Key Points
Frequent apheresis platelet donors may have very low CD4+ and CD8+ T-cell counts.
The cytopenias do not appear to be harmful.
Abstract
More than 1 million apheresis platelet collections are performed annually in the United States. After 2 healthy plateletpheresis donors were incidentally found to have low CD4+ T-lymphocyte counts, we investigated whether plateletpheresis causes lymphopenia. We conducted a cross-sectional single-center study of platelet donors undergoing plateletpheresis with the Trima Accel, which removes leukocytes continuously with its leukoreduction system chamber. We recruited 3 groups of platelet donors based on the total number of plateletpheresis sessions in the prior 365 days: 1 or 2, 3 to 19, or 20 to 24. CD4+ T-lymphocyte counts were <200 cells per microliter in 0/20, 2/20, and 6/20 donors, respectively (P = .019), and CD8+ T-lymphocyte counts were low in 0/20, 4/20, and 11/20 donors, respectively (P < .001). The leukoreduction system chamber’s lymphocyte-extraction efficiency was ∼15% to 20% for all groups. Immunophenotyping showed decreases in naive CD4+ T-lymphocyte and T helper 17 (Th17) cell percentages, increases in CD4+ and CD8+ effector memory, Th1, and regulatory T cell percentages, and stable naive CD8+ and Th2 percentages across groups. T-cell receptor repertoire analyses showed similar clonal diversity in all groups. Donor screening questionnaires supported the good health of the donors, who tested negative at each donation for multiple pathogens, including HIV. Frequent plateletpheresis utilizing a leukoreduction system chamber is associated with CD4+ and CD8+ T-cell lymphopenia in healthy platelet donors. The mechanism may be repeated extraction of these cells during plateletpheresis. The cytopenias do not appear to be harmful.
Introduction
Apheresis platelet donors undergo a procedure called plateletpheresis to collect platelets that can be transfused to patients who are bleeding or at risk for bleeding. More than 1 million plateletpheresis procedures are performed each year in the United States alone.1 Currently, apheresis platelet donors in the United States can donate up to 24 times in a rolling 12-month period. During plateletpheresis, white blood cells that would otherwise contaminate platelet units are removed in a process termed leukoreduction. Leukoreduction reduces febrile reactions2 and HLA alloimmunization3 in susceptible recipients. A commonly used leukoreduction method, utilized in the Trima Accel Automated Blood Collection System (Terumo BCT, Lakewood, CO), involves a leukoreduction system chamber that sequesters large numbers of white blood cells, mainly T lymphocytes.4-6 These cells are not returned to the donor.
In the past, concerns have been raised about lymphocyte depletion in platelet donors.7-12 A 1988 US Food and Drug Administration (FDA) guidance required that informed consent for platelet donation include a statement that the long-term effect of lymphocyte reduction was not clear.7 The FDA issued a draft guidance in 2005 that proposed additional restrictions on platelet donation, including limiting the number of units that could be donated in a rolling 12-month period13 ; however, these restrictions were not mandated in the final 2007 guidance.14 This was likely due to an absence of high-quality data to suggest harm and a belief that improvements in apheresis technology had mitigated the risk of lymphocyte depletion.7,15-17 Indeed, in 1 contemporary study, lymphocyte counts in frequent platelet donors were not observed to change over a relatively short study period (≤130 weeks), with the limitation that absolute lymphocyte counts were not reported.15 Given that lymphocyte depletion was no longer felt to be occurring, the FDA dropped the requirement that donor consent forms include lymphocyte reduction as a potential risk in its 2007 guidance.14
In May of 2017, a frequent apheresis platelet donor was referred to 1 of us (J.M.G.) for outpatient hematology consultation due to CD4+ T-cell lymphopenia. The donor had undergone 24 plateletpheresis sessions in the prior year using the Trima Accel and had a lifetime history of 212 plateletpheresis donations. While volunteering as a healthy control in a research study, he had been found to have a CD4+ T-lymphocyte count <200 cells per microliter. A second frequent apheresis platelet donor had been excluded from that study for the same reason. The degree of CD4+ T-cell lymphopenia in these donors was concerning, being characteristic of advanced immunodeficiency associated with HIV infection, but both had repeatedly tested negative for HIV using sensitive nucleic acid tests. We conducted a study to assess the prevalence of CD4+ T-cell lymphopenia in plateletpheresis donors and to identify its cause and clinical implications.
Methods
Study design
We performed a cross-sectional study evaluating immune cells in apheresis platelet donors at 1 hospital-based donor center. The research protocol was approved by the Partners HealthCare Institutional Review Board (2017P001432). All participants provided written informed consent. A total of 60 subjects were recruited prospectively, 20 in each of 3 prespecified groups defined by the number of plateletpheresis sessions in the prior 365 days, including the day of study participation: 1 or 2, 3 to 19, or 20 to 24 sessions. We reasoned that participants in the 1 or 2 sessions group would provide a reasonably strict control group, because they had not undergone plateletpheresis before entry into the study (in the prior 365 days, including the day of study participation) or they had undergone only 1 plateletpheresis session, which was viewed as unlikely to affect the results. To maximize the chance of detecting a difference in CD4+ T-lymphocyte counts between groups, and with the knowledge that the index case had undergone 24 plateletpheresis sessions in the prior 365 days (the maximum permitted by the FDA), we elected to create a group of 20 to 24 sessions. This left a broad intermediate group of 3 to 19 sessions. Only successful plateletpheresis sessions were counted; all day-of-participation sessions were successful. Study participants provided samples on 1 occasion and were not followed thereafter. Study data were managed using REDCap.18
Eligibility
Healthy volunteer platelet donors ≥18 years of age who met standard eligibility requirements to donate platelets were eligible. Subjects were excluded if they had donated platelets at any other site in the past.
Plateletpheresis
Plateletpheresis was performed according to the donor center's routine using the Trima Accel, which was deployed at the donor center in 2002 and has been the only instrument used since 2006. The Trima Accel replaced the COBE Spectra Apheresis System, which used similar apheresis technology but did not sequester as many lymphocytes in its leukoreduction system chamber.19 The anticoagulant ratio, draw management, return management, and maximal draw flows varied. Donors were programmed for single, double, or triple product collections depending on their baseline platelet count, tolerance of the anticoagulant, and time availability. For yield calculations, only 10 prior years of records were available electronically. Donation units were defined as follows based on actual yields from each session: singles, <6.4 × 1011 platelets; doubles, 6.4 to 9.5 × 1011 platelets; and triples, >9.5 × 1011 platelets.
Blood counts
Blood samples were obtained immediately before and after plateletpheresis. Postplateletpheresis samples were used only for extraction efficiency calculations. Cells sequestered in the leukoreduction system chamber were analyzed after plateletpheresis. These cells were isolated by cutting the plastic tubing near both ends and allowing the blood contents to empty directly into EDTA tubes for further analysis. Basic flow cytometry to obtain lymphocyte subset counts was performed using antibodies to CD3, CD4, CD8, CD16, CD56, and CD19 (BD 337166). T cells were identified as CD3+ and then divided into CD4+ and CD8+ populations. Laboratory reference ranges were 441 to 2156 cells per microliter for CD4+ T lymphocytes and 125 to 1312 cells per microliter for CD8+ T lymphocytes. A cutoff of 200 cells per microliter was used for CD4+ T lymphocytes, because this number is clinically actionable in certain settings. Complete blood counts with white cell differential counts were performed on a hematology analyzer (Sysmex, Lincolnshire, IL). White blood cell counts on the platelet products were performed using an ADAM-rWBC instrument (NanoEntek, Waltham, MA).
Extraction efficiency calculation
Cell-extraction efficiency by the leukoreduction system chamber was calculated as previously described.20 A chamber volume of 11.35 mL was assumed based on manufacturer specifications.
Detailed flow cytometry
Flow cytometry using larger panels of cell markers was performed as described in the supplemental Methods (available on the Blood Web site). A list of antibodies and other reagents used can be found in supplemental Table 1. Two samples were lost during processing due to tube breakage (one from the 1 or 2 sessions group and one from the 3 to 19 sessions group); 2 samples contained insufficient viable cells after thawing to be analyzed (one from the 1 or 2 sessions group and one from the 3 to 19 sessions group).
T-cell receptor diversity
Genomic DNA was extracted from 3 mL of peripheral blood (60 samples) and matched leukoreduction system chamber blood (2 samples) using a QIAGEN Gentra Puregene kit (QIAGEN 158467) with RNA removal. Purified DNA was submitted to Adaptive Biotechnologies (Seattle, WA) for immunoSEQ TCRB analysis. The survey method was used with the exception of leukoreduction system chamber blood, for which deep and survey methods were used. Productive clonality was calculated using Adaptive Biotechnologies immunoSEQ analyzer v3.0.
Statistical analysis
We investigated differences in CD4+ T-lymphocyte counts and other blood cell parameters using the 3 groups of donors specified in our study design. We initially examined CD4+ T-lymphocyte counts in a binary manner as <200 or ≥200 cells per microliter and CD8+ T-lymphocyte counts in a binary manner as <125 or ≥125 cells per microliter. For all subsequent analyses (including the P values in Figure 1A-B), blood count data were analyzed as continuous data. Donation category was viewed as an ordered categorical variable, and the Kruskal-Wallis test was used except where indicated. Nominal P values are presented. P values retaining or not retaining statistical significance at the P < .050 level after adjustment for multiplicity of testing using the Bonferroni method are indicated in the text and figure legends. We considered 3 linear regression models for each outcome. In the first, we used our 3 categories of donations, with those who were enrolled at the first or second donation in 365 days as the base category. In the second model, we considered decade of age and found no differences in CD4+ or CD8+ T-lymphocyte counts by decade in those donors who were <50 years of age, but significant differences associated with ages 50 to 59 years, 60 to 69 years, and ≥70 years and older. Our modeling then went forward with combined ages <50 years and potential differences by decade for older donors. Our combined multivariable model included indicators for the categorical variables for the 3 to 19 sessions group and the 20 to 24 sessions group in the prior 365 days, as well as for decade of age for those donors aged ≥50 years.
Blood counts. (A-B) CD4+ and CD8+ counts for the 3 groups. Donors who have undergone 20 to 24 successful plateletpheresis sessions in a 365-day period at any point in the prior 20 years are indicated by blue symbols. The dotted line indicates 200 cells per microliter (A) and the lower limit of normal (B). (C) CD4+ counts relative to the total number of plateletpheresis sessions in the prior 20 years; the horizontal dotted line indicates 200 cells per microliter; the vertical dotted line indicates 50 sessions. (D) CD8+ counts relative to the total number of plateletpheresis sessions in the prior 20 years; the horizontal dotted line indicates the lower limit of normal; the vertical dotted line indicates 50 sessions. Absolute lymphocyte (E) and monocyte (F) counts for the 3 groups; the dotted lines indicate the lower limit of normal. (G) White blood cell count, hematocrit, and platelet count for the 3 groups. Short horizontal lines indicate the median and interquartile range. All blood counts in this figure were obtained immediately before plateletpheresis. Asterisks denote P values that retain significance after Bonferroni correction for multiple testing. The caret (^) denotes a P value that does not retain significance after Bonferroni correction for multiple testing.
Blood counts. (A-B) CD4+ and CD8+ counts for the 3 groups. Donors who have undergone 20 to 24 successful plateletpheresis sessions in a 365-day period at any point in the prior 20 years are indicated by blue symbols. The dotted line indicates 200 cells per microliter (A) and the lower limit of normal (B). (C) CD4+ counts relative to the total number of plateletpheresis sessions in the prior 20 years; the horizontal dotted line indicates 200 cells per microliter; the vertical dotted line indicates 50 sessions. (D) CD8+ counts relative to the total number of plateletpheresis sessions in the prior 20 years; the horizontal dotted line indicates the lower limit of normal; the vertical dotted line indicates 50 sessions. Absolute lymphocyte (E) and monocyte (F) counts for the 3 groups; the dotted lines indicate the lower limit of normal. (G) White blood cell count, hematocrit, and platelet count for the 3 groups. Short horizontal lines indicate the median and interquartile range. All blood counts in this figure were obtained immediately before plateletpheresis. Asterisks denote P values that retain significance after Bonferroni correction for multiple testing. The caret (^) denotes a P value that does not retain significance after Bonferroni correction for multiple testing.
Results
Donor population
A total of 60 apheresis platelet donors were recruited from September of 2017 through November of 2017, with 20 subjects in each of 3 prespecified groups. Donor demographic characteristics are shown in Table 1. Donor age increased across groups as donation frequency increased (P = .003), but donor sex was not statistically different. Donors were predominantly white in all groups. In the prior 365-day period, a minority of donors had a history of concurrent platelet and fresh-frozen plasma collection by apheresis, concurrent platelet and red cell collection by apheresis, and/or concurrent platelet, fresh-frozen plasma, and red cell collection by apheresis. Such sessions were counted when determining group assignments. A minority of donors also had a history of whole blood donation during the prior 365-day period; these sessions were not counted, because they did not involve apheresis.
Characteristics of the donors
| Characteristic . | 1 or 2 Sessions group (n = 20) . | 3 to 19 Sessions group (n = 20) . | 20 to 24 Sessions group (n = 20) . | P . |
|---|---|---|---|---|
| Age, year, median (range) | 37.5 (19-72) | 60.5 (21-76) | 62.5 (28-79) | .003 |
| Sex, n (%) | .096 | |||
| Female | 10 (50) | 5 (25) | 4 (20) | |
| Male | 10 (50) | 15 (75) | 16 (80) | |
| Race, n (%)* | .105† | |||
| White | 14 (70) | 18 (90) | 19 (95) | |
| Other | 3 (15) | 1 (5) | 0 (0) | |
| Not reported | 3 (15) | 1 (5) | 1 (5) | |
| Plateletpheresis sessions, n (range) | ||||
| Median in prior 365 d | 1 (1-2) | 9 (3-19) | 23 (20-24) | |
| Median in prior 20 y | 2 (1-76) | 95 (3-322) | 256.5 (21-461) | <.001 |
| Characteristic . | 1 or 2 Sessions group (n = 20) . | 3 to 19 Sessions group (n = 20) . | 20 to 24 Sessions group (n = 20) . | P . |
|---|---|---|---|---|
| Age, year, median (range) | 37.5 (19-72) | 60.5 (21-76) | 62.5 (28-79) | .003 |
| Sex, n (%) | .096 | |||
| Female | 10 (50) | 5 (25) | 4 (20) | |
| Male | 10 (50) | 15 (75) | 16 (80) | |
| Race, n (%)* | .105† | |||
| White | 14 (70) | 18 (90) | 19 (95) | |
| Other | 3 (15) | 1 (5) | 0 (0) | |
| Not reported | 3 (15) | 1 (5) | 1 (5) | |
| Plateletpheresis sessions, n (range) | ||||
| Median in prior 365 d | 1 (1-2) | 9 (3-19) | 23 (20-24) | |
| Median in prior 20 y | 2 (1-76) | 95 (3-322) | 256.5 (21-461) | <.001 |
Race was reported by the donor. “Other” includes Asian, black or African American, and more than 1 race.
The P value does not take into account the “Not reported” category.
Donor blood counts
Flow cytometric analyses revealed striking decreases in CD4+ (Figure 1A) and CD8+ (Figure 1B) T-lymphocyte counts as donation frequency increased across groups. Zero of 20 donors (0%) in the 1 or 2 sessions group, 2 of 20 donors (10%) in the 3 to 19 sessions group, and 6 of 20 donors (30%) in the 20 to 24 sessions group had a CD4+ T-lymphocyte count < 200 cells per microliter (P = .019). CD8+ T-lymphocyte counts were below normal in 0 of 20 donors (0%), 4 of 20 donors (20%), and 11 of 20 donors (55%), respectively (P < .001). These findings remained significant when age by decade was considered as an additional variable in a least-squares regression model of quantitative CD4+ or CD8+ T-lymphocyte counts (Table 2). Absolute CD16+CD56+ natural killer (NK) cells and CD19+ B cells were similar across groups (supplemental Figure 1).
Linear regression models incorporating donation group and age by decade
| Model . | CD4+ T cells . | CD8+ T cells . | ||
|---|---|---|---|---|
| Estimate (cells per microliter) . | P . | Estimate (cells per microliter) . | P . | |
| Donation model | ||||
| Constant | 812 | 478 | ||
| 3-19 group | −256 | .004 | −176 | .005 |
| 20-24 group | −472 | <.001 | −308 | <.001 |
| Age model | ||||
| Constant | 801 | 511 | ||
| 50-59 y | −315 | .003 | −311 | <.001 |
| 60-69 y | −369 | <.001 | −328 | <.001 |
| ≥70 y | −507 | <.001 | −363 | <.001 |
| Multivariable model | ||||
| Constant | 890 | 560 | ||
| 3-19 group | −162 | .050 | −92 | .062 |
| 20-24 group | −305 | .001 | −155 | .004 |
| 50-59 y | −232 | .021 | −270 | <.001 |
| 60-69 y | −251 | .005 | −267 | <.001 |
| ≥70 y | −370 | <.001 | −293 | <.001 |
| Model . | CD4+ T cells . | CD8+ T cells . | ||
|---|---|---|---|---|
| Estimate (cells per microliter) . | P . | Estimate (cells per microliter) . | P . | |
| Donation model | ||||
| Constant | 812 | 478 | ||
| 3-19 group | −256 | .004 | −176 | .005 |
| 20-24 group | −472 | <.001 | −308 | <.001 |
| Age model | ||||
| Constant | 801 | 511 | ||
| 50-59 y | −315 | .003 | −311 | <.001 |
| 60-69 y | −369 | <.001 | −328 | <.001 |
| ≥70 y | −507 | <.001 | −363 | <.001 |
| Multivariable model | ||||
| Constant | 890 | 560 | ||
| 3-19 group | −162 | .050 | −92 | .062 |
| 20-24 group | −305 | .001 | −155 | .004 |
| 50-59 y | −232 | .021 | −270 | <.001 |
| 60-69 y | −251 | .005 | −267 | <.001 |
| ≥70 y | −370 | <.001 | −293 | <.001 |
When donor records dating back 20 years were examined, we determined that the 2 donors with a CD4+ T-lymphocyte count <200 cells per microliter in the 3 to 19 sessions group (Figure 1A) and all 4 donors with a CD8+ T-lymphocyte count <125 cells per microliter in the 3 to 19 sessions group (Figure 1B) had a history of undergoing 20 to 24 plateletpheresis sessions in a 365-day period other than the 1 immediately before study participation. Of 8 donors with a CD4+ T-lymphocyte count <200 cells per microliter, 7 had a CD8+ T-lymphocyte count below the normal range. These 8 donors had begun donating platelets by 1994 (4 donors), in 1999 (1 donor), in 2005 (1 donor), in 2007 (1 donor), and in 2008 (1 donor). All donors with a low CD4+ and/or CD8+ T-lymphocyte count were older than 55 years of age.
As an exploratory analysis, we examined the relationship between CD4+ and CD8+ T-lymphocyte counts and the number of plateletpheresis sessions over the last 20 years. The prevalence of CD4+ and CD8+ T-cell lymphopenia increased as the number of plateletpheresis sessions increased; there was a notable decrease in counts once 50 plateletpheresis sessions had been performed (Figure 1C-D). When 20-year donation history and age by decade were considered in a least-squares regression model, a history of ≥50 or more donations was associated with a significant decrease in CD4+ and CD8+ T lymphocytes (supplemental Table 2).
We also examined whether differences in the number of platelet products donated at each session impacted CD4+ and CD8+ T-lymphocyte counts. Session yields for the prior 10 years were reviewed and categorized as single-unit donations, double-unit donations, or triple-unit donations (supplemental Figure 2). A higher percentage of sessions during which double or triple unit donations were made over the previous 10 years was not associated with lower CD4+ and CD8+ T-lymphocyte counts (supplemental Figure 2). However, donors with higher total actual platelet yields over the prior 10 years tended to have lower CD4+ and CD8+ T-lymphocyte counts (supplemental Figure 2). Of the 4 donors with the highest total yields, the one with the lowest CD4+ and CD8+ T-lymphocyte counts was older than age 55 years; the others were younger, perhaps explaining why they diverged from the overall trend. There was no linear association between time since last donation in donors within the 20 to 24 sessions group and number of CD4+ or CD8+ T lymphocytes (P = .644 and P = .754, respectively). In each case, time since last donation explained ∼1% of the variability in T-lymphocyte counts (data not shown). We did not perform this analysis within the other 2 groups because the time since last plateletpheresis cannot be defined in 9 of 20 donors in the 1 or 2 sessions group (it was their very first donation), and the 3 to 19 sessions group is relatively heterogeneous, making it likely that variation in the number of recent donations would confound the analysis.
We observed a decrease in absolute lymphocyte counts across groups as donation frequency increased (P < .001) (Figure 1E). Absolute monocyte counts increased across groups, but this did not retain significance after adjustment for multiple testing (Figure 1F). Total white blood cell counts, hematocrits, and platelet counts were not significantly different across the 3 groups (Figure 1G). Absolute neutrophil, eosinophil, and basophil counts were similar across groups (supplemental Figure 3).
One donor in the 20 to 24 sessions group had a normal white blood cell count but a high CD16+CD56+ lymphocyte count (supplemental Figure 3). This donor intermittently spilled excessive numbers of white blood cells into platelet products collected during the prior 365-day period. Given the donor’s age (>70 years old), good health, and lack of anemia and thrombocytopenia, this suggested a potential diagnosis of chronic lymphoproliferative disorder of NK cells. Follow-up testing was compatible with this indolent disorder, although a nonspecific diagnosis of NK cell lymphocytosis was also considered. The consulting hematologist did not feel that bone marrow aspirate or biopsy was necessary, and mutational analysis for STAT3 and other mutations was not performed. The donor’s CD4+ T-lymphocyte count was >300 cells per microliter, and the CD8+ T-lymphocyte count was normal. Other than this 1 case, white blood cells were not lost in any significant number to the platelet products, and the products met criteria for being leukoreduced (supplemental Table 3).
Leukoreduction system chamber extraction efficiency
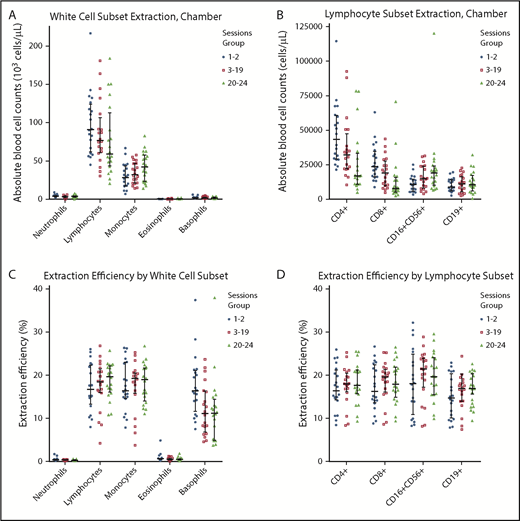
To investigate the potential mechanism of plateletpheresis-associated CD4+ and CD8+ T-cell lymphopenia in frequent platelet donors, we analyzed the blood cells in the leukoreduction system chamber at the end of each plateletpheresis session and calculated the efficiency of blood cell extraction. Leukoreduction system chambers contained high numbers of lymphocytes, including CD4+ and CD8+ T lymphocytes, and moderate numbers of monocytes, consistent with independent reports (Figure 2A-B; supplemental Figure 4; supplemental Table 4).4,5,19 The median number of lymphocytes in the leukoreduction system chambers was 10.3 × 108 for the 1 or 2 sessions group, 8.7 × 108 for the 3 to 19 sessions group, and 6.7 × 108 for the 20 to 24 sessions group (supplemental Table 4). The median number of CD4+ T lymphocytes in the leukoreduction system chambers was 4.9 × 108 cells, 3.6 × 108 cells and 1.9 × 108 cells, respectively (supplemental Table 4). The extraction efficiency of the chambers was ∼15% to 20% for lymphocytes and monocytes, ∼10% to 15% for basophils, and <1% for neutrophils and eosinophils (Figure 2C). There was no selectivity in the extraction efficiency of lymphocytes by 2-way analysis of variance (P = .203) (Figure 2D). There was also no difference in lymphocyte subset extraction efficiency by donor group (Figure 2D).
Leukoreduction system chamber extraction characteristics. Absolute number of white blood cells, including neutrophils, lymphocytes, monocytes, eosinophils, and basophils (A) and absolute number of various lymphocyte subsets, including CD4+, CD8+, CD16+CD56+, and CD19+ lymphocytes (B) that are present in the chamber at the end of plateletpheresis, based on group. (C) Extraction efficiency for neutrophils, lymphocytes, monocytes, eosinophils, and basophils by group. (D) Extraction efficiency for CD4+, CD8+, CD16+CD56+, and CD19+ lymphocytes by group. Horizontal lines indicate the median and interquartile range.
Leukoreduction system chamber extraction characteristics. Absolute number of white blood cells, including neutrophils, lymphocytes, monocytes, eosinophils, and basophils (A) and absolute number of various lymphocyte subsets, including CD4+, CD8+, CD16+CD56+, and CD19+ lymphocytes (B) that are present in the chamber at the end of plateletpheresis, based on group. (C) Extraction efficiency for neutrophils, lymphocytes, monocytes, eosinophils, and basophils by group. (D) Extraction efficiency for CD4+, CD8+, CD16+CD56+, and CD19+ lymphocytes by group. Horizontal lines indicate the median and interquartile range.
Detailed lymphocyte immunophenotyping
Flow cytometry to examine lymphocyte subsets in more detail revealed a decrease in the percentage of naive CD4+ T lymphocytes (P = .017) and increases in the percentages of CD4+ and CD8+ effector memory cells (P = .007 and P = .002, respectively) as donation frequency increased across groups (Figure 3A). There was no change in the percentage of naive CD8+ T lymphocytes (Figure 3A). The percentage of T helper 1 (Th1) and regulatory T (Treg) cells increased as donation frequency increased across groups (P = .024 and P < .001, respectively) (Figure 3B). The absolute numbers of Tregs stayed relatively constant (supplemental Figure 5). The percentage of Th2 cells was not significantly different across groups (Figure 3B). The percentage of Th17 cells decreased across groups (P = .024) (Figure 3B). The frequency of innate-like T-cell populations, including mucosal associated invariant T cells and γ-δ T-cells, were generally similar across groups (supplemental Figure 5). Other cell populations are included in supplemental Figure 5; supplemental Figure 6 shows select lymphocyte subsets from a set of control patients similar in median age to the donors in the 20 to 24 sessions group.
Detailed lymphocyte phenotyping. (A) Percentages of naive and effector memory T-cell populations among CD4+ T cells or CD8+ T cells based on group. (B) Percentages of Th subsets and Treg cells among CD4+ T cells based on group. (C) T-cell receptor diversity based on group, as measured by productive clonality. Horizontal lines indicate the median and interquartile range. All blood counts in this figure were obtained immediately before plateletpheresis.
Detailed lymphocyte phenotyping. (A) Percentages of naive and effector memory T-cell populations among CD4+ T cells or CD8+ T cells based on group. (B) Percentages of Th subsets and Treg cells among CD4+ T cells based on group. (C) T-cell receptor diversity based on group, as measured by productive clonality. Horizontal lines indicate the median and interquartile range. All blood counts in this figure were obtained immediately before plateletpheresis.
T-cell receptor diversity
To determine whether frequent plateletpheresis was associated with decreased T-cell receptor diversity in the peripheral blood, we performed immunoSEQ TCRB analysis. This showed no difference in clonal diversity across donor groups (Figure 3C). Due to concerns that T-cell receptor diversity measurements might be artificially restricted in subjects with low CD4+ T-lymphocyte counts, we submitted paired samples from leukoreduction system chambers for 2 donors in the 20 to 24 sessions group who had CD4+ T-lymphocyte counts below the normal range. These samples were enriched for lymphocytes. There was a strong correlation between productive clones identified from peripheral blood and the leukoreduction system chamber specimens (R = 0.998 and 0.948) (supplemental Figure 7).
Donor health
Standard donor history questionnaires completed by the donors before each plateletpheresis session were reviewed. There were no reports of opportunistic lung infections or unusual cancers. Nine treated nonmelanoma skin cancers in 6 donors and 1 instance of prostate cancer in another donor were reported (Table 3). More donors in the 20 to 24 sessions group reported ever having a problem with their heart or lungs, but these included conditions that are generally benign (eg, asymptomatic mitral valve prolapse and asymptomatic premature ventricular contractions) (Table 3). Donors were screened for HIV and other infectious agents at each donation. No donor had a positive screening test.
Summary of donor answers to 2 relevant health-related questions on the donor history questionnaire
| Donor questions and affirmative responses . | 1 or 2 Sessions group (n = 20) . | 3 to 19 Sessions group (n = 20) . | 20 to 24 Sessions group (n = 20) . |
|---|---|---|---|
| Have you ever had any type of cancer, including leukemia? | |||
| Basal cell carcinoma (excised and healed) | 2 (10) | 2* (10) | 2 (10) |
| Squamous cell cancer (excised and healed) | 0 (0) | 1* (5) | 0 (0) |
| Prostate cancer | 0 (0) | 0 (0) | 1 (5) |
| Individuals reporting cancer | 2 (10) | 2 (10) | 3 (15) |
| Have you ever had any problem with your heart or lungs? | |||
| Asymptomatic mitral valve prolapse | 0 (0) | 0 (0) | 1 (5) |
| Coronary artery stent | 0 (0) | 0 (0) | 1 (5) |
| Exercise-induced asthma | 0 (0) | 0 (0) | 1 (5) |
| Asymptomatic premature ventricular contractions | 0 (0) | 0 (0) | 1 (5) |
| Hypertension (controlled with medication) | 0 (0) | 1 (5) | 0 (0) |
| Irregular heart beat | 1 (5) | 0 (0) | 0 (0) |
| Individuals reporting problems with heart or lungs | 1 (5) | 1 (5) | 4 (20) |
| Donor questions and affirmative responses . | 1 or 2 Sessions group (n = 20) . | 3 to 19 Sessions group (n = 20) . | 20 to 24 Sessions group (n = 20) . |
|---|---|---|---|
| Have you ever had any type of cancer, including leukemia? | |||
| Basal cell carcinoma (excised and healed) | 2 (10) | 2* (10) | 2 (10) |
| Squamous cell cancer (excised and healed) | 0 (0) | 1* (5) | 0 (0) |
| Prostate cancer | 0 (0) | 0 (0) | 1 (5) |
| Individuals reporting cancer | 2 (10) | 2 (10) | 3 (15) |
| Have you ever had any problem with your heart or lungs? | |||
| Asymptomatic mitral valve prolapse | 0 (0) | 0 (0) | 1 (5) |
| Coronary artery stent | 0 (0) | 0 (0) | 1 (5) |
| Exercise-induced asthma | 0 (0) | 0 (0) | 1 (5) |
| Asymptomatic premature ventricular contractions | 0 (0) | 0 (0) | 1 (5) |
| Hypertension (controlled with medication) | 0 (0) | 1 (5) | 0 (0) |
| Irregular heart beat | 1 (5) | 0 (0) | 0 (0) |
| Individuals reporting problems with heart or lungs | 1 (5) | 1 (5) | 4 (20) |
All data are n (%).
One subject reported 3 separate occurrences of basal cell carcinoma and 1 occurrence of squamous cell carcinoma.
Discussion
In this study of 60 healthy platelet donors undergoing plateletpheresis with the Trima Accel instrument, we found an association between frequent platelet donation and lower CD4+ and CD8+ T-lymphocyte counts. In 8 donors, the CD4+ T-lymphocyte count was <200 cells per microliter. Each of these donors was over the age of 55 years, had completed a large number of plateletpheresis sessions (≥170), and had a history of frequent donation (20 to 24 sessions in at least one 365-day period). We confirmed that large numbers of lymphocytes are sequestered by the leukoreduction system chamber, which has a lymphocyte-extraction efficiency of ∼15% to 20%. These data are consistent with the hypothesis that the T-cell lymphopenia observed in some donors is related to recurrent removal of T lymphocytes by the leukoreduction system chamber.
Although platelets can be collected from whole blood donations, ∼94% of platelet units in the United States are apheresis derived.21 The market share of various plateletpheresis instruments is not publicly documented, but the Trima Accel and Fenwal Amicus (Fresenius Kabi, Lake Zurich, IL) instruments are commonly used across the United States and abroad. In a recent publication describing 36 regional American Red Cross blood centers, 16 used the Fenwal Amicus only, 9 used the Trima Accel only, and 11 used both instruments.22
Plateletpheresis instruments separate platelets from blood using slightly different technologies, which may impact transfusion recipients. Platelet units collected on the Fenwal Amicus have recently been associated with more septic transfusion reactions than platelet units collected on the Trima Accel, resulting in software modifications to the Fenwal Amicus.22 With the Fenwal Amicus, most white blood cells are returned to the donor. The effect of frequent plateletpheresis on CD4+ T-lymphocyte counts using instruments other than the Trima Accel is unknown.
Our statistical model suggests that older age contributes to decreased CD4+ and CD8+ T-lymphocyte counts; however, previous data suggest that aging is associated with smaller decreases in these cell counts than predicted by our model.23-27 Even if there remains concern for bias related to an age imbalance among our groups, the finding of CD4+ T-lymphocyte counts <200 cells per microliter in a healthy population, regardless of age, is surprising. The homeostatic mechanisms that maintain a given peripheral blood T-cell count are incompletely understood but appear to depend on cytokines like interleukin-7 and interleukin-15, which were not measured in this study.28,29 Importantly, most CD4+ and CD8+ T lymphocytes reside in lymphoid tissues rather than peripheral blood.30 These tissues take on an increasingly important role in T-lymphocyte homeostasis as thymic output declines.31
The leukoreduction system chamber blood counts reported here (Figure 2A-B; supplemental Figure 4; supplemental Table 4) are similar to those reported in other studies involving the Trima Accel. One group reported a median white blood cell chamber concentration of 112.1 × 103 cells per microliter (range, 65.2-205.2 × 103 cells per microliter) with a median lymphocyte yield of 5.33 × 108 cells (range, 2.50-8.38 × 108 cells).19 A second publication reported a median lymphocyte yield of 7.1 × 108 cells (range, 0-14.4 × 108 cells).5 Only 1 publication has reported mean CD4+ and CD8+ T-lymphocyte yields, which were 8.3 × 107 and 5.3 × 107 cells, respectively.32 These numbers are lower than in our current study but might be explained by processing of the cells that occurred in the former study. Our findings suggest that up to 10% of circulating CD4+ T lymphocytes are lost during each plateletpheresis session based on an estimated total number of 5 × 109 CD4+ T lymphocytes in the peripheral blood.30
The long-term kinetics of CD4+ and CD8+ T-cell lymphopenia in donors undergoing plateletpheresis and who cease plateletpheresis after becoming lymphopenic is unclear. It is likely that some donors in our study have had persistent lymphopenia for months, if not years, without negative health consequences. It is unknown whether low CD4+ T-lymphocyte counts return to normal after stopping plateletpheresis. The index case continued to have a CD4+ T-lymphocyte count <200 cells per microliter 5 months after cessation of plateletpheresis, suggesting that increases may occur slowly if they do occur. A lack of a return to baseline counts has been noted in a small number of leukapheresis and plateletpheresis donors at 8 months.11 It is possible that the low T-lymphocyte counts in peripheral blood represent a new clinically harmless “set point,” and that normal levels of T lymphocytes are maintained in lymphoid tissues. A history of frequent plateletpheresis should be considered when evaluating idiopathic CD4+ T-cell lymphopenia, a heterogeneous clinical syndrome defined as a CD4+ T-lymphocyte count <300 cells per microliter (or <20% of total lymphocytes) on >1 occasion in the absence of an identifiable cause of immunodeficiency.33
A strength of this study is its exclusion of donors who donated platelets at other sites, which might have resulted in confounding from the use of other plateletpheresis instruments. In addition, our detailed white cell immunophenotyping and T-cell receptor diversity data support the clinical impression of the donors’ good health. Because our donors were subjectively and objectively healthy, no donor was deferred from subsequent donation due to a low CD4+ or CD8+ T-lymphocyte count. Our study has several limitations. All data were collected at 1 site, and replication by other centers would be appropriate. Only healthy platelet donors who qualified to donate platelets could participate. In addition, donor health problems were self-reported and, therefore, subject to underreporting. These limitations are being addressed in ongoing studies.
In conclusion, frequent plateletpheresis performed with a leukoreduction system chamber is associated with a decrease in circulating T-cell numbers but not in their antigen receptor diversity. It is likely that some donors in our study have had persistent lymphopenia for months, if not years, without apparent adverse effects. Although further study is needed, our current data do not suggest that plateletpheresis-associated lymphopenia is harmful to donors.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Donna Crown for help with conducting the study and Graham Dudley, Sabrina La Fave, Mai Drew, Lisa Golemme, Rodel Rosales, and Jose Menor for help with flow cytometry. The authors are grateful to the platelet donors for their selfless anonymous support of patients and for their participation in this study.
This work was supported by a Clinical Translational Science Award (UL1RR025758) to Harvard University and Brigham and Women’s Hospital from the National Center for Research Resources. J.M.G. was supported by a K12 award from the National Heart, Lung, and Blood Institute, National Institutes of Health (HL087164). D.A.R. was supported by a K08 award from the National Institute of Arthritis and Musculoskeletal and Skin Diseases, National Institutes of Health (AR072791). D.N. was supported by a Dana-Farber/Harvard Cancer Center Comprehensive Cancer Center grant from the National Cancer Institute, National Institutes of Health (5P30 CA006516). The MR1 tetramer technology was developed jointly by James McCluskey, Jamie Rossjohn, and David Fairlie, and the material was produced by the National Institutes of Health Tetramer Core Facility as permitted to be distributed by the University of Melbourne.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Authorship
Contribution: J.M.G. was the principal investigator, helped to design the study, recruited subjects, arranged for the T-cell receptor diversity analyses, contributed to the overall analysis and interpretation of the data, generated the first draft of the manuscript, and incorporated feedback from the other authors; M.R. recruited subjects and helped to conduct the study; A.H.J. helped to design the study, performed flow cytometry experiments, and revised the manuscript; B.M.F. helped with subject recruitment, data management, and generation of tables and revised the manuscript; I.B. helped with subject recruitment and data management; M.E. and R.S.-W. helped to design and conduct the study; Z.J.L. performed flow cytometry experiments; J.M.S. and R.Y.-F. helped to design the study; M.B.B. provided reagents and guidance; S.R.S., L.R.B., D.L.L., and D.R.W. helped to design the study and revised the manuscript; N.B. and N.C.I. helped to design the study; D.N. helped to design the study, performed the statistical analyses, helped with the overall analysis and interpretation of the data, and revised the manuscript; D.A.R. helped to design the study, oversaw the detailed immunophenotyping experiments, and revised the manuscript; R.M.K. helped to design the study, recruited a subject, contributed to the overall analysis and interpretation of the data, and revised the manuscript; and all authors reviewed and provided final approval of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: John M. Gansner, Hematology Division, Brigham and Women’s Hospital, 75 Francis St, Boston, MA 02115; e-mail: jgansner@bwh.harvard.edu; and Richard M. Kaufman, Brigham and Women’s Hospital Transfusion Service, Blood Bank, Amory 260, 75 Francis St, Boston, MA 02115; e-mail: rmkaufman@bwh.harvard.edu.