Key Points
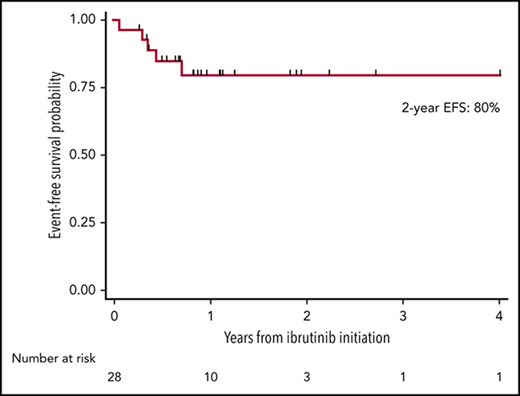
Ibrutinib was administered to 28 patients with BNS.
Ibrutinib showed rapid and durable symptomatic and radiologic responses in patients with BNS.
Abstract
The treatment of patients with Bing-Neel syndrome (BNS) is not standardized. We included patients with Waldenström macroglobulinemia (WM) and a radiologic and/or cytologic diagnosis of BNS treated with ibrutinib monotherapy. Response assessment was based on criteria for BNS from the 8th International Workshop for WM. Survival from BNS diagnosis (BNS survival), survival from ibrutinib initiation to last follow-up or death (ibrutinib survival), and time from ibrutinib initiation to ibrutinib discontinuation for toxicity, progression, or death (event-free survival [EFS]) were estimated. Twenty-eight patients were included in our study. The median age at BNS diagnosis was 65 years. Ibrutinib was the first line of treatment for BNS in 39% of patients. Ibrutinib was administered orally at a dose of 560 and 420 mg once daily in 46% and 54% of patients, respectively; symptomatic and radiologic improvements were seen in 85% and 60% of patients within 3 months of therapy. At best response, 85% of patients had improvement or resolution of BNS symptoms, 83% had improvement or resolution of radiologic abnormalities, and 47% had cleared the disease in the cerebrospinal fluid. The 2-year EFS rate with ibrutinib was 80% (95% confidence interval [CI], 58%-91%), the 2-year ibrutinib survival rate was 81% (95% CI, 49%-94%), and the 5-year BNS survival rate was 86% (95% CI, 63%-95%). Ibrutinib therapy is effective in patients with BNS and should be considered as a treatment option in these patients.
Introduction
Bing-Neel syndrome (BNS) is a rare condition seen in ∼1% of patients with a diagnosis of Waldenström macroglobulinemia (WM).1 BNS is a clinicopathologic entity characterized by WM cells gaining access to the central nervous system (CNS) and causing a variety of neurologic deficits in patients affected by this process.2,3 Interestingly, Bing and Neel4 described 2 cases of neurologic deterioration occurring in patients with macroglobulinemia in 1937, 7 years before the seminal description of patients with macroglobulinemia, anemia, coagulopathy, and incipient myelomatosis by Waldenström.5
The therapy for BNS is not standardized and limited to antineoplastic agents with good CNS penetration.1 However, agents such as high-dose methotrexate, cytarabine, and fludarabine are associated with well-known toxicities such as myelosuppression, immunosuppression, and mucositis.6 The oral Bruton tyrosine kinase (BTK) inhibitor ibrutinib is approved in the United States and Europe for the treatment of patients with symptomatic WM. There have been a few cases reporting clinical efficacy of ibrutinib in patients with BNS.7-10 In 1 patient, we demonstrated blood-brain barrier (BBB) penetration by ibrutinib in a dose-dependent manner.9 However, data on response to and survival outcomes with ibrutinib in BNS remain limited.
Therefore, we carried out a retrospective study of patients who received ibrutinib for a diagnosis of symptomatic BNS. The aim of our study was to report on response rates, survival outcomes, and safety of ibrutinib therapy in patients with BNS.
Methods
Patient selection
We identified patients age >18 years with a clinicopathologic diagnosis of WM11 who also had a diagnosis of BNS made at the time of or after the diagnosis of WM.1 BNS was defined as the presence of malignant lymphoplasmacytic cells in the cerebrospinal fluid (CSF) demonstrated by cytology and/or flow cytometry or brain tissue biopsy, with or without evidence of leptomeningeal enhancement or brain masses using magnetic resonance imaging (MRI) techniques. Finally, patients must have received ibrutinib for the treatment of BNS. The institutional review boards from all participating centers approved the present study.
Data collection
Pertinent clinical data were gathered at the time of WM diagnosis, at the time of BNS diagnosis, and at the time of ibrutinib therapy initiation for BNS. Response to ibrutinib therapy was evaluated based on current response criteria for BNS and WM.1,12 Briefly, the response criteria for BNS define complete response as the complete resolution of symptoms as well as radiologic and cytologic evidence of BNS, partial response as radiologic and/or symptomatic improvement but with clearance of cytologic findings, and no response as no improvement in symptoms or radiologic or cytologic abnormalities. Data on toxicity were gathered retrospectively, and reporting was based on Common Terminology Criteria for Adverse Events (version 5.0).13 We calculated time from WM diagnosis to BNS diagnosis; time from BNS diagnosis to ibrutinib initiation; time from ibrutinib initiation to unacceptable toxicity, progressive disease, or death resulting from any cause (event-free survival [EFS]); time from ibrutinib initiation to last follow-up or death resulting from any cause (ibrutinib survival); and time from BNS diagnosis to last follow-up or death resulting from any cause (BNS survival).
Statistical analysis
Clinical characteristics are presented using descriptive statistics such as medians and ranges and numbers and proportions. Times to events were estimated using the Kaplan-Meier method for incomplete observations.14 Comparisons between groups were assessed using the log-rank test.15 All calculations and graphs were obtained using STATA (version 13.1; College Station, TX).
Results
Patient characteristics
Twenty-eight patients were included in this study and were diagnosed with WM between 1992 and 2018. The median age at diagnosis of WM was 60 years (range, 38-80 years). Sixteen patients (57%) were men. The patients were diagnosed with BNS between 2011 and 2018. At the time of BNS diagnosis, the median age was 65 years (range, 38-81 years). The median time from WM to BNS diagnosis was 48 months (range, 0-320 months). Ten patients (36%) were diagnosed with BNS within 12 months of WM diagnosis. Eleven patients (39%) were previously untreated for their WM at the time of BNS diagnosis. Of the 17 patients who were previously treated for their WM, the median number of WM therapies was 3 (range, 1-7). All patients had received anti-CD20 monoclonal antibody therapy, 16 (94%) had received alkylating agents, 8 (47%) had received nucleoside analogs, 4 (24%) had received proteasome inhibitors, 3 (18%) had received immunomodulating agents, and 2 (12%) had undergone autologous stem-cell transplantation.
BNS presenting symptoms included motor deficits in 13 patients (46%), cognitive deficits in 11 (39%), sensory deficits in 11 (39%), headaches in 6 (21%), ataxia in 5 (18%), cranial nerve deficits in 5 (18%), and seizures in 5 (18%). Brain and spinal MRI were normal in 3 patients (11%). Of the 25 patients (89%) with MRI findings, 20 (80%) showed leptomeningeal enhancement, 3 (12%) had intraparenchymal masses, and 2 had intraparenchymal masses with concurrent leptomeningeal enhancement (8%). CSF cytology was positive for lymphoplasmacytic cells in 27 patients (96%). The diagnosis of BNS was made on a brain biopsy in 1 patient. CSF flow cytometry revealed clonal B cells with positive expression of CD19 and/or CD20 and negative expression of CD5 and/or CD10 in 21 patients (75%). The MYD88 L265 mutation was detected in 23 (96%) of 24 patients investigated; 17 (94%) of 18 in bone marrow or other tissues, and 11 (100%) of 11 in CSF. The patient in whom the MYD88 L265P mutation was not detected in the bone marrow had minimal bone marrow involvement by WM, and this could have been a false-negative result. In summary, 24 patients had positive findings on MRI, CSF cytology, and CSF flow cytometry and/or molecular testing, and 4 patients had positive findings on MRI and CSF cytology.
The patients were started on ibrutinib therapy between 2014 and 2018. Eleven patients (39%) had not previously received therapy for their BNS. Of the 17 patients who were previously treated for their BNS, the median number of therapies before ibrutinib was 1 (range, 1-5). The median time from last BNS therapy to ibrutinib was 5 months (range, 1-81 months). Nine patients (53%) had received intrathecal chemotherapy, 8 (48%) had received high-dose methotrexate, 3 (18%) had received bendamustine, and 3 (18%) had received radiotherapy. At the time of ibrutinib initiation, the median serum immunoglobulin M (IgM) level was 1148 mg/dL (range, 110-9360 mg/dL), and the median hemoglobin level was 11.8 g/dL (range, 7.7-15.2 g/dL). Thirteen patients (46%) received ibrutinib at a dose of 560 mg orally once per day, and 15 (54%) received ibrutinib at a dose of 420 mg orally once per day. Patient-level characteristics at the time of ibrutinib initiation are listed in Table 1.
Selected clinical characteristics and outcomes of 28 patients with BNS treated with ibrutinib
| ID . | Age WM diagnosis, y . | Sex . | Prior WM therapy . | Age BNS diagnosis, y . | Prior BNS therapy . | Dose, mg . | Best response . | Stopped ibrutinib . | EFS, mo* . | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Symptomatic . | Radiologic . | Cytologic . | |||||||||
| 1 | 56 | M | No | 65 | Yes | 560 | Improved | Improved | NA | No | 23 |
| 2 | 60 | F | No | 60 | No | 560 | Unchanged | Unchanged | Cleared | Yes | 5 |
| 3 | 61 | F | Yes | 76 | No | 560 | Improved | Improved | NA | No | 23 |
| 4 | 72 | M | Yes | 78 | No | 420 | Improved | Resolved | NA | No | 27 |
| 5 | 48 | F | No | 49 | Yes | 560 | Improved | Improved | Cleared | No | 10 |
| 6 | 73 | F | Yes | 76 | Yes | 560 | Unchanged | Improved | Cleared | No | 4 |
| 7 | 65 | M | Yes | 75 | Yes | 560 | Improved | Improved | Persistent | No | 48 |
| 8 | 63 | M | No | 64 | No | 560 | Improved | Improved | Persistent | No | 6 |
| 9 | 61 | F | No | 61 | No | 560 | Improved | Improved | Persistent | No | 6 |
| 10 | 72 | M | No | 72 | Yes | 560 | Improved | Improved | NA | No | 3 |
| 11 | 53 | M | Yes | 65 | Yes | 420 | Improved | Unchanged | Cleared | Yes | 4 |
| 12 | 62 | M | No | 62 | No | 420 | Improved | Unchanged | Persistent | No | 13 |
| 13 | 56 | M | Yes | 65 | No | 420 | Unchanged | Improved | NA | No | 22 |
| 14 | 56 | M | Yes | 63 | Yes | 560 | Resolved | NA | Cleared | No | 10 |
| 15 | 52 | M | Yes | 78 | Yes | 420 | Improved | NA | Cleared | Yes | 1 |
| 16 | 49 | F | No | 50 | Yes | 560 | Improved | NA | Persistent | No | 8 |
| 17 | 55 | F | Yes | 59 | No | 560 | Improved | NA | Persistent | Yes | 4 |
| 18 | 64 | M | No | 64 | Yes | 420 | Resolved | Resolved | Persistent | No | 11 |
| 19 | 38 | M | Yes | 38 | Yes | 420 | Resolved | Improved | NA | No | 10 |
| 20 | 61 | F | No | 61 | Yes | 420 | Unchanged | Improved | Persistent | Yes | 8 |
| 21 | 67 | F | Yes | 71 | No | 420 | Resolved | NA | NA | No | 8 |
| 22 | 60 | M | Yes | 67 | Yes | 420 | Resolved | Improved | Persistent | No | 33 |
| 23 | 61 | M | Yes | 76 | No | 420 | Improved | NA | Cleared | No | 15 |
| 24 | 42 | F | Yes | 60 | Yes | 420 | Improved | NA | NA | No | 13 |
| 25 | 76 | M | No | 80 | Yes | 420 | Improved | NA | NA | No | 11 |
| 26 | 53 | F | Yes | 66 | Yes | 420 | Improved | NA | Cleared | No | 8 |
| 27 | 80 | F | Yes | 81 | No | 420 | Improved | NA | NA | No | 4 |
| 28 | 57 | M | Yes | 66 | Yes | 560 | Improved | Improved | NA | No | 13 |
| ID . | Age WM diagnosis, y . | Sex . | Prior WM therapy . | Age BNS diagnosis, y . | Prior BNS therapy . | Dose, mg . | Best response . | Stopped ibrutinib . | EFS, mo* . | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Symptomatic . | Radiologic . | Cytologic . | |||||||||
| 1 | 56 | M | No | 65 | Yes | 560 | Improved | Improved | NA | No | 23 |
| 2 | 60 | F | No | 60 | No | 560 | Unchanged | Unchanged | Cleared | Yes | 5 |
| 3 | 61 | F | Yes | 76 | No | 560 | Improved | Improved | NA | No | 23 |
| 4 | 72 | M | Yes | 78 | No | 420 | Improved | Resolved | NA | No | 27 |
| 5 | 48 | F | No | 49 | Yes | 560 | Improved | Improved | Cleared | No | 10 |
| 6 | 73 | F | Yes | 76 | Yes | 560 | Unchanged | Improved | Cleared | No | 4 |
| 7 | 65 | M | Yes | 75 | Yes | 560 | Improved | Improved | Persistent | No | 48 |
| 8 | 63 | M | No | 64 | No | 560 | Improved | Improved | Persistent | No | 6 |
| 9 | 61 | F | No | 61 | No | 560 | Improved | Improved | Persistent | No | 6 |
| 10 | 72 | M | No | 72 | Yes | 560 | Improved | Improved | NA | No | 3 |
| 11 | 53 | M | Yes | 65 | Yes | 420 | Improved | Unchanged | Cleared | Yes | 4 |
| 12 | 62 | M | No | 62 | No | 420 | Improved | Unchanged | Persistent | No | 13 |
| 13 | 56 | M | Yes | 65 | No | 420 | Unchanged | Improved | NA | No | 22 |
| 14 | 56 | M | Yes | 63 | Yes | 560 | Resolved | NA | Cleared | No | 10 |
| 15 | 52 | M | Yes | 78 | Yes | 420 | Improved | NA | Cleared | Yes | 1 |
| 16 | 49 | F | No | 50 | Yes | 560 | Improved | NA | Persistent | No | 8 |
| 17 | 55 | F | Yes | 59 | No | 560 | Improved | NA | Persistent | Yes | 4 |
| 18 | 64 | M | No | 64 | Yes | 420 | Resolved | Resolved | Persistent | No | 11 |
| 19 | 38 | M | Yes | 38 | Yes | 420 | Resolved | Improved | NA | No | 10 |
| 20 | 61 | F | No | 61 | Yes | 420 | Unchanged | Improved | Persistent | Yes | 8 |
| 21 | 67 | F | Yes | 71 | No | 420 | Resolved | NA | NA | No | 8 |
| 22 | 60 | M | Yes | 67 | Yes | 420 | Resolved | Improved | Persistent | No | 33 |
| 23 | 61 | M | Yes | 76 | No | 420 | Improved | NA | Cleared | No | 15 |
| 24 | 42 | F | Yes | 60 | Yes | 420 | Improved | NA | NA | No | 13 |
| 25 | 76 | M | No | 80 | Yes | 420 | Improved | NA | NA | No | 11 |
| 26 | 53 | F | Yes | 66 | Yes | 420 | Improved | NA | Cleared | No | 8 |
| 27 | 80 | F | Yes | 81 | No | 420 | Improved | NA | NA | No | 4 |
| 28 | 57 | M | Yes | 66 | Yes | 560 | Improved | Improved | NA | No | 13 |
NA, not available.
Toxicity, progression, or death.
Response outcomes with ibrutinib
At best response, the median serum IgM level decreased to 373 mg/dL (range, 54-5010 mg/dL), and the median hemoglobin level increased to 14.2 g/dL (range, 8.8-16 g/dL). On the basis of WM response criteria, 1 patient (4%) achieved very good partial response (PR), 12 (46%) achieved PR, 8 (31%) achieved minor response, and 5 (19%) had stable disease. Data on IgM levels were not available in 2 patients. Data on BNS symptoms were available in 28 patients: 5 patients (18%) had resolution, 19 (68%) had improvement, and 4 (14%) had no changes. Data on MRI results were available in 18 patients: 2 patients (11%) had resolution, 13 (72%) had improvement, and 3 (17%) had no changes. Data on cytologic findings were available in 17 patients: 8 patients (47%) cleared, and 9 patients (53%) had persistence of disease. On the basis of current response criteria, 1 patient (6%) achieved complete response (CR), 6 (35%) achieved PR, and 10 (59%) had no response. However, of the 10 patients who did not respond, 7 had clinical benefit, because 5 had improvement and 2 had resolution of symptoms. Symptomatic, radiologic, and cytologic responses at 3, 6, and 12 months and at best response to ibrutinib therapy are summarized in Table 2.
Symptomatic, radiologic, and cytologic responses in 28 patients with BNS treated with single-agent ibrutinib
| . | n/N (%) . | |||
|---|---|---|---|---|
| 3 mo . | 6 mo . | 12 mo . | Best response . | |
| Symptomatic | ||||
| Resolved | 1/26 (4) | 3/20 (15) | 2/10 (20) | 5/28 (18) |
| Improved | 21/26 (81) | 15/20 (75) | 7/10 (70) | 19/28 (68) |
| Unchanged | 4/26 (15) | 2/20 (10) | 1/10 (10) | 4/28 (14) |
| Radiologic | ||||
| Resolved | 0/15 (0) | 1/9 (11) | 2/8 (25) | 2/18 (11) |
| Improved | 9/15 (60) | 7/9 (78) | 6/8 (75) | 13/18 (72) |
| Unchanged | 6/15 (40) | 1/9 (11) | 0/8 (0) | 3/18 (17) |
| Cytologic | ||||
| Cleared | 7/12 (58) | 2/7 (29) | 0/1 (0) | 8/17 (47) |
| Persistent | 5/12 (42) | 5/7 (71) | 1/1 (100) | 9/17 (53) |
| . | n/N (%) . | |||
|---|---|---|---|---|
| 3 mo . | 6 mo . | 12 mo . | Best response . | |
| Symptomatic | ||||
| Resolved | 1/26 (4) | 3/20 (15) | 2/10 (20) | 5/28 (18) |
| Improved | 21/26 (81) | 15/20 (75) | 7/10 (70) | 19/28 (68) |
| Unchanged | 4/26 (15) | 2/20 (10) | 1/10 (10) | 4/28 (14) |
| Radiologic | ||||
| Resolved | 0/15 (0) | 1/9 (11) | 2/8 (25) | 2/18 (11) |
| Improved | 9/15 (60) | 7/9 (78) | 6/8 (75) | 13/18 (72) |
| Unchanged | 6/15 (40) | 1/9 (11) | 0/8 (0) | 3/18 (17) |
| Cytologic | ||||
| Cleared | 7/12 (58) | 2/7 (29) | 0/1 (0) | 8/17 (47) |
| Persistent | 5/12 (42) | 5/7 (71) | 1/1 (100) | 9/17 (53) |
There were no detectable statistical differences in the rates of symptomatic (P = .42), radiologic (P = .13), or CSF cytologic response (P = .82) in BNS patients treated with an ibrutinib dose of 420 vs 560 mg. There was also no detectable difference based on current response criteria (P = .37), nor were there differences in symptomatic (P = .60), radiologic (P = .49), or CSF cytologic response (P = .40) or response based on International Workshop on WM criteria (P = .30) in BNS patients who were previously untreated vs previously treated for BNS.
Survival outcomes with ibrutinib
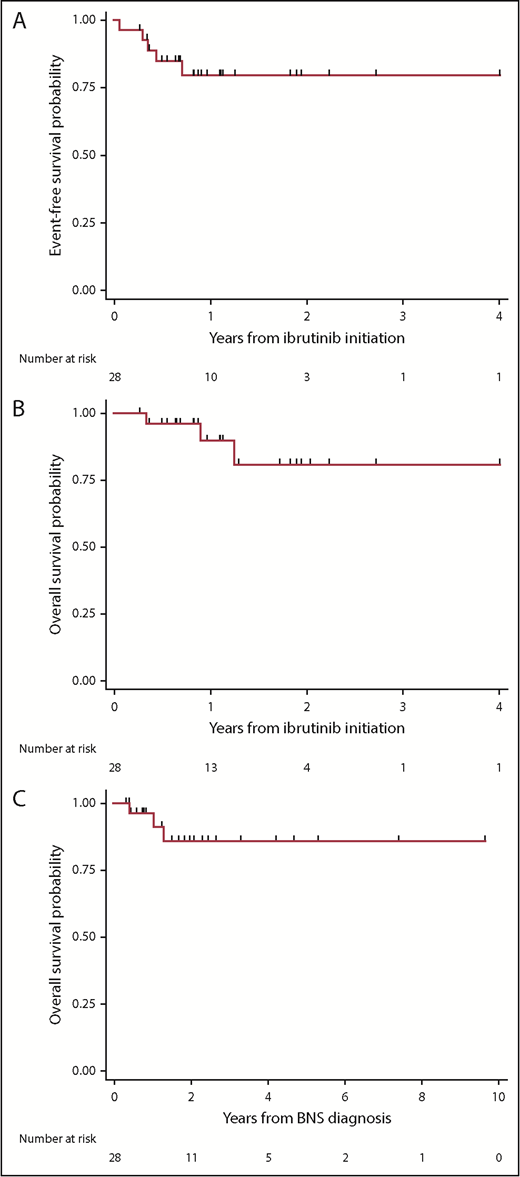
The median follow-up time from WM diagnosis was 8.7 years (95% confidence interval [CI], 4.1-11.5 years), the median follow-up time from BNS diagnosis was 1.9 years (95% CI, 1.2-2.3 years), and the median follow-up time from ibrutinib initiation was 1 year (95% CI, 0.6-1.7 years). At the time of this report, 5 patients had stopped taking ibrutinib because of progression or toxicity, and 3 patients had died. The estimated 2-year EFS rate from ibrutinib initiation was 80% (95% CI, 58%-91%; Figure 1A). The estimated 2-year survival rate from ibrutinib initiation was 81% (95% CI, 49%-94%; Figure 1B). The estimated 5-year survival rate from BNS diagnosis was 86% (95% CI, 63%-95%; Figure 1C). There were no detectable differences between patients who were previously treated or untreated for their BNS or between patients who received an ibrutinib dose of 560 or 420 mg, with regard to EFS (log-rank P = .97 and .88, respectively), survival from ibrutinib initiation (log-rank P = .71 and .82, respectively), or survival from BNS diagnosis (log-rank P = .95 and .70, respectively). Of the 5 BNS patients who progressed on ibrutinib, treatments after ibrutinib discontinuation included high-dose methotrexate (n = 2), bendamustine and rituximab (n = 1), fludarabine and rituximab (n = 1), and methotrexate and temozolomide (n = 1).
Kaplan-Meier estimates in 28 patients with BNS treated with ibrutinib. (A) EFS on ibrutinib, (B) survival from ibrutinib initiation, and (C) survival from BNS diagnosis.
Kaplan-Meier estimates in 28 patients with BNS treated with ibrutinib. (A) EFS on ibrutinib, (B) survival from ibrutinib initiation, and (C) survival from BNS diagnosis.
Safety
Thirteen patients (45%) had a reported adverse event. Grade 4 adverse events included neutropenia (n = 1). Grade 3 adverse events included pneumonia (n = 2), muscle cramps (n = 1), bleeding (n = 1), atrial fibrillation (n = 1), and ventricular tachycardia (n = 1). Grade 1 or 2 adverse events included diarrhea (n = 3), fatigue (n = 2), muscle cramps (n = 1), headache (n = 1), nausea (n = 1), mouth sores (n = 1), rash (n = 1), bruising (n = 1), and neutropenia (n = 1). Adverse events leading to ibrutinib discontinuation included muscle cramps (n = 1) and ventricular tachycardia (n = 1). The causes of death were disease progression (n = 1), infection (n = 1), and multiple comorbidities (n = 1).
Discussion
Ibrutinib is an oral BTK inhibitor approved by the US Food and Drug Administration and the European Medicines Agency for the treatment of patients with symptomatic WM. The approval was based on the results of a multicenter single-arm prospective phase 2 study in which ibrutinib at a dose of 420 mg orally once daily induced an overall response rate of 91%, a major response rate of 73%, and a 24-month progression-free survival rate of 69% in 63 previously treated patients.16 Two additional studies in WM patients, 1 in 31 rituximab-refractory and 1 in 30 previously untreated patients, showed similar results.17,18 However, patients with BNS were excluded from these studies.
Herein, we present clinical characteristics as well as response and survival outcomes and safety of 28 WM patients with a diagnosis of BNS treated with ibrutinib. Symptomatic and radiologic improvements were seen in 84% and 57% of patients within 3 months of ibrutinib therapy, respectively. At best response, ibrutinib induced improvement or resolution of BNS-associated symptoms and improvement or resolution of MRI abnormalities in 85% and 82% of patients, respectively. The estimated 2-year probability of BNS patients continuing ibrutinib without toxicity, progression, or death was 80%. The toxicity of ibrutinib in this study was consistent with prior studies. Previous studies have reported 3-year survival rates from BNS diagnosis between 60% to 70% and a median time to progression or death from first-line treatment of BNS of 2 years.2,3 We believe the outcomes reported here are encouraging and strongly suggest a benefit for the use of ibrutinib in patients with BNS.
Observational data have supported clinical efficacy of ibrutinib in patients with mantle cell lymphoma and chronic lymphocytic leukemia involving the CNS.19,20 A few case reports have also suggested clinical efficacy of ibrutinib specifically in WM and BNS.7-10 In a single-case study, plasma and CSF were obtained synchronously from a patient with WM and BNS receiving 560 mg of ibrutinib orally once daily and showed that the ibrutinib levels in this patient’s CSF were >50% the inhibitory concentration for BTK inhibition.9 A preclinical murine model showed that ibrutinib rapidly crosses the BBB, with a median time of 0.3 hours.21 Additionally, intracerebral entry of ibrutinib directly correlated with ibrutinib plasma concentrations and ibrutinib dose. Of interest, the CNS distribution of ibrutinib was not different in ventricular and cortical areas.
The optimal dose of ibrutinib in BNS has not yet been defined. Recently, a phase 1 study in patients with relapsed and/or refractory CNS lymphoma showed high efficacy of ibrutinib in this setting.22 In this study, mean CSF ibrutinib concentrations at 2 hours after ibrutinib dose were higher in patients who received ibrutinib at 840 mg than in patients who received 560 mg (2 and 0.8 ng/mL, respectively). The mean CSF ibrutinib concentrations after 1 month of therapy also appeared higher in patients on 840 mg than in patients on 560 mg (3.2 and 1.7 ng/mL, respectively). One could hypothesize that higher doses of ibrutinib would translate into deeper and more durable responses in BNS patients. However, in our study, with a median follow-up time on ibrutinib of 1 year, there did not seem to be a difference in response rate or duration of response between patients who received 420 or 560 mg of ibrutinib orally once daily. Therefore, 420 mg orally once daily is a reasonable starting dose for patients with BNS, and if a desired response is not seen, increasing ibrutinib to 560 mg by mouth once daily may be advisable.
As per current BNS response criteria, PR is defined by persistence of symptoms and/or radiologic abnormalities, but there should be CSF cytologic clearance. Therefore, patients with persistent CSF cytologic findings would be considered nonresponders, despite the clinical benefit obtained with therapy. An important point of controversy is the persistence of malignant cells observed in the CSF of BNS patients who otherwise had symptomatic and/or radiologic improvement with ibrutinib therapy. A similar phenomenon was observed in studies evaluating ibrutinib in patients with symptomatic WM, in which there was bone marrow persistence of disease despite marked improvements in symptoms, hematologic parameters, and serum IgM levels.16-18 A modulating rather than cytotoxic effect of ibrutinib could, at least in part, explain this phenomenon. However, in a previous study, approximately half of BNS patients treated with standard therapies had CSF cytologic persistence of disease.2 This finding would suggest revising the current response criteria to permit persistence of CSF cytologic findings in the partial response category.
Limitations of our study include the small sample size and its retrospective nature. BNS is a rare condition, and our sample size of 28 patients treated with ibrutinib could be considered large. As an example, 2 (relatively) large case series on BNS included 35 and 44 patients, respectively.2,3 Also, several practice-changing prospective studies in WM have had similar sample sizes.17,18,23-26 Our study could have been affected by case selection bias and may have overestimated ibrutinib efficacy. However, our cohort included BNS patients from several centers and with clinical features consistent with prior studies. For example, in our study, the median age at BNS diagnosis was 60 years, which is similar to previous reports.2,3 Therefore, our sample could be considered representative of the population under study. Also, given the rarity of BNS, it is unlikely a prospective study evaluating ibrutinib specifically in BNS will be performed. Therefore, a retrospective study can provide observations of important clinical value.
In conclusion, our results show that ibrutinib monotherapy, administered at a dose of 420 or 560 mg orally once daily, can induce durable responses with acceptable toxicity in patients with BNS. Therefore, ibrutinib should be considered a treatment option for these patients in the frontline and relapsed settings. Longer follow-up is needed to better define the optimal dose of ibrutinib in BNS patients.
Presented in part at the 22nd Annual Meeting of the European Hematology Association, Stockholm, Sweden, 14-17 June 2018; 10th International Workshop for Waldenström Macroglobulinemia, New York, NY, 11-13 October 2018; and 60th Annual Meeting of the American Society of Hematology, San Diego, CA, 1-4 December 2018. Australian data were presented at the Blood 2018 Meeting, Brisbane, Australia, 21-24 October 2018.
For original data, please contact jorgej_castillo@dfci.harvard.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
G.I. was awarded a Young Investigator Award at the 10th International Workshop for Waldenström Macroglobulinemia for this research. J.J.C. thanks the WMR Fund for support.
Authorship
Contribution: J.J.C., G.I., and S.P.T. designed the study; all the authors provided patient data; G.I. maintained the database; J.J.C. and G.I. performed the analysis; J.J.C. wrote the initial manuscript draft; and all the authors reviewed and approved the final manuscript.
Conflict-of-interest disclosure: J.J.C. received research funds from AbbVie, Beigene, Janssen, and Pharmacyclics and honoraria from Beigene, Janssen, Merck, and Pharmacyclics; M.V. received honoraria from Janssen; C.B. has received research funds from Bayer, Janssen, and Roche and honoraria from Gilead, Janssen, Pfizer, and Roche; T.A.E. received honoraria from AbbVie, Gilead, and Janssen; J.C.C. received honoraria from Bayer, Genentech, Janssen, Kite, Merck, and Novartis; K.H.S. received research funds from AbbVie and honoraria from Amgen, Bristol-Myers Squibb, Celgene, Janssen, and Takeda; M.L.P. received honoraria from Celgene, Merck, and Pharmacyclics, and a family member of M.L.P. received royalties from Juno and Seres; D.T. received research funds from Roche and honoraria from Janssen and Roche; C.S.T. received research funding from Janssen and AbbVie and honoraria from Janssen, Beigene, Pharmacyclics, and AbbVie; L.N. has received honoraria from Bristol-Myers Squibb; and S.P.T. received research funding from Bristol-Myers Squibb and Pharmacyclics and honoraria from Pharmacyclics. The remaining authors have no competing financial interests.
Correspondence: Jorge J. Castillo, 450 Brookline Ave, Mayer 221, Boston, MA 02215; e-mail: jorgej_castillo@dfci.harvard.edu.
REFERENCES
Author notes
J.J.C. and G.I. contributed equally to this study.