Abstract
Mantle cell lymphoma (MCL) is a unique type of non-Hodgkin lymphoma characterized by the overexpression of cyclin D1. MCL patients typically live for years but experience multiple relapses. Acalabrutinib is a novel second-generation oral Bruton tyrosine kinase inhibitor approved by the US Food and Drug Administration for relapsed MCL based on a clinical trial demonstrating an overall response rate of 81%. It provides a valuable new treatment option for MCL patients and is now being tested upfront.
Introduction
Mantle cell lymphoma (MCL) is a type of B-cell non-Hodgkin lymphoma (NHL) characterized by a t(11;14) leading to cyclin 1 overexpression. MCL represents ∼8% of all NHL and has features somewhat intermediate between indolent and aggressive NHL. MCL prognosis is assessed with the Mantle Cell International Prognostic Index (MIPI).1 There are 3 key pathology variables that are important to the prognosis: cell morphology as assessed by light microscopy, TP53 mutations, and cell proliferation rate of the tumor cells. Pleomorphic and blastoid variants by light microscopy represent ∼10% of MCL cases.2 TP53 mutations are detected in 10% of cases.3 MCL is the only NHL that incorporates cell proliferation in standard prognostic indices, and the usual method is immunohistochemistry staining of tumor tissue for the Ki-67 antigen, which identifies cells in the G1/S/G2M phases of the cell cycle.4 All 3 of these features, blastoid/pleomorphic morphology, TP53 mutations, and high growth rate fraction, predict for poor prognosis with standard chemoimmunotherapy. Initial treatment is determined by these variables, patient fitness, and eligibility for autologous stem cell transplant (SCT). Those considered transplant candidates are typically treated aggressively with regimens containing rituximab along with a high-dose cytarabine-containing combination chemotherapy regimen.5-8 Patients who respond proceed to autologous SCT in first remission. Patients who are elderly, or younger patients unfit for SCT, are usually given bendamustine with rituximab.9,10 Most patients in both groups receive rituximab maintenance. Although the aggressive approach can result in long-term remission for some patients, unfortunately, long-term follow-up of these trials does not show a plateau on the progression-free survival (PFS) curves.11 Patients who achieve minimal residual disease (MRD) negativity do better.11,12 The current national trial in the United States, EA4151 (NCT03267433), is testing the hypothesis that adjuvant SCT in first MRD− complete remission provides a better long-term outcome than rituximab maintenance alone with the possibility of a SCT at the time of subsequent relapse. Patients in this trial who are MRD− in the blood are randomized to SCT followed by 3 years of rituximab maintenance vs 3 years of rituximab maintenance without the SCT.
Patients who relapse are typically treated with salvage chemoimmunotherapy or other approved agents. These include the proteasome inhibitor bortezomib,13,14 the immunomodulatory agent lenalidomide,15-19 the mammalian target of rapamycin complex 1 inhibitor temsirolimus (Europe, not United States),20-24 and the Bruton tyrosine kinase (BTK) inhibitors discussed in the next section.25-29 The bcl-2 inhibitor venetoclax has orphan drug designation (but not approval) for MCL.30,31 Despite the plethora of agents for this disease, most patients will need all of them during their lifetime. Thus, there is always a need for additional agents as illustrated by the case described in Figure 1.
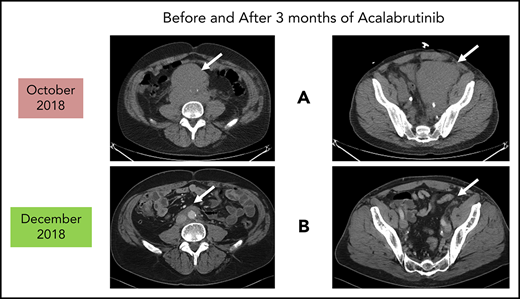
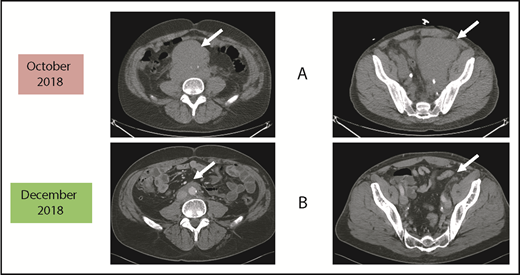
Before and after 3 months of acalabrutinib. A 54-year-old man was diagnosed with stage IVB MCL in 2014. He received chemoimmunotherapy including cytosine arabinoside and an adjuvant autologous SCT. (A) In 2018, he relapsed with left leg swelling due to bulky pelvic disease and a new deep vein thrombosis. (B) After biopsy proof of relapsed MCL, he received acalabrutinib 100 mg twice daily, rituximab, and apixaban; within 3 months, he achieved a marked response with no side effects of the combination. He has returned to full-time work and continues on therapy as of January 2019.
Before and after 3 months of acalabrutinib. A 54-year-old man was diagnosed with stage IVB MCL in 2014. He received chemoimmunotherapy including cytosine arabinoside and an adjuvant autologous SCT. (A) In 2018, he relapsed with left leg swelling due to bulky pelvic disease and a new deep vein thrombosis. (B) After biopsy proof of relapsed MCL, he received acalabrutinib 100 mg twice daily, rituximab, and apixaban; within 3 months, he achieved a marked response with no side effects of the combination. He has returned to full-time work and continues on therapy as of January 2019.
BTK inhibitors with a focus on acalabrutinib
The B-cell–signaling pathway plays an important role in the growth and expansion of malignant B cells. This pathway has recently been reviewed with specific reference to MCL.32 All of the agents mentioned in the Introduction that are approved for MCL therapy target at some level between the membrane and the nucleus. Selecting the optimal pathway member to target in the clinic can be difficult and requires clinical trial testing. For example, the Syk and BTK kinases are located very close to each other in the signal pathway and have been targets of new agents but with quite different outcomes. Fostamatinib, an inhibitor of Syk, produced an overall response rate (ORR) of only 11% (1 of 9) in relapsed MCL patients.33 Inhibiting the nearby BTK with ibrutinib has been much more successful with an ORR of 68% (75 of 111).25 This study was updated in 2015 and the 2-year PFS and overall survival (OS) rates were 31% and 47%, respectively.34
There are 2 oral BTK inhibitors approved for use in relapsed MCL: ibrutinib (Pharmacyclics and Janssen)25,34 and acalabrutinib (AstraZeneca). Acalabrutinib, originally referred to as ACP-196 (Acerta Pharmaceuticals), is a novel, irreversible BTK inhibitor that was designed to be more kinase-selective than ibrutinib.35,36 Like ibrutinib, acalabrutinib binds covalently to Cys481 located at the edge of the adenosine triphosphate–binding site of BTK, producing irreversible inhibition of BTK. Acalabrutinib is more selective than ibrutinib for BTK with less off-target effects. For example, Barf et al35 found that with concentrations of 1 µM acalabrutinib, only 1.5% of nonmutant protein kinases were inhibited; with ibrutinib, it was 8.9%. Acalabrutinib is less likely to affect other kinases such as TEC-, epidermal growth factor receptor–, and SRC-family kinases.35 These results were confirmed in a mouse model of chronic lymphocytic leukemia (CLL).37 In normal human volunteer studies, acalabrutinib is rapidly absorbed after oral administration and reaches peak concentrations within 30 to 60 minutes. Doses of 75 mg and 100 mg reliably produced >90% occupancy of BTK with a single dose. The 100-mg dose provided longer duration of BTK occupancy and better inhibition of B-cell receptor function.35 This dose and schedule was first tested in a phase 1/2 trial for patients with relapsed CLL.38 The recommended dose thus is 100 mg twice daily continuously and indeed this is the current US Food and Drug Administration (FDA)–approved dose.
Resistance to BTK inhibitors has been described in CLL patients and involves the development of BTK or phospholipase Cγ2 (PLCG2) mutations.39-41 These mutations have also been reported to have developed in MCL patients treated with ibrutinib.42 There are likely to be other mechanisms of BTK inhibitor failure without actual mutations in MCL cells.41
Studies of acalabrutinib in relapsed MCL
Acalabrutinib received approval for relapsed MCL patients based on a phase 2 open-label single-arm multicenter trial.43 Patients were required to have failed at least 1 prior therapy. They received single-agent acalabrutinib at 100 mg by mouth twice daily. There were 124 patients enrolled with a median age of 68 years (range, 42-90 years); 80% were men; and 93% were performance status 0 or 1. The median number of prior therapies was 2 (range, 1-5) and the median number of months from diagnosis to enrollment was 46 months (range, 2.5-170 months). MIPI scores were low risk in 39%, intermediate in 44%, high risk in 17%, and missing in 1 case. Only 18% had received a SCT and few had received other FDA-approved therapies for relapsed MCL such as bortezomib or carfilzomib (19%) or lenalidomide (7%). The ORR was 81% with 40% complete remission. Responses occurred quickly with a median of 1.9 months. Longer-term follow-up of this cohort was recently reported.44 The median time on study is now 26.3 months (range, 0.3-35.1 months), the median duration of response was 25.7 months (95% confidence interval, 17.5 months to not reached), the median PFS was 19.5 months (95% confidence interval, 16.5, 27.7), and the median OS has not yet been reached. At 24 months of follow-up, 72% of the responders remained alive. In this report, there is no mention of whether any of these cases were blastoid morphology or whether any of the participants had TP53 mutations in the MCL tumor cells.
Side effects
As reported in the clinical trial,43 acalabrutinib at the recommended dose of 100 mg twice daily is well-tolerated as demonstrated by only 1.6% of patients requiring dose reductions and only 6.5% of patients discontinuing acalabrutinib due to adverse events. Of note, atrial fibrillation was not observed in any patient. The most common side effects were headaches (36%) and diarrhea (38%), both of which were typically grades 1-2 and self-limited.43,44 If treatment of these is needed, then oral nonnarcotic agents that do not impair platelet function are recommended. These would include acetaminophen alone or with caffeine. Bleeding events were usually grade 1-2 and consisted of bruising and petechiae; there was 1 case of grade 3 gastrointestinal hemorrhage. Significant myelosuppression can occur and grade 4 neutropenia occurred in 6%. For these grade 3/4 toxicities, dose reductions to 100 mg daily may be required. It is not known what effect dose reduction has on tumor response. In patients such as the 1 described in Figure 1, acalabrutinib at full dose can be given with direct oral anticoagulants with appropriate instructions to watch for bleeding complications.
In early studies of ibrutinib for patients with heavily pretreated CLL, it was noted that some patients with CLL developed unusual infections including aspergillosis.45 In a subsequent study of single-agent ibrutinib in patients with treatment-naive (n = 5) or relapsed (n = 13) primary central nervous system (CNS) lymphoma, aspergillosis developed in 39% (7 of 18; 2 were treatment naive). This was not found to be correlated with ibrutinib blood levels. These primary CNS lymphoma (PCNSL) patients were also on corticosteroids at the time of the ibrutinib; however, Aspergillosis, in our experience, is very unusual in PCNSL patients treated with conventional methotrexate-based chemoimmunotherapy regimens that also use corticosteroids. Further investigation using a BTK knockout mouse model demonstrated that BTK signaling is important for maintaining adequate macrophage function and to prevent death from Aspergillus exposure.46 This led to more studies that have confirmed that ibrutinib has an inhibitory effect on macrophage function.47 Fortunately, the incidence of Aspergilloses in CLL patients treated with ibrutinib appears to be much lower, in the 2.5% range.48 A recent report of 42 patients with CLL treated with acalabrutinib noted 1 case of Aspergillus infection.49 In the MCL trial with Acala,43 no cases of Aspergilloses have been reported to date. There have not been any reported trials of acalabrutinib in PCNSL. Thus, it appears that BTK inhibitors can increase the risk of fungal infection of the lungs or brain and that this risk is highest in PCNSL patients receiving steroids with the BTK inhibitor. There are inadequate data to estimate this risk specifically with acalabrutinib but it appears to be rare in CLL and MCL; and there are no data on the risk in PCNSL. Further investigations into macrophage function in PCNSL patients are also warranted.
Summary
Acalabrutinib represents another welcome addition to the treatment armamentarium for relapsed MCL. This oral agent produces high response rates with a well-tolerated side-effect profile. Because there have not been direct trials of acalabrutinib with ibrutinib in relapsed MCL, comparing the 2 BTK inhibitors is not possible. With respect to the differences in ORR (Table 1) in the 2 large clinical trials, it should be noted that the patient population treated and reported with acalbrutinib43 was lower risk with few patients having been transplanted, nor had many received the other FDA-approved agents for relapsed MCL. In addition, there are no data regarding the efficacy in populations of MCL patients that are higher risk (TP53 mutant; blastoid variant). Acalabrutinib was designed to be more selective for BTK and indeed it appears that acalabrutinib is less toxic than ibrutinib at least with respect to the incidence of atrial fibrillation (Table 1). However, grade 1-2 headache appears more common with acalabrutinib43,44 than with ibrutinib.25 It is reassuring that even with long-term treatment of MCL patients with either agent, fungal infections are not being observed as frequently as they are in PCNSL. The studies of both BTK inhibitors in MCL remain small and the information from the new trials, as well as longer-term follow-up of the current acalabrutinib trials in MCL and CLL, will be needed. The side-effect profile of acalabrutinib is allowing studies in combination with standard chemoimmunotherapy for new, untreated MCL. Indeed, the future is now brighter for MCL patients in relapse. The more precise role of acalabrutinib in MCL will be defined by further experience in clinical practice and the ongoing research trials.
Results of published clinical trials of BTK inhibitors in patients with relapsed MCL
| Parameter . | Ibrutinib25,34 . | Acalabrutinib43,44 . |
|---|---|---|
| No. of patients | 111 | 124 |
| Phase of study | Single-arm phase 2 | Single-arm phase 2 |
| Median age, y | 68 | 68 |
| MIPI score: low/intermediate/high, % | 14/38/49 | 39/44/17 |
| Median no. of prior therapies | 3 | 2 |
| Prior rituximab, % | 89 | 95 |
| Prior stem cell transplant, % | 11 | 18 |
| Prior proteasome inhibitor, % | 43 | 19 |
| Prior lenalidomide, % | 24 | 7 |
| Dose | 560 mg postoperatively daily indefinitely | 100 mg postoperatively twice daily indefinitely |
| ORR, % | 68 | 81 |
| Complete remission, % | 21 | 40 |
| Median duration of response, mo | 17.5 | 25.7 |
| Median PFS, mo | 13.9 | 19.5 |
| Median OS, mo | Median not reached; 47% OS at 24 mo | Median not reached; 72% OS at 24 mo |
| Stopped drug due to adverse events, % | 7 | 6 |
| New atrial fibrillation, % | 11 | 0 |
| Bleeding | 5 with grade 3; no grade 4-5 | 22% petechiae/purpura (all grades 1-2) 3 grade 3 (GI bleed; hematuria, hematoma on each) |
| Grade 3/4 neutropenia, % | 6/10 | 5/6 |
| Grade 2/3 pneumonia, % | 6 | 1/5 |
| Parameter . | Ibrutinib25,34 . | Acalabrutinib43,44 . |
|---|---|---|
| No. of patients | 111 | 124 |
| Phase of study | Single-arm phase 2 | Single-arm phase 2 |
| Median age, y | 68 | 68 |
| MIPI score: low/intermediate/high, % | 14/38/49 | 39/44/17 |
| Median no. of prior therapies | 3 | 2 |
| Prior rituximab, % | 89 | 95 |
| Prior stem cell transplant, % | 11 | 18 |
| Prior proteasome inhibitor, % | 43 | 19 |
| Prior lenalidomide, % | 24 | 7 |
| Dose | 560 mg postoperatively daily indefinitely | 100 mg postoperatively twice daily indefinitely |
| ORR, % | 68 | 81 |
| Complete remission, % | 21 | 40 |
| Median duration of response, mo | 17.5 | 25.7 |
| Median PFS, mo | 13.9 | 19.5 |
| Median OS, mo | Median not reached; 47% OS at 24 mo | Median not reached; 72% OS at 24 mo |
| Stopped drug due to adverse events, % | 7 | 6 |
| New atrial fibrillation, % | 11 | 0 |
| Bleeding | 5 with grade 3; no grade 4-5 | 22% petechiae/purpura (all grades 1-2) 3 grade 3 (GI bleed; hematuria, hematoma on each) |
| Grade 3/4 neutropenia, % | 6/10 | 5/6 |
| Grade 2/3 pneumonia, % | 6 | 1/5 |
GI, gastrointestinal.
Acknowledgment
The Mayo Clinic received research funding from Acerta Pharmaceuticals to conduct the trials. No direct remuneration was provided to the authors.
Authorship
Contribution: T.E.W. wrote the review; and D.I. reviewed and critiqued the manuscript.
Conflict-of-interest disclosure: T.E.W. and D.I. have participated in clinical trials of acalabrutinib.
Correspondence: Thomas E. Witzig, Mayo Clinic, 200 First St SW, Rochester, MN 55905; e-mail: witzig.thomas@mayo.edu.