Abstract
Evidence-based recommendations have been established for treatment of chronic myeloid leukemia (CML) in adults treated with tyrosine kinase inhibitors (TKIs), but the rarity of this leukemia in children and adolescents makes it challenging to develop similar recommendations in pediatrics. In addition to imatinib, which was approved for pediatric CML in 2003, the second-generation TKIs dasatinib and nilotinib were recently approved for use in children, expanding the therapeutic options and pushing allogeneic stem cell transplantation to a third-line treatment of most pediatric cases. Yet, without sufficient data on efficacy and safety specific to pediatric patients, the selection of a TKI continues to rely on clinical experience in adults. Here, we present 4 case scenarios highlighting common yet challenging issues encountered in the treatment of pediatric CML (suboptimal response, poor treatment adherence, growth retardation, and presentation in advanced phases). Limited experience with very young children, the transition of teenagers to adult medicine, and the goal of achieving treatment-free remission for this rare leukemia are additional significant obstacles that require further clinical investigation through international collaboration.
Introduction
Chronic myeloid leukemia (CML) rarely occurs in the first few decades of life, accounting for 2% to 3% of leukemias in children and adolescents.1,2 Epidemiologic data show an incidence of 0.6 to 1.2 per million children/year that increases with age: CML is exceptionally rare in infancy, and occurs in 0.7 per million children 1 to 14 years of age/year and in 1.2 per million adolescents/year.1
CML in children and adolescents is different from CML in adults.1,3 Children and adolescents with CML tend to present with more aggressive features (higher white blood cell count, larger spleen size in proportion to body size, higher frequency of advanced phases at diagnosis)1,4 ; show a distribution of BCR-ABL1 breakpoints resembling Philadelphia chromosome-positive acute lymphoblastic leukemia (ALL)5 ; and have a higher proportion of mutated cancer driver genes.6,7 Risk scores that are well-established in adults for predicting outcomes8-10 (eg, Sokal, EURO, European Treatment and Outcome Study, and European Treatment and Outcome Study long-term survival score) do not apply in children,11 with the exception of European Treatment and Outcome Study long-term survival, which may predict progression-free survival but not overall survival (OS).12
The advent of tyrosine kinase inhibitors (TKIs) in the past 2 decades has changed the landscape of CML treatment dramatically, with life expectancy in adult patients now almost identical to that of the age-matched healthy population.13 Treatment with imatinib has also improved outcomes in children.14-16 The second-generation TKIs (2G-TKIs) dasatinib and nilotinib were approved recently as first-line treatment in children, which has expanded treatment options and made allogeneic stem cell transplantation (allo-SCT) a third-line treatment.17,18 However, because children are actively growing during TKI treatment, they face unique side effects not seen in adults, such as growth disturbance.19-23 Discontinuing TKIs in patients with a deep and sustained molecular response is feasible in adult patients.24-26 In pediatrics, this approach would likely be more beneficial in terms of reducing TKI-related side effects, but limited data are available, coming primarily from anecdotal experiences in patients who stopped TKI because of poor adherence and a small proportion of children who have discontinued TKI successfully.14,27 Prospective studies of TKI discontinuation in pediatric patients with CML are needed.
Here, we discuss our personal approach to treating CML in children and adolescents, considering various key issues that contribute to the complexity of treating this disease.
Case 1
An 8-year-old, previously healthy female presented to her pediatrician’s office after experiencing fatigue for a few months. She was found to have a large spleen extending to 10 cm below the costal margin and an enlarged liver. Blood tests showed white blood cell count, 150 × 109/L; hemoglobin, 10.2 g/dL; and platelet count, 928 × 109/L; she was referred to a tertiary care center for further workup. The result of bone marrow aspirate and biopsy was consistent with CML in the chronic phase (CML-CP). She was started on imatinib 340 mg/m2 (Table 1) orally once daily.16 She tolerated the treatment without significant toxicity initially and her blood counts normalized within a month; however, her BCR-ABL1 transcript level by quantitative polymerase chain reaction (PCR) assay at 3 months was 25.9% (on the international scale [IS]). It decreased gradually, but was still 2.5% at 12 months. Her parents confirmed that she was taking imatinib daily without missing any doses. Mutational analysis showed no BCR-ABL1 mutations.
Recommended TKI doses approved for children and proportion of patients achieving MMR
| Recommended TKI dose for CML-CP treatment . | Proportion of pediatric patients with CML-CP treated with first-line TKI who achieved MMR . | ||||
|---|---|---|---|---|---|
| Patients, no. . | 12 mo . | 18 mo . | 24 mo . | References . | |
| Imatinib | |||||
| 340 mg/m2/dose, once daily | 51 | NR | NR | NR | 16 |
| 300 mg/m2/dose, once daily | 140 | 42%* | 59%* | 69%* | 14 |
| 260 mg/m2/dose, once daily | 44 | 31%† | 55%* | 60%* | 15 |
| Nilotinib | |||||
| 230 mg/m2/dose, twice daily | 25 | 64%* | 68%§ | NR | 18 |
| Dasatinib | |||||
| 60 mg/m2/dose, once daily | 84 | 52%* | 65%* | 70%* | 17 |
| Recommended TKI dose for CML-CP treatment . | Proportion of pediatric patients with CML-CP treated with first-line TKI who achieved MMR . | ||||
|---|---|---|---|---|---|
| Patients, no. . | 12 mo . | 18 mo . | 24 mo . | References . | |
| Imatinib | |||||
| 340 mg/m2/dose, once daily | 51 | NR | NR | NR | 16 |
| 300 mg/m2/dose, once daily | 140 | 42%* | 59%* | 69%* | 14 |
| 260 mg/m2/dose, once daily | 44 | 31%† | 55%* | 60%* | 15 |
| Nilotinib | |||||
| 230 mg/m2/dose, twice daily | 25 | 64%* | 68%§ | NR | 18 |
| Dasatinib | |||||
| 60 mg/m2/dose, once daily | 84 | 52%* | 65%* | 70%* | 17 |
NR, not reported.
Results are reported as a cumulative rate at the indicated time point.
Results are reported as a response rate at the indicated time point.
Cumulative response rate by data cutoff at 16.6 mo.
Recommendations for initial management and follow-up
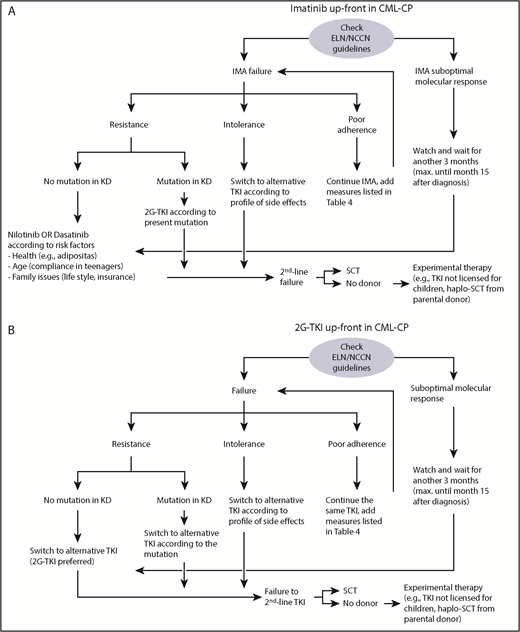
The procedure for establishing a diagnosis of CML in children does not differ from that used in adults, and recommendations in pediatrics have been published (Table 2).2,28,29 Four TKIs (imatinib, dasatinib, nilotinib, and bosutinib) are currently available as first-line treatment of adults. In addition to imatinib, which was approved in 2003, the 2G-TKIs dasatinib and nilotinib were recently approved for children by US Food and Drug Administration and European Medicines Agency.17,18,30,31 The availability of 3 TKI options may make it challenging to choose a first-line TKI, but it also gives clinicians additional treatment options in the case of a suboptimal response (Figure 1). Several factors must be taken into consideration when choosing a TKI. In pediatrics, there is more experience with the efficacy, toxicity profiles, and comorbidities of imatinib than with the other TKIs.14-16 Drug availability, ease of administration, and financial issues should also be considered. Imatinib and dasatinib are given once daily and can be given with food, whereas nilotinib is given twice daily and food should be avoided 2 hours before and 1 hour after each dose. These restrictions may be challenging for young children and teenagers, depending on lifestyle. Generic imatinib is much less expensive than the branded product32 and 2G-TKIs cost significantly more than imatinib.33
Initial and follow-up evaluations
| Evaluations at diagnosis . | Evaluations at follow-up visits . |
|---|---|
| Complete blood count (including differentials) and comprehensive metabolic profile | Complete blood count (including differentials) and comprehensive metabolic profile |
| Spleen and liver size (should be measured as below costal margin) | Spleen and liver size (should be measured as below costal margin) |
| Bone marrow aspirate and biopsy (differentials and karyotype) | Bone marrow every 6 mo until complete cytogenetic response |
| Baseline molecular genetics (qRT-PCR for BCR-ABL1) with transcript type (eg, e14a2) | Molecular monitoring from peripheral blood by qRT-PCR (monthly in the first 3 mo, every 3 mo thereafter) |
| Categorize stage of disease (CML-CP, CML-AP, CML-BP), identify additional risk factors (eg, additional chromosomal aberrations) | Classify response according to treatment time as recommended by the ELN44 or NCCN42 (Table 3) In advanced phases of CML, also monitor bone marrow cytogenetics to detect clonal evolution |
| Evaluations at diagnosis . | Evaluations at follow-up visits . |
|---|---|
| Complete blood count (including differentials) and comprehensive metabolic profile | Complete blood count (including differentials) and comprehensive metabolic profile |
| Spleen and liver size (should be measured as below costal margin) | Spleen and liver size (should be measured as below costal margin) |
| Bone marrow aspirate and biopsy (differentials and karyotype) | Bone marrow every 6 mo until complete cytogenetic response |
| Baseline molecular genetics (qRT-PCR for BCR-ABL1) with transcript type (eg, e14a2) | Molecular monitoring from peripheral blood by qRT-PCR (monthly in the first 3 mo, every 3 mo thereafter) |
| Categorize stage of disease (CML-CP, CML-AP, CML-BP), identify additional risk factors (eg, additional chromosomal aberrations) | Classify response according to treatment time as recommended by the ELN44 or NCCN42 (Table 3) In advanced phases of CML, also monitor bone marrow cytogenetics to detect clonal evolution |
qRT, quantitative reverse transcription.
Algorithms reflecting the authors’ experience treating children in CML-CP once failure or suboptimal response is detected by regular monitoring of the BCR-ABL1 transcript ratio. (A) First-line treatment with imatinib; (B) first-line treatment with a 2G-TKI (either dasatinib or nilotinib). IMA, imatinib; KD, kinase domain; SCT, stem cell transplantation.
Algorithms reflecting the authors’ experience treating children in CML-CP once failure or suboptimal response is detected by regular monitoring of the BCR-ABL1 transcript ratio. (A) First-line treatment with imatinib; (B) first-line treatment with a 2G-TKI (either dasatinib or nilotinib). IMA, imatinib; KD, kinase domain; SCT, stem cell transplantation.
In randomized studies in adults, 2G-TKIs induced a significantly faster and deeper molecular remission than imatinib, although no survival advantage was observed.34-36 No randomized studies have compared imatinib with 2G-TKIs in pediatric patients, but the molecular response rates with 2G-TKIs seem to be comparable to those seen adults.17,18 Limited data from pediatric cohorts demonstrate that molecular responses at months 6 and 12 months are deeper with 2G-TKIs, but at 18 months are similar for imatinib and 2G-TKIs (Table 1).15,17,18 The 2G-TKIs may also have higher toxicity, including a small number of serious vascular occlusive events in adults,35,37-39 but to date, pediatric cases (eg, pleural or pericardial effusions with dasatinib) have been reported only rarely.17,18,30 Therefore, if higher cost is not an issue, 2G-TKIs might be a good option for first-line therapy in children for whom future TKI discontinuation needs to be considered. The efficacy, safety, availability, cost, and the goal of treatment should all be weighed carefully when choosing the first-line TKI for pediatric CML. In adults, it remains a matter of debate which TKI should be used for first-line treatment of CML-CP.40,41
When to switch TKIs
Currently, no pediatric-specific response criteria are available; National Comprehensive Cancer Network (NCCN) guidelines42,43 or ELN criteria44 may be used (Table 3). Taking case 1 as an example, the patient exhibited suboptimal response to her initial TKI treatment. According to NCCN guidelines, a BCR-ABL1 transcript level of 2.5% at 12 months is graded as warning of possible TKI resistance and is considered treatment failure by European Leukemia Net (ELN) criteria. Although NCCN guidelines give the option of switching TKI or continuing the same TKI, it would be appropriate in this situation to do at least the following: (1) evaluate adherence to treatment (see “Adherence issues in children and adolescents”), (2) order BCR-ABL1 mutational analysis and cytogenetics as well as BCR-ABL1 transcript ratio in bone marrow, and (3) consider a donor search for allo-SCT.
Definition of molecular response based on the IS; adopted from ELN guidelines and NCCN guidelines, which reasonably can be used in children
| Milestone, mo. . | Optimal response . | Warning . | Failure . |
|---|---|---|---|
| 3 | BCR-ABL1 <10% IS | BCR-ABL1 ≥10% IS | |
| 6 | BCR-ABL1 <1%* IS | BCR-ABL1 1-10% IS | BCR-ABL1 ≥10% IS |
| 12 | BCR-ABL1 <0.1% IS | BCR-ABL1 0.1-1% IS | BCR-ABL1 ≥1% IS |
| Milestone, mo. . | Optimal response . | Warning . | Failure . |
|---|---|---|---|
| 3 | BCR-ABL1 <10% IS | BCR-ABL1 ≥10% IS | |
| 6 | BCR-ABL1 <1%* IS | BCR-ABL1 1-10% IS | BCR-ABL1 ≥10% IS |
| 12 | BCR-ABL1 <0.1% IS | BCR-ABL1 0.1-1% IS | BCR-ABL1 ≥1% IS |
Mutations of the BCR-ABL1 kinase domain may cause TKI resistance, but there are also BCR-ABL1‒independent causes.45 Mutation screening is recommended in patients with poor response (ie, primary resistance) as well as those who lose initial response (ie, secondary resistance).46 A sharp and sudden increase in the BCR-ABL1 transcript ratio should always raise suspicion of poor adherence47 because TKI resistance from mutations in the ABL1 kinase domain are characterized by a slower expansion of the mutated clone. Each TKI has different patterns of inactivity against defined mutations; therefore, the specific mutation needs to be considered when selecting a TKI.46,48 Ponatinib is the only TKI to effectively treat T315I mutation in BCR-ABL1+ leukemias, but is not approved in pediatric patients. There are few anecdotal experiences with ponatinib,49,50 with no safe dose having been determined in children.
For imatinib treatment failure in patients without an identified mutation, we recommend switching to a 2G-TKI followed by monthly monitoring in the first 3 months. If response according to adult guidelines is observed, treatment may be continued indefinitely, with monitoring every 3 months. If suboptimal response or failure to 2G-TKIs according to ELN/NCCN guidelines is observed, allo-SCT should be initiated (Figure 1).
Case 2
After diagnosis of CML-CP in a 13-year-old boy, daily imatinib 300 mg/m2 was started.14,28 He achieved milestones of response according to the ELN guidelines.44 Bone pain and muscle cramps were treated effectively with ibuprofen and disappeared after the first 12 months of treatment. MR4 (BCR-ABL1 transcript ≤0.01% IS) was achieved at 24 months. However, at month 30, PCR showed loss of MR4 with a log-fold increase. When the BCR-ABL1 transcript was reassessed a month later, it had doubled (0.27%). No kinase domain mutations were detected. When the laboratory findings were discussed, the patient finally admitted he had not taken imatinib during the past 4 months because he had read on the Internet that patients in deep molecular response could safely stop TKI treatment. He did not discuss his decision with his parents or his doctor.47 After prolonged counseling, he agreed to restart imatinib treatment at the same dosage and achieved MR4 in 6 months.
Adherence issues in children and adolescents
In patients with chronic but potentially life-threatening illnesses (eg, cystic fibrosis, epilepsy, asthma, diabetes), poor adherence to treatment is a common clinical experience and a major source of frustration for pediatricians. Extended treatment duration, multiple medications, and periods of symptomatic remission contribute to poor adherence in 30% to 70% of patients with chronic disease.51 In pediatric malignancies such as ALL, intentional nonadherence to maintenance treatment occurs rarely (8%), but repeated forgetfulness is noted in 15% of patients.52 CML is no exception and nonadherence is demonstrated in 15% to 30% of adult patients.53,54 Within this clinical reality, it should be emphasized to patients that omitting 10% of the cumulative monthly imatinib dose (eg, 3 days per month) results in a significantly inferior achievement of relevant treatment milestones.55,56
Adolescence is another well-known challenge to compliance in pediatric patients with chronic diseases47,57 because it is associated with increased risk-taking behaviors. Patients may resist frequent physician visits or, as in this patient, secretly stop taking medication because of unwanted side effects.27 As a consequence, if treatment failure in a teenaged patient occurs, poor adherence to treatment should be considered first, before exploring the possibility of treatment resistance and ordering extensive laboratory tests.
Studies have demonstrated excellent long-term outcomes with early optimal response to first-line treatment with imatinib.58 As in this patient, the possibility for treatment-free remission after stopping imatinib, as demonstrated in adults,25,26 is reported in the popular lay media. However, only the depth of response needed for interruption is frequently noted in these reports, which neglects the necessary framework conditions for success (see “Stopping TKI in deep and sustained molecular response”).
Physicians should be also aware that poor adherence is increased by side effects. Often, those side effects that are considered negligible by physicians (eg, “mild” diarrhea “only” twice weekly), are seen by patients as unacceptable, especially by teenagers who are involved in various activities and do not want to have to deal with CML and its treatment.59,60 Thus, at every visit, it is critical to ask pediatric patients with CML about whether they are taking their medication as prescribed and to discuss the importance of adherence to treatment in achieving optimal outcomes.55
Addressing the issues listed in Table 4 can enhance adherence to treatment among teenagers. Any short-term changes in the caregiver’s responsibility or oversight (eg, participation in summer vacation camps; children of divorce visiting the noncustodial parent over a weekend) can lead to forgetfulness and nonadherence. Medication diaries are practical tools that can help promote adherence.51 In randomized clinical trials, children and adolescents improved their self-care after playing health education and disease management video games.54 Such tools are still awaiting development for pediatric patients with CML. The decision to stop TKI treatment may also be influenced by the financial burden of these expensive drugs; this issue may vary among different families and countries depending on the insurance system and the availability and price of imatinib generics.32,61,62
Approaches to improve medication adherence in teenagers with CML
| Physicians . | Patients . |
|---|---|
| Communicate the recommended schedule for TKI therapy (eg, once or twice daily, with or without food, on an empty stomach). | Incorporate taking medication into daily routines and take it at the same time each day (eg, before brushing teeth in the evening). |
| Have others (eg, pharmacists, nursing staff, medical assistants, subspecialists) reinforce the information. | Use a pillbox. In addition, keep a diary of doses taken or missed or mark a calendar. |
| Provide patient information handouts. | Use visual reminders (eg, notes on the refrigerator, on the mirror). |
| Keep clear documentation in the patient’s chart (eg, contracts, reevaluation policies). | Ask family member or close friend to remind you. |
| Discuss medication safety issues with the patient and the parents. | Set an alarm (on a clock, your watch, or mobile phone). |
| Renew the prescription to a fixed schedule (eg, monthly, every 3 mo). | Make a contract with your parents, with rewards for not forgetting to take the medication. |
| Be aware of the relatively high financial costs for the dose prescribed. When prescribing generics, do not switch for financial reasons more frequently than once a year. | Communicate with your physician about the doses you did not take and the reasons why you did not take them. |
| Electronic devices | |
| Pill box with medication timer and alarm. | |
| Pill box with sensors that alert family members through Bluetooth if a pill was not taken on time. | |
| Smartphone app that sends a reminder to take medication. | |
| Electronic microchip attached to the pill that acts as a pH sensor to gastric acid. Once the pill is swallowed, a signal is sent to a smartphone app for documentation. |
| Physicians . | Patients . |
|---|---|
| Communicate the recommended schedule for TKI therapy (eg, once or twice daily, with or without food, on an empty stomach). | Incorporate taking medication into daily routines and take it at the same time each day (eg, before brushing teeth in the evening). |
| Have others (eg, pharmacists, nursing staff, medical assistants, subspecialists) reinforce the information. | Use a pillbox. In addition, keep a diary of doses taken or missed or mark a calendar. |
| Provide patient information handouts. | Use visual reminders (eg, notes on the refrigerator, on the mirror). |
| Keep clear documentation in the patient’s chart (eg, contracts, reevaluation policies). | Ask family member or close friend to remind you. |
| Discuss medication safety issues with the patient and the parents. | Set an alarm (on a clock, your watch, or mobile phone). |
| Renew the prescription to a fixed schedule (eg, monthly, every 3 mo). | Make a contract with your parents, with rewards for not forgetting to take the medication. |
| Be aware of the relatively high financial costs for the dose prescribed. When prescribing generics, do not switch for financial reasons more frequently than once a year. | Communicate with your physician about the doses you did not take and the reasons why you did not take them. |
| Electronic devices | |
| Pill box with medication timer and alarm. | |
| Pill box with sensors that alert family members through Bluetooth if a pill was not taken on time. | |
| Smartphone app that sends a reminder to take medication. | |
| Electronic microchip attached to the pill that acts as a pH sensor to gastric acid. Once the pill is swallowed, a signal is sent to a smartphone app for documentation. |
Case 3
A 7-year-old female presented with CML-CP and was started on dasatinib 60 mg/m2 daily. Her response was favorable: BCR-ABL1 was 5.6% IS at 3 months, 0.9% at 6 months, and 0.08% at 12 months, achieving major molecular response (MMR). At 24 months, BCR-ABL1 was MR4 and remained MR4 for 5 years. Her height at the time of diagnosis was 95th percentile and she was Tanner 1. After 12 months of dasatinib treatment, growth started to slow, and her height was 75th percentile after 2 years of treatment and 50th percentile after 3 years of treatment (age 10 years). She started puberty at age 11 (breast development and pubic hair, Tanner 2) and then experienced a growth spurt. She reached menarche at age 13 years. At age 14 years, her height was 75th percentile and she was Tanner stage 5.
Monitoring side effects of TKI in children
This patient had an excellent response to TKI treatment, but a significant growth delay. Unlike adults, children with CML are exposed to TKI during a critical period of active growth and physical development. Various degrees of growth deceleration have been reported, possibly caused by alterations in bone mineral and vitamin D metabolism, and disruption of the growth hormone-insulin-like growth factor 1 hormonal axis.20-23,63-68 Preclinical research in animals suggests that treatment with TKIs can affect bone strength and both male and female gonadal function.69 Long-term effects of TKIs on gonadal function are largely unknown. In teenagers with long-term TKI exposure, it seems reasonable to follow pubertal development every 4 to 6 months. If puberty is delayed or evidence of sex steroid deficiency is noted, referral to an endocrinologist and further workup are strongly recommended (Table 5).21 Very little research has investigated the long-term effects of TKI on puberty and most patients in small case series seem to have normal pubertal development. Because findings have been inconclusive, we recommend close monitoring of growth and pubertal development.21
Recommended monitoring for endocrine toxicities in children and adolescents with CML on TKI
| Parameter . | Potential changes . | Recommended monitoring . | Management . |
|---|---|---|---|
| Growth | Growth attenuation | Accurate height and weight at each visit | Referral to endocrinologist for possible GH stimulation testing |
| Close monitoring of growth velocity | |||
| Calculate prospective height from mid parental height | |||
| Bone | Dysregulation of bone remodeling | DEXA scan if radiograph indicates low bone mineral density or unprovoked fractures occur | Referral to endocrinologist |
| Altered calcium, phosphate, and vitamin D metabolism | |||
| Thyroid | Hypothyroidism | TSH and free T4 levels every 4-6 wk after initiation of therapy; every 6-12 mo thereafter or with symptoms suggestive of hypo- or hyperthyroidism | Referral to endocrinologist and consider thyroid hormone replacement therapy |
| Hyperthyroidism | |||
| Gonadal function | Delayed puberty | Accurate Tanner staging at reasonable intervals | Referral to endocrinologist for delayed puberty |
| Gonadal dysfunction | Check gonadotropins and sex steroids for delayed puberty or gonadal dysfunction | Offer sperm cryopreservation to pubertal males | |
| Potentially decreased fertility | Fertility preservation before therapy may be discussed | ||
| Pregnancy outcome | Fetal abnormalities | Pregnancy test at initiation of therapy for female patients of childbearing age | Recommend counseling on contraceptives for female patients of childbearing age. Efforts should be made to increase the chance of TKI discontinuation to facilitate safe pregnancies in adult life. |
| Parameter . | Potential changes . | Recommended monitoring . | Management . |
|---|---|---|---|
| Growth | Growth attenuation | Accurate height and weight at each visit | Referral to endocrinologist for possible GH stimulation testing |
| Close monitoring of growth velocity | |||
| Calculate prospective height from mid parental height | |||
| Bone | Dysregulation of bone remodeling | DEXA scan if radiograph indicates low bone mineral density or unprovoked fractures occur | Referral to endocrinologist |
| Altered calcium, phosphate, and vitamin D metabolism | |||
| Thyroid | Hypothyroidism | TSH and free T4 levels every 4-6 wk after initiation of therapy; every 6-12 mo thereafter or with symptoms suggestive of hypo- or hyperthyroidism | Referral to endocrinologist and consider thyroid hormone replacement therapy |
| Hyperthyroidism | |||
| Gonadal function | Delayed puberty | Accurate Tanner staging at reasonable intervals | Referral to endocrinologist for delayed puberty |
| Gonadal dysfunction | Check gonadotropins and sex steroids for delayed puberty or gonadal dysfunction | Offer sperm cryopreservation to pubertal males | |
| Potentially decreased fertility | Fertility preservation before therapy may be discussed | ||
| Pregnancy outcome | Fetal abnormalities | Pregnancy test at initiation of therapy for female patients of childbearing age | Recommend counseling on contraceptives for female patients of childbearing age. Efforts should be made to increase the chance of TKI discontinuation to facilitate safe pregnancies in adult life. |
GH, growth hormone; DEXA, dual-energy x-ray absorptiometry; T4, thyroxine TSH, thyroid-stimulating hormone.
No significant cardiovascular morbidities of TKIs have been observed in children,17,18,30,31 as have been reported in adults,38,70 but follow-up data are limited to the past several years and it is possible that these effects will manifest after several decades of TKI exposure. Lifelong TKI treatment could also have a significant effect on young patients’ quality of life; therefore, strategies to minimize long-term toxicities are even more important in children than in adults.71
Stopping TKI in deep and sustained molecular response
Recent studies in adults have shown the feasibility of discontinuing TKI and maintaining treatment-free remission when deep and sustained molecular remission is achieved (Table 6).24,26,72-77 Overall, TKI treatment can be discontinued successfully in about one-half of patients who fulfill the following prerequisites: (1) TKI treatment has continued for a minimum of 3 years and (2) MR4 or better has been maintained continuously over the past 2 years.
Criteria and outcome in studies on treatment-free remission (TFR) in adults
| Study . | N . | Eligibility for discontinuation . | Prior treatment . | Criteria for reinitiation of therapy . | TFR . |
|---|---|---|---|---|---|
| Stop Imatinib72 | 100 | Undetectable transcript ≥2 y | Imatinib ≥3 y* | Loss of MR3 or ≥1 log fold increase in transcript | 43% (95% CI, 33-52) at 6 mo |
| 38% (95% CI, 29-47) at 60 mo | |||||
| TWISTER73 | 40 | Undetectable transcript ≥2 y | Imatinib ≥3 y† | Loss of MR3 or MR4.5 | 47.1% (95% CI, 31.5-62.7) at 2 y |
| A-STIM74 | 80 | CMR (undetectable transcript) ≥2 y‡ | Imatinib ≥3 y§ | Loss of MR3 | 64% (95% CI, 54-75) at 12 and 24 mo |
| 61% (95% CI, 51-73) at 36 mo | |||||
| ENEST freedom75 | 190 | MR4.5 ≥1 y | Nilotinib frontline ≥3 y | Loss of MR3 | 51.6% (95% CI, 44.2-8.9) at 48 wk |
| STOP 2G-TKI97 | 60 | MR4.5 ≥2 y | Nilotinib or dasatinib ≥3 y first or second line | Loss of MR3 | 63.33% (95% CI, 51.14-75.53) at 12 mo |
| 53.57% (95% CI, 40.49-66.65) at 48 mo | |||||
| EURO-SKI24 | 755 | MR4 or undetectable transcript ≥1 y | Any TKI ≥3 y | Loss of MR3 | 61% (95% CI, 57-64) at 6 mo |
| 50% (95% CI, 46-54) at 24 mo |
| Study . | N . | Eligibility for discontinuation . | Prior treatment . | Criteria for reinitiation of therapy . | TFR . |
|---|---|---|---|---|---|
| Stop Imatinib72 | 100 | Undetectable transcript ≥2 y | Imatinib ≥3 y* | Loss of MR3 or ≥1 log fold increase in transcript | 43% (95% CI, 33-52) at 6 mo |
| 38% (95% CI, 29-47) at 60 mo | |||||
| TWISTER73 | 40 | Undetectable transcript ≥2 y | Imatinib ≥3 y† | Loss of MR3 or MR4.5 | 47.1% (95% CI, 31.5-62.7) at 2 y |
| A-STIM74 | 80 | CMR (undetectable transcript) ≥2 y‡ | Imatinib ≥3 y§ | Loss of MR3 | 64% (95% CI, 54-75) at 12 and 24 mo |
| 61% (95% CI, 51-73) at 36 mo | |||||
| ENEST freedom75 | 190 | MR4.5 ≥1 y | Nilotinib frontline ≥3 y | Loss of MR3 | 51.6% (95% CI, 44.2-8.9) at 48 wk |
| STOP 2G-TKI97 | 60 | MR4.5 ≥2 y | Nilotinib or dasatinib ≥3 y first or second line | Loss of MR3 | 63.33% (95% CI, 51.14-75.53) at 12 mo |
| 53.57% (95% CI, 40.49-66.65) at 48 mo | |||||
| EURO-SKI24 | 755 | MR4 or undetectable transcript ≥1 y | Any TKI ≥3 y | Loss of MR3 | 61% (95% CI, 57-64) at 6 mo |
| 50% (95% CI, 46-54) at 24 mo |
A-STIM, According to Stop Imatinib; CMR, complete molecular response; ENEST freedom, Nilotinib Treatment-free Remission Study in CML (Chronic Myeloid Leukemia) Patients; EURO-SKI, European Stop Tyrosine Kinase Inhibitor Study; MR3, BCR-ABL1 ≤0.1% IS; MR4, BCR-ABL1 ≤0.01% IS; MR4, BCR-ABL1 ≤0.0032% IS; STOP 2G-TKI, STOP second generation-tyrosine kinase inhibitor study; TWISTER, a phase II study to determine relapse-free interval after withdrawal of imatinib therapy in adult patients with chronic phase chronic myeloid leukaemia in stable complete molecular remission.
Patients who had previous stem cell transplantation or who had been treated with immunomodulatory agents other than interferon-α were excluded.
Prior treatment was permitted, except for allogeneic stem cell transplantation or other kinase inhibitors.
Patients with confirmed CMR with occasional weekly positive samples before study entry were also considered eligible.
Prior treatment was permitted, except for allogeneic stem cell transplantation.
Older versions of NCCN guidelines recommended TKI discontinuation only in the clinical trial setting (NCCN 2016, version 1), but version 1.2017 and later versions list stringent criteria for discontinuation in adult patients that may be applied in a nonstudy setting. However, CML in children tends to have a more aggressive clinical presentation and different biology from CML in adults.1,2 Outcomes after TKI discontinuation in children were suboptimal in reports from small cohorts.14,27 In addition, discontinuing TKI could cause withdrawal symptoms including musculoskeletal pain,26 for which there are so far no data in children. Neurocognitive impairment after TKI discontinuation has also been described in adult patients,78 possibly caused by a rebound increase in neuronal Abl activity after stopping TKI therapy. Such changes may be more significant in young children.
TKI discontinuation should be considered as experimental in children at this point and we recommend it be done only in a prospective clinical trial setting until further data are available. Children’s Oncology Group is planning to open a pilot study to examine the feasibility of TKI discontinuation (NCT 03817398).
Case 4
A diagnosis of ALL was suspected in a 9-year-old girl (liver 3 cm and spleen 5 cm below costal margin, white blood cell count 320 000/µL (59% blasts, 10% promyelocytes, 8% metamyelocytes, 5% granulocytes, 1% monocytes, 11% basophils, 5% eosinophils, and 1% lymphocytes), hemoglobin 8.5 g/dL, and platelets 42 000/µL. A bone marrow aspirate showed 80% blasts, which were lymphoblastic (CD19+, CD20+, TdT+, HLA-DR+) by flow cytometry. Cytogenetic analysis showed t(9;22)(q34;q11) in 14/15 metaphases analyzed and additional chromosomal aberrations comprising trisomy 8 and inv(7). She had the e1a2 BCR-ABL1 transcript, which encodes p190. A diagnosis of lymphoid blast phase CML was made. No mutation of the BCR-ABL1 kinase domain was found. She received modified ALL induction treatment (prednisolone, vincristine on days 8 and 15, but no anthracyclines). Imatinib 500 mg/m2 once daily28 was added on day 16. Bone marrow aspirate on day 15 showed 15% blasts and on day 33 complete remission was achieved (<5% blasts). The BCR-ABL1/ABL1 transcript ratio was 40% and with continued imatinib treatment further declined to 12% at month 3. There were no HLA-matched donors for allo-SCT, and treatment with imatinib alone was continued. Monthly PCR monitoring showed a steady decline in the BCR-ABL1/ABL1 transcript ratio (month 6, 2%; month 9, 0.3%; month 12, 0.15%). When ≤0.1% was achieved at month 15, the TKI dose was reduced to 300 mg/m2 once daily28 and monitoring was continued every 3 months. Four years after diagnosis, the BCR-ABL1/ABL1 transcript ratio remains ≤0.1% with ongoing imatinib treatment. A donor for allo-SCT still has not been identified despite an active search.
Management of children who present with advanced stage disease
Experience in children with CML in advanced stages (accelerated phase [CML-AP], blast phase [CML-BP]) is very limited because of the small number of cases. In 5 children treated with TKI who achieved a morphologic and cytogenetic remission before allo-SCT, followed by restart of TKIs post allo-SCT, 100% survival at an average of 38 months (range, 14-51 months) was reported.79 Recently, data from the International Registry for Chronic Myeloid Leukemia (CML) in Children and Adolescents study including 37 children with de novo CML-AP (n = 20) or de novo CML-BP (n = 17, 70% CML lymphoid blast phase) was presented.4 Among the 17 patients with CML-BP, 14 children were treated with a combination of TKI and chemotherapy and 3 patients received TKI only. Eleven of these 17 patients were taken to allo-SCT and, similar to case 4, 6 patients received TKI with or without chemotherapy (3 patients received TKI only). At a median follow-up of 28 months (range, 16-78 months), 13 of the 17 patients were alive (5-year OS: 74%; 95% confidence interval [CI], 44-89). The 4 deaths (3/11 with SCT, 1/6 without SCT) were all attributed to CML relapse (Frederic Millot, Poitiers University Hospital, oral communication, 9 December 2018). Thus, if no HLA-matched stem cell donor is available, a strategy of treatment with imatinib or a 2G-TKI without transplant, although not ideal, is a potential option. Although the number of pediatric patients in the study was very small, these data suggest that the prognosis of CML-BP in pediatric patients may be better than reported in adults, for whom the current evidence shows that allo-SCT may be the best chance of cure but with an OS of only 30%.80,81 As a word of caution, relapse of CML-BP can occur rapidly82 ; therefore, close monitoring of the response, especially defining the intervals for long-term molecular monitoring in the peripheral blood, remains challenging in these children. Monitoring in 3-month intervals might be reasonable once MR4 or better is accomplished; until this milestone is achieved, we recommend monthly monitoring.
Among the 20 patients who presented with de novo CML-AP, 17 were initially treated with TKI and 3 were treated with TKI and chemotherapy.4 Six of the 20 patients received allo-SCT; for the entire cohort, the 5-year OS was 94% (95% CI, 66-99). The data are obviously very limited, with a small cohort size, but suggest that the majority of pediatric patients with de novo CML-AP survive without allo-SCT.4 Given the opportunity for extended treatment with dasatinib and nilotinib in pediatric patients, we would select a 2G-TKI as first-line treatment of de novo CML-AP.
For patients who experience TKI treatment failure with progression to CML-BP, according to the blasts’ immunophenotype, ALL or acute myeloid leukemia induction treatment including intrathecal prophylaxis should be applied to achieve another CML-CP. At diagnosis, mutation analysis is recommended, and treatment with a suitable TKI selected according to the mutation profile should follow induction treatment. Once a suitable stem cell donor is identified, allo-SCT should be performed.83
CML-AP evolving from CML-CP under imatinib is somewhat poorly defined,84 making it difficult to compare outcomes by different treatment approaches. A search for an HLA-matched donor may be initiated, but if the milestones of response are achieved, we would not recommend immediate allo-SCT for children. Monitoring of CML-AP should include bone marrow cytogenetics in addition to molecular response assessment from peripheral blood. In addition, in patients with CML-AP that evolves under first-line imatinib, an optimal response to a 2G-TKI should make it possible to postpone allo-SCT as long as no failure to second-line treatment is suspected.14,85
Indication for allo-SCT in children with CML
Before TKIs were introduced, allo-SCT was the only curative treatment of CML, but the indication for allo-SCT is now limited (Table 7). Allo-SCT may play a bigger role in children with CML compared with adults for several reasons. Children potentially need life-long TKI, much longer than in adults. TKIs have known side effects and as yet unknown long-term side effects from several decades of TKI treatment,1 therefore, allo-SCT benefits pediatric patients by avoiding life-long TKI treatment. In general, outcomes of allo-SCT in children are more favorable than in adults and survival after allo-SCT in children with CML-CP is close to 90% in some studies.86,87 Improvement in supportive care in recent years and advances in reduced-toxicity SCT may further improve the outcomes of allo-SCT in the pediatric population.
Indication of allo-SCT for children with CML in TKI era
| Indication . | Comment . |
|---|---|
| No consensus | Data on long-term outcomes in children remain sparse. |
| Advanced phase (CML-AP or CML-BP) at diagnosis | 2G-TKI is recommended first. If a suitable donor is not available and milestones are achieved, TKI may be continued under close monitoring. |
| Progression to CML-AP or CML-BP | Undertake attempt to achieve a second CP with 2G-TKI first. |
| Resistance to 2 TKIs; unacceptable intolerance to all TKIs licensed; T315I mutation | Give ponatinib first, if available, and only for bridging to SCT.* |
| Indication . | Comment . |
|---|---|
| No consensus | Data on long-term outcomes in children remain sparse. |
| Advanced phase (CML-AP or CML-BP) at diagnosis | 2G-TKI is recommended first. If a suitable donor is not available and milestones are achieved, TKI may be continued under close monitoring. |
| Progression to CML-AP or CML-BP | Undertake attempt to achieve a second CP with 2G-TKI first. |
| Resistance to 2 TKIs; unacceptable intolerance to all TKIs licensed; T315I mutation | Give ponatinib first, if available, and only for bridging to SCT.* |
Ponatinib is not approved for use in children. No pediatric studies have been conducted and no safe pediatric dose can be recommended.
However, early morbidities and mortality associated with allo-SCT can be significant, making TKI treatment more advantageous. If a patient fails imatinib, there are 2G-TKIs available; it was demonstrated that 43% of adult patients who were resistant to imatinib and switched to dasatinib attained MMR.88 In a retrospective data analysis of 27 children who were switched from imatinib to the 2G-TKIs dasatinib or nilotinib for poor response or intolerance, deeper molecular responses were observed in 63% and maintained in 37% of patients.89 Nonetheless, it would be reasonable to consider allo-SCT if a patient fails treatment with 2 TKIs.
Transformation from CML-CP to blast phase while on TKI therapy is rare; however, once this happens, survival is poorer than in de novo CML-BP. Allo-SCT seems to offer better outcomes than other treatments in adult patients.80,81 The current NCCN guidelines42 suggest allo-SCT for patients who progress to CML-BP and that it should be performed within a 3- to 6-month interval from diagnosis.80,81,90,91 Although several previous studies have demonstrated that the use of TKI before transplantation does not seem to have a negative effect on the outcome of allo-SCT in CML,92,93 a more recent study including adult patients receiving 1, 2, or 3 TKIs before allo-SCT clearly showed that the nonrelapse mortality rate was higher in patients with 3 TKIs than in patients treated with 1 or 2 TKIs.94 Also in adults, it was demonstrated that patients with CML-BP harboring a T315I mutation had better outcomes with allo-SCT than with ponatinib.95,96 Thus, in younger patients with CML-CP who have resistance to second-line TKI therapy, allo-SCT from an appropriate donor could be considered.
Also, in accordance with the NCCN guidelines,42 we recommend starting a donor search when a patient progresses from CML-CP to CML-AP. There are only sparse pediatric data for these patients; thus, the recommendations for adults should be followed: allo-SCT should be considered if there is a matched sibling donor or full-matched unrelated donor. However, if the patient achieves molecular response with a 2G-TKI, it is also reasonable to continue observation without allo-SCT.
Conclusion
Because of the rarity of CML in children, guidelines for therapeutic decisions and definitions of response have been adopted from recommendations made for adult patients. This approach seems to be feasible in pediatric cohorts; however, long-term morbidities unique in the pediatric population require different approaches to adherence and SCT. In addition, long-term outcomes of TKI treatment and late effects of life-long TKI treatment need to be defined. Progress in the treatment of adult patients with CML during the past 2 decades has resulted in the possibility of TKI therapy discontinuation under well-defined conditions. The feasibility of this approach in children is still being studied. The rarity of pediatric CML necessitates further pediatric data collection that can only be achieved by international collaboration.
Acknowledgments
The authors thank Frederic Millot for valuable discussions. Stacey Tobin, The Tobin Touch, Inc., was compensated for her assistance in language editing.
Authorship
Contribution: N.H. and M.S. contributed equally to conceiving, writing, and reviewing the manuscript.
Conflict-of-interest disclosure: N.H. received honoraria from Novartis for serving on the Data Monitoring Committee and research support (statistical support) from Bristol-Myers Squibb. M.S. received research support (Institutional) from Novartis and reimbursement for attending symposia organized by Bristol-Myers Squibb, Novartis, and Pfizer.
Correspondence: Nobuko Hijiya, Department of Pediatrics, Columbia University Medical Center, 161 Fort Washington Ave, New York, NY 10032; e-mail: nh2636@cumc.columbia.edu; and Meinolf Suttorp, Medical Faculty, Pediatric Hemato-Oncology, Fetscherstr 74, D-01307 Dresden, Germany; e-mail: meinolf.suttorp@uniklinikum-dresden.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal