug of interest (in this case, the GSK-3 inhibitor).4 The particles adhere to the surface of hematopoietic cells during an in vitro coincubation period by covalent bonding. The nanoparticles are not endocytosed and therefore remain attached to the surface of stem cells and continuously release the GSK-3 inhibitor. This results in continuous exposure of the HSCs to the GSK-3 inhibitor until the particle naturally degrades, which effectively improves engraftment of the transplanted HSCs compared with both untreated cells and cells treated in vitro only with the GSK-3 inhibitor in the cell culture media.
Improved engraftment with nanoparticle delivery of GSK-3 inhibitor following in utero HSC transplantation. Transient exposure of HSCs to the GSK-3 inhibitor in culture prior to transplantation leads to modest engraftment in the in utero setting (A). However, encapsulation of the GSK-3 inhibitor with nanoparticles that adhere to the HSCs and release the inhibitor over time improves HSC engraftment (B). Professional illustration by Somersault18:24.
Improved engraftment with nanoparticle delivery of GSK-3 inhibitor following in utero HSC transplantation. Transient exposure of HSCs to the GSK-3 inhibitor in culture prior to transplantation leads to modest engraftment in the in utero setting (A). However, encapsulation of the GSK-3 inhibitor with nanoparticles that adhere to the HSCs and release the inhibitor over time improves HSC engraftment (B). Professional illustration by Somersault18:24.
The concepts presented in this work are notable and have the potential to significantly impact the field of hematology. First, although the drug delivered in this study is a GSK-3 inhibitor, there are likely other molecules or combinations of molecules that can be similarly encapsulated and delivered to achieve higher efficacy. Second, the material used in this nanoparticle delivery system is lipid based and biodegradable. This probably contributes to the tolerance of the treatment and may help limit the host inflammatory response, thereby protecting the HSCs from damaging cytokines. Finally, the coincubation step to adhere the nanoparticles is simple and relatively quick, characteristics that will be highly valued when translating this approach to the clinic. It should be noted that a variety of other nanoparticle drug delivery systems are being developed, including particles made of other inert materials, dual-drug delivery systems that incorporate hydrophilic and hydrophobic drugs in different regions of the nanoparticle, particles that are endocytosed via ligands conjugated to the particle surface for intracellular delivery, and particles that are degraded and deliver their payload based on the pH differences inside a cell.5 This rapidly advancing field of drug delivery is encouraging, particularly now that preclinical models are demonstrating their potential to improve therapeutic efficacy.
The specific impact of the work by Loukogeorgakis and colleagues is most relevant to diseases that can be detected in utero and that can otherwise be cured by allogeneic transplantation. Advances in molecular techniques, such as next-generation sequencing, make it feasible to perform rapid diagnostic testing early in pregnancy despite the small quantities of DNA that can be collected. Of course, the natural inclination after achieving early diagnosis is the desire to treat as expeditiously as possible. However, postnatal allogeneic transplantation involving conditioning chemotherapy and radiation is currently the only routinely available treatment of applicable prenatally diagnosed conditions. In utero transplantation potentially avoids the need for chemotherapy by engrafting the incompletely filled marrow space of a fetus.6 To date, there have been concerns about maintained long-term engraftment of cells following in utero transplant, because preclinical models have shown insufficient engraftment to cure disease. The microchimerism achieved with in utero transplant does seem to allow a less intense conditioning regimen in the postnatal period, and a postnatal “boost” of hematopoietic cells from the same donor used for the in utero transplant can lead to stable engraftment of a therapeutic number of cells.7 In a disease like sickle cell disease, where 20% to 25% donor cell engraftment is sufficient to result in disease resolution, this approach may be considered a less toxic way to achieve cure.8,9 However, with the in vivo GSK-3 inhibition approach, it may be feasible to achieve a therapeutic level of fetal engraftment without needing to perform a second transplant “boost” after birth.
As the authors allude to, more investigation is warranted to determine if this model is durable and capable of long-term hematopoiesis. This study’s findings also need to be recapitulated with human HSCs preclinically and ultimately further tested in postnatal and in utero clinical trials. Ultimately, with the right delivery, we are likely to see diseases being cured in utero, resulting in the best delivery one could ask for: a healthy newborn.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal