Key Points
ENDEAVOR reported clinically meaningful PFS and OS improvements with Kd56 vs Vd in RRMM patients with varying degrees of renal impairment.
Patients with complete renal response had superior PFS and OS outcomes compared with nonresponders across treatment groups.
Abstract
In ENDEAVOR, carfilzomib (56 mg/m2) and dexamethasone (Kd56) demonstrated longer progression-free survival (PFS) over bortezomib and dexamethasone (Vd) in patients with relapsed/refractory multiple myeloma (RRMM). Here we evaluated Kd56 vs Vd by baseline renal function in a post hoc exploratory subgroup analysis. The intent-to-treat population included 929 patients (creatinine clearance [CrCL] ≥15 to <50 mL/min, n = 85 and n = 99; CrCL 50 to <80 mL/min, n = 186 and n = 177; and CrCL ≥80 mL/min, n = 193 and n = 189 for Kd56 and Vd arms, respectively). In these respective subgroups, median PFS was 14.9 vs 6.5 months (hazard ratio [HR], 0.49; 95% confidence interval [CI], 0.320-0.757), 18.6 vs 9.4 months (HR, 0.48; 95% CI, 0.351-0.652), and not reached (NR) vs 12.2 months (HR, 0.60; 95% CI, 0.434-0.827) for those receiving Kd56 vs Vd, respectively; median overall survival (OS) was 42.1 vs 23.7 months (HR, 0.66; 95% CI, 0.443-0.989), 42.5 vs 32.8 months (HR, 0.83; 95% CI, 0.626-1.104), and NR vs 42.3 months (HR, 0.75; 95% CI, 0.554-1.009). Complete renal response (ie, CrCL improvement to ≥60 mL/min in any 2 consecutive visits if baseline CrCL <50 mL/min) rates were 15.3% (95% CI, 8.4-24.7) and 14.1% (95% CI, 8.0-22.6) for those receiving Kd56 vs Vd, respectively. In a combined Kd56 and Vd analysis, complete renal responders had longer median PFS (14.1 vs 9.4 months; HR, 0.805; 95% CI, 0.438-1.481) and OS (35.3 vs 29.7 months; HR, 0.91; 95% CI, 0.524-1.577) vs nonresponders. Grade ≥3 adverse event rates in the respective subgroups were 87.1% vs 79.4%, 84.4% vs 71.8%, and 77.1% vs 65.9% for those receiving Kd56 vs Vd, respectively. Thus, Kd56 demonstrated PFS and OS improvements over Vd in RRMM patients regardless of their baseline renal function. The ENDEAVOR trial was registered at www.clinicaltrials.gov as #NCT01568866.
Introduction
Renal impairment is a common complication of multiple myeloma (MM) and is associated with poor prognosis and shorter survival in patients with MM.1,2 Although the underlying causes are not fully understood, renal failure in MM may result from a variety of factors, including dehydration, hypercalcemia, hyperuricemia, and acute tubular necrosis.2,3 Persistent renal failure is most commonly due to the precipitation of monoclonal light chains in renal tubules, which can lead to cast nephropathy.3,4 Renal impairment can complicate drug dosing and limit treatment options, leading to higher incidence or worsening of adverse events (AEs).5,6 Furthermore, administration of antimyeloma therapy and key supportive medications (eg, bisphosphonates, analgesics, antibiotics) is affected by renal insufficiency, because these treatments can be nephrotoxic.7 Three large studies evaluating a combined total of 1975 patients with newly diagnosed MM found that 17% to 21% of patients had renal failure, defined as serum creatinine ≥2 mg/dL or creatinine clearance (CrCL) <40 mL/min, at the time of diagnosis.1,2,8 In addition, median survival of patients with renal failure was less than half that of patients without renal failure (19.5 vs 40.4 months, respectively; P < .001).2 Other studies found that in patients with newly diagnosed MM and baseline renal failure, improved renal function was associated with improved survival.9-11
Novel agents have led to improved outcomes in patients with relapsed/refractory MM (RRMM), including in patients with renal impairment. Immunomodulatory agents (eg, lenalidomide, pomalidomide) and monoclonal antibodies (eg, daratumumab, elotuzumab) have demonstrated efficacy in MM patients in phase 1 to 2 trials who had varying degrees of renal impairment.12-15 Lenalidomide has been shown to have high activity in patients with MM,16,17 but 1 pharmacokinetic (PK) study found that total and renal lenalidomide clearances decreased drastically in individuals with moderate or severe renal disease.18 Therefore, the PK profile of lenalidomide necessitates adjusting the dose to the degree of renal function.6,19 The proteasome inhibitor bortezomib has been shown to be effective in MM patients with renal impairment.9,20,21 Bortezomib is currently a standard treatment for MM patients with renal impairment of any grade.22 However, bortezomib is associated with high rates of peripheral neuropathy, limiting its use and duration of treatment in MM.23,24 Given these limitations, there is a need for new therapies that are effective and well tolerated for MM patients with renal impairment.
Carfilzomib is a second-generation proteasome inhibitor that has been approved in the United States, Europe, and other countries for the treatment of RRMM in combination with lenalidomide plus dexamethasone or with dexamethasone alone.25 The PKs of carfilzomib have been evaluated in MM patients with normal renal function and in those with renal impairment.26,27 In a phase 2 study of patients with MM and varying degrees of renal impairment, there were no differences in carfilzomib (15 or 20 mg/m2) clearance or exposure between patients with normal renal function and those with varying degrees of renal impairment, including those with end-stage renal disease (ESRD).26 When PKs were evaluated at higher doses of carfilzomib (27 and 56 mg/m2) in an open-label, single-arm, phase 1 study, there were no meaningful PK differences between patients with normal renal function and those with ESRD.27 In the phase 3 ENDEAVOR trial, carfilzomib (56 mg/m2) and dexamethasone (Kd56) demonstrated a clinically and statistically significant improvement compared with bortezomib and dexamethasone (Vd) in progression-free survival (PFS; primary end point; median, 18.7 vs 9.4 months; hazard ratio [HR], 0.53; 95% confidence interval [CI], 0.44-0.65; P < .0001)28 and overall survival (OS; secondary end point; median, 47.6 vs 40.0 months; HR, 0.791; 95% CI, 0.648-0.964; P = .010).29 In addition, the proportion of patients achieving an objective response in ENDEAVOR was greater in the Kd56 group compared with the Vd group (77% vs 63%; odds ratio, 2.03; 95% CI, 1.52-2.72; P < .0001).28
Although patients with advanced renal impairment have been excluded from a number of immunomodulatory agent-based phase 3 MM trials,30-32 ENDEAVOR enrolled patients with varying degrees of renal impairment, including patients with CrCL <30 mL/min who were not dialysis dependent.28 Here we performed a post hoc exploratory subgroup analysis of ENDEAVOR to evaluate the efficacy and safety of Kd56 vs Vd in RRMM patients with impaired renal function at randomization.
Methods
Study design and participants
The ENDEAVOR trial has been previously described by Dimopoulos et al.28 Briefly, ENDEAVOR was a prospective, open-label, multicenter, randomized, phase 3 trial that enrolled patients from 27 countries in North America, South America, Europe, and the Asia-Pacific region. Eligible patients were ≥18 years of age with RRMM. Patients must have received 1 to 3 previous treatments and had an Eastern Cooperative Oncology Group performance status of 0 to 2, left ventricular ejection fraction of at least 40%, and CrCL ≥15 mL/min. Prior therapy with a proteasome inhibitor (carfilzomib or bortezomib) was allowed if the patient achieved at least a partial response to treatment, did not receive proteasome inhibitor treatment for an interval of ≥6 months before enrollment, and did not discontinue the proteasome inhibitor because of toxicity. Exclusion criteria included grade 3 or 4 peripheral neuropathy (or grade 2 peripheral neuropathy with pain) within 14 days before randomization, New York Heart Association class 3 or 4 heart failure, and myocardial infarction 4 months before randomization. The study protocol was approved by the ethics committees or institutional review boards of all participating institutions, and all patients provided written informed consent. All authors had access to the primary clinical trial data.
Randomization
Patients were randomized 1:1 to receive Kd56 or Vd and were stratified by previous proteasome inhibitor therapy (yes vs no), previous treatment lines (1 vs 2 or 3), International Staging System (ISS) stage (1 vs 2-3), and planned route of bortezomib administration (IV or subcutaneous) if randomized to the Vd group. The Kd56 group received carfilzomib (20 mg/m2 on days 1 and 2 of cycle 1; 56 mg/m2 thereafter; IV infusion over 30 minutes) on days 1, 2, 8, 9, 15, and 16 and dexamethasone (20 mg oral or IV infusion) on days 1, 2, 8, 9, 15, 16, 22, and 23 of a 28-day cycle. IV hydration of 250 to 500 mL before and after carfilzomib administration was given during cycle 1 and at the investigator’s discretion thereafter. The Vd group received bortezomib (1.3 mg/m2; IV bolus over 3 to 5 seconds or subcutaneous injection) on days 1, 4, 8, and 11 and dexamethasone (20 mg oral or IV infusion) on days 1, 2, 4, 5, 8, 9, 11, and 12 of a 21-day cycle. Treatment was given until disease progression, physician decision, unacceptable toxicity, withdrawal of consent, or death.
Assessments of renal function
The present analyses examined efficacy and safety outcomes in patients grouped according to baseline renal function (CrCL ≥15 to <50, 50 to <80, and ≥80 mL/min). On the basis of International Myeloma Working Group criteria,22 a complete renal response was defined as CrCL ≥60 mL/min in any 2 consecutive study visits for patients who had baseline CrCL <50 mL/min. The Cockcroft-Gault (C-G) formula was used to calculate baseline and on-study renal function. This formula was calculated using actual body weight. The C-G formula does not require data on race, allowing all patient data to be used in the analysis regardless of missing data on race.
Statistical analyses
The Kaplan-Meier method was used to analyze time-to-event end points, including PFS, OS, and duration of response. The data cutoff for the evaluation of PFS was 10 November 2014; for OS and safety, the data cutoff was 19 July 2017. Multivariate logistic models were used to assess the association between achievement of a complete renal response and the following patient or disease characteristics: age (<75 vs ≥75 years), sex (female vs male), ISS stage (1 vs 2-3), number of prior lines of treatment (1 vs 2-3), response to treatment status (very good partial response or better at PFS primary analysis: no vs yes), lactate dehydrogenase category (<300 vs ≥300 U/L), baseline corrected calcium (BCCA; <10.5 vs ≥10.5 mg/dL), and presence of light chain only (no vs yes). Safety and tolerability assessments included AEs and extent of exposure to study treatment. AEs were not adjusted for exposure.
Results
The intent-to-treat population included 929 patients who were enrolled between 20 June 2012 and 30 June 2014.28,29 Of these, 184 patients had CrCL ≥15 to <50 mL/min (Kd56, n = 85; Vd, n = 99), 363 patients had CrCL 50 to <80 mL/min (Kd56, n = 186; Vd, n = 177), and 382 patients had CrCL ≥80 mL/min (Kd56, n = 193; Vd, n = 189; supplemental Figure 1, available on the Blood Web site). Demographic and baseline disease characteristics were generally balanced between treatment arms within each CrCL subgroup, with the exception of the CrCL ≥15 to <50 mL/min subgroup, in which more patients (≥10% difference) in the Vd arm compared with the Kd56 arm had high-risk cytogenetics or were 65 to 74 years of age; the percentage of patients with missing/unknown cytogenetic data was higher in the Kd56 arm than in the Vd arm (22.4% vs 12.1%; Table 1). In the CrCL ≥15 to <50 mL/min subgroup, the proportion of patients age ≥65 years was similar between treatment arms (Kd56, 74.1%; Vd, 77.8%). Overall, a higher percentage of patients with impaired renal function (CrCL ≥15 to <50 mL/min) were older and had more advanced disease (ISS stage 3) compared with the other CrCL subgroups.
Patient demographic and baseline disease characteristics
| . | N (%) . | |||||
|---|---|---|---|---|---|---|
| CrCL, mL/min . | ||||||
| ≥15 to <50 . | 50 to <80 . | ≥80 . | ||||
| Kd56 (n = 85) . | Vd (n = 99) . | Kd56 (n = 186) . | Vd (n = 177) . | Kd56 (n = 193) . | Vd (n = 189) . | |
| Age, y | ||||||
| Median | 72.0 | 72.0 | 68.0 | 68.0 | 60.0 | 61.0 |
| Range | 41-89 | 45-86 | 39-89 | 44-88 | 35-81 | 30-81 |
| <65 | 22 (25.9) | 22 (22.2) | 64 (34.4) | 53 (29.9) | 137 (71.0) | 135 (71.4) |
| 65-74 | 28 (32.9) | 44 (44.4) | 85 (45.7) | 97 (54.8) | 51 (26.4) | 48 (25.4) |
| ≥75 | 35 (41.2) | 33 (33.3) | 37 (19.9) | 27 (15.3) | 5 (2.6) | 6 (3.2) |
| Sex | ||||||
| Female | 45 (52.9) | 62 (62.6) | 96 (51.6) | 105 (59.3) | 83 (43.0) | 69 (36.5) |
| Male | 40 (47.1) | 37 (37.4) | 90 (48.4) | 72 (40.7) | 110 (57.0) | 120 (63.5) |
| Race | ||||||
| White | 54 (63.5) | 75 (75.8) | 143 (76.9) | 133 (75.1) | 156 (80.8) | 153 (81.0) |
| Black | 1 (1.2) | 0 | 3 (1.6) | 5 (2.8) | 3 (1.6) | 4 (2.1) |
| Asian | 18 (21.2) | 16 (16.2) | 20 (10.8) | 19 (10.7) | 18 (9.3) | 22 (11.6) |
| Not reported | 12 (14.1) | 8 (8.1) | 20 (10.8) | 20 (11.3) | 14 (7.3) | 9 (4.8) |
| Other | 0 | 0 | 0 | 0 | 2 (1.0) | 1 (0.5) |
| Cytogenetic risk by FISH at study entry* | ||||||
| High | 11 (12.9) | 26 (26.3) | 45 (24.2) | 41 (23.2) | 41 (21.2) | 46 (24.3) |
| Standard | 55 (64.7) | 61 (61.6) | 111 (59.7) | 117 (66.1) | 118 (61.1) | 113 (59.8) |
| Unknown/missing | 19 (22.4) | 12 (12.1) | 30 (16.1) | 19 (10.7) | 34 (17.6) | 30 (15.9) |
| ISS stage at baseline | ||||||
| 1 | 11 (12.9) | 9 (9.1) | 76 (40.9) | 69 (39.0) | 125 (64.8) | 127 (67.2) |
| 2 | 28 (32.9) | 28 (28.3) | 66 (35.5) | 75 (42.4) | 45 (23.3) | 48 (25.4) |
| 3 | 46 (54.1) | 62 (62.6) | 44 (23.7) | 33 (18.6) | 23 (11.9) | 14 (7.4) |
| N of prior regimens | ||||||
| 1 | 39 (45.9) | 43 (43.4) | 96 (51.6) | 85 (48.0) | 96 (49.7) | 101 (53.4) |
| 2-3 | 46 (54.1) | 56 (56.6) | 90 (48.4) | 92 (52.0) | 97 (50.3) | 88 (46.6) |
| . | N (%) . | |||||
|---|---|---|---|---|---|---|
| CrCL, mL/min . | ||||||
| ≥15 to <50 . | 50 to <80 . | ≥80 . | ||||
| Kd56 (n = 85) . | Vd (n = 99) . | Kd56 (n = 186) . | Vd (n = 177) . | Kd56 (n = 193) . | Vd (n = 189) . | |
| Age, y | ||||||
| Median | 72.0 | 72.0 | 68.0 | 68.0 | 60.0 | 61.0 |
| Range | 41-89 | 45-86 | 39-89 | 44-88 | 35-81 | 30-81 |
| <65 | 22 (25.9) | 22 (22.2) | 64 (34.4) | 53 (29.9) | 137 (71.0) | 135 (71.4) |
| 65-74 | 28 (32.9) | 44 (44.4) | 85 (45.7) | 97 (54.8) | 51 (26.4) | 48 (25.4) |
| ≥75 | 35 (41.2) | 33 (33.3) | 37 (19.9) | 27 (15.3) | 5 (2.6) | 6 (3.2) |
| Sex | ||||||
| Female | 45 (52.9) | 62 (62.6) | 96 (51.6) | 105 (59.3) | 83 (43.0) | 69 (36.5) |
| Male | 40 (47.1) | 37 (37.4) | 90 (48.4) | 72 (40.7) | 110 (57.0) | 120 (63.5) |
| Race | ||||||
| White | 54 (63.5) | 75 (75.8) | 143 (76.9) | 133 (75.1) | 156 (80.8) | 153 (81.0) |
| Black | 1 (1.2) | 0 | 3 (1.6) | 5 (2.8) | 3 (1.6) | 4 (2.1) |
| Asian | 18 (21.2) | 16 (16.2) | 20 (10.8) | 19 (10.7) | 18 (9.3) | 22 (11.6) |
| Not reported | 12 (14.1) | 8 (8.1) | 20 (10.8) | 20 (11.3) | 14 (7.3) | 9 (4.8) |
| Other | 0 | 0 | 0 | 0 | 2 (1.0) | 1 (0.5) |
| Cytogenetic risk by FISH at study entry* | ||||||
| High | 11 (12.9) | 26 (26.3) | 45 (24.2) | 41 (23.2) | 41 (21.2) | 46 (24.3) |
| Standard | 55 (64.7) | 61 (61.6) | 111 (59.7) | 117 (66.1) | 118 (61.1) | 113 (59.8) |
| Unknown/missing | 19 (22.4) | 12 (12.1) | 30 (16.1) | 19 (10.7) | 34 (17.6) | 30 (15.9) |
| ISS stage at baseline | ||||||
| 1 | 11 (12.9) | 9 (9.1) | 76 (40.9) | 69 (39.0) | 125 (64.8) | 127 (67.2) |
| 2 | 28 (32.9) | 28 (28.3) | 66 (35.5) | 75 (42.4) | 45 (23.3) | 48 (25.4) |
| 3 | 46 (54.1) | 62 (62.6) | 44 (23.7) | 33 (18.6) | 23 (11.9) | 14 (7.4) |
| N of prior regimens | ||||||
| 1 | 39 (45.9) | 43 (43.4) | 96 (51.6) | 85 (48.0) | 96 (49.7) | 101 (53.4) |
| 2-3 | 46 (54.1) | 56 (56.6) | 90 (48.4) | 92 (52.0) | 97 (50.3) | 88 (46.6) |
FISH, fluorescence in situ hybridization.
High-risk patients have genetic subtype t(4; 14), t(14;16), or del(17p); standard-risk patients do not. The unknown risk group includes patients who have FISH assessment, but the results for ≥1 genetic subtype are not available.
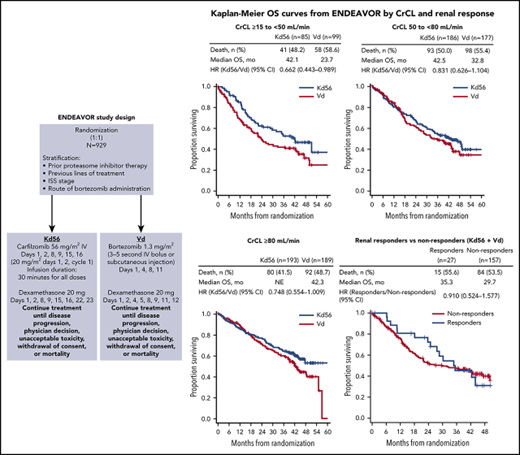
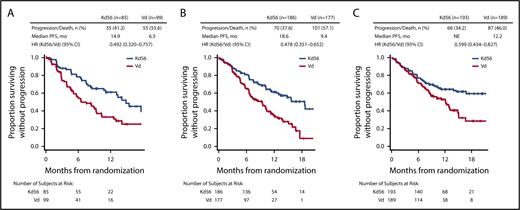
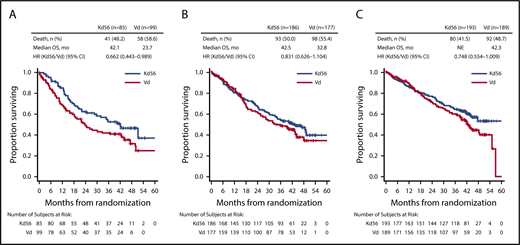
PFS and OS were consistently improved with Kd56 compared with Vd within each renal subgroup (Table 2; Figures 1 and 2). For the CrCL ≥15 to <50 mL/min group, median PFS was 14.9 months for Kd56 vs 6.5 months for Vd (HR, 0.49; 95% CI, 0.320-0.757). For the CrCL 50 to <80 mL/min group, median PFS was 18.6 vs 9.4 months (HR, 0.48; 95% CI, 0.351-0.652), and for the CrCL ≥80 mL/min group, median PFS was not reached vs 12.2 months (HR, 0.60; 95% CI, 0.434-0.827). For the CrCL ≥15 to <50 mL/min group, median OS was 42.1 (Kd56) vs 23.7 months (Vd; HR, 0.66; 95% CI, 0.443-0.989). For the CrCL 50 to <80 mL/min group, median OS was 42.5 vs 32.8 months (HR, 0.83; 95% CI, 0.626-1.104), and for the CrCL ≥80 mL/min group, median OS was not reached vs 42.3 months (HR, 0.75; 95% CI, 0.554-1.009).
Efficacy outcomes by renal impairment subgroup
| . | CrCL, mL/min . | |||||
|---|---|---|---|---|---|---|
| ≥15 to <50 . | 50 to <80 . | ≥80 . | ||||
| Kd56 (n = 85) . | Vd (n = 99) . | Kd56 (n = 186) . | Vd (n = 177) . | Kd56 (n = 193) . | Vd (n = 189) . | |
| Median PFS, mo* | 14.9 | 6.5 | 18.6 | 9.4 | Not reached | 12.2 |
| HR for Kd56 vs Vd (95% CI) | 0.49 (0.320-0.757) | 0.48 (0.351-0.652) | 0.60 (0.434-0.827) | |||
| Median OS, mo | 42.1 | 23.7 | 42.5 | 32.8 | Not reached | 42.3 |
| HR for Kd56 vs Vd (95% CI) | 0.66 (0.443-0.989) | 0.83 (0.626-1.104) | 0.75 (0.554-1.009) | |||
| ORR, %* | 74.1 | 49.5 | 78.5 | 69.5 | 76.7 | 63.0 |
| Odds ratio (95% CI) | 2.922 (1.564-5.460) | 1.602 (0.997-2.574) | 1.935 (1.239-3.021) | |||
| ≥CR, %* | 8.2 | 4.0 | 9.1 | 7.3 | 17.6 | 6.3 |
| ≥VGPR, %* | 51.8 | 28.3 | 55.4 | 26.0 | 54.4 | 31.2 |
| Median DOR, mo* | 16.6 | 9.3 | 17.6 | 9.3 | Not reached | 14.0 |
| Complete renal response, % | 15.3 | 14.1 | — | — | — | — |
| PFS by prior line of therapy | ||||||
| 1 prior line, n | 39 | 43 | 96 | 85 | 96 | 101 |
| Median PFS, mo | 17.7 | 9.3 | 18.7 | 9.5 | Not reached | 12.1 |
| HR for Kd56 vs Vd (95% CI) | 0.44 (0.212-0.918) | 0.46 (0.288-0.731) | 0.42 (0.251-0.685) | |||
| 2-3 prior lines | 46 | 56 | 90 | 92 | 97 | 88 |
| Median PFS, mo | 11.1 | 6.1 | 15.7 | 7.5 | Not reached | 12.4 |
| HR for Kd56 vs Vd (95% CI) | 0.47 (0.274-0.817) | 0.51 (0.339-0.778) | 0.77 (0.505-1.186) | |||
| OS by prior line of therapy | ||||||
| 1 prior line, n | 39 | 43 | 96 | 85 | 96 | 101 |
| Median OS, mo | 51.3 | 26.5 | 48.8 | 43.2 | Not reached | 44.5 |
| HR for Kd56 vs Vd (95% CI) | 0.53 (0.272-1.029) | 0.88 (0.577-1.353) | 0.77 (0.491-1.203) | |||
| 2-3 prior lines | 46 | 56 | 90 | 92 | 97 | 88 |
| Median OS, mo | 28.6 | 21.7 | 36.1 | 28.2 | Not reached | 32.9 |
| HR for Kd56 vs Vd (95% CI) | 0.79 (0.475-1.298) | 0.81 (0.552-1.186) | 0.70 (0.469-1.059) | |||
| . | CrCL, mL/min . | |||||
|---|---|---|---|---|---|---|
| ≥15 to <50 . | 50 to <80 . | ≥80 . | ||||
| Kd56 (n = 85) . | Vd (n = 99) . | Kd56 (n = 186) . | Vd (n = 177) . | Kd56 (n = 193) . | Vd (n = 189) . | |
| Median PFS, mo* | 14.9 | 6.5 | 18.6 | 9.4 | Not reached | 12.2 |
| HR for Kd56 vs Vd (95% CI) | 0.49 (0.320-0.757) | 0.48 (0.351-0.652) | 0.60 (0.434-0.827) | |||
| Median OS, mo | 42.1 | 23.7 | 42.5 | 32.8 | Not reached | 42.3 |
| HR for Kd56 vs Vd (95% CI) | 0.66 (0.443-0.989) | 0.83 (0.626-1.104) | 0.75 (0.554-1.009) | |||
| ORR, %* | 74.1 | 49.5 | 78.5 | 69.5 | 76.7 | 63.0 |
| Odds ratio (95% CI) | 2.922 (1.564-5.460) | 1.602 (0.997-2.574) | 1.935 (1.239-3.021) | |||
| ≥CR, %* | 8.2 | 4.0 | 9.1 | 7.3 | 17.6 | 6.3 |
| ≥VGPR, %* | 51.8 | 28.3 | 55.4 | 26.0 | 54.4 | 31.2 |
| Median DOR, mo* | 16.6 | 9.3 | 17.6 | 9.3 | Not reached | 14.0 |
| Complete renal response, % | 15.3 | 14.1 | — | — | — | — |
| PFS by prior line of therapy | ||||||
| 1 prior line, n | 39 | 43 | 96 | 85 | 96 | 101 |
| Median PFS, mo | 17.7 | 9.3 | 18.7 | 9.5 | Not reached | 12.1 |
| HR for Kd56 vs Vd (95% CI) | 0.44 (0.212-0.918) | 0.46 (0.288-0.731) | 0.42 (0.251-0.685) | |||
| 2-3 prior lines | 46 | 56 | 90 | 92 | 97 | 88 |
| Median PFS, mo | 11.1 | 6.1 | 15.7 | 7.5 | Not reached | 12.4 |
| HR for Kd56 vs Vd (95% CI) | 0.47 (0.274-0.817) | 0.51 (0.339-0.778) | 0.77 (0.505-1.186) | |||
| OS by prior line of therapy | ||||||
| 1 prior line, n | 39 | 43 | 96 | 85 | 96 | 101 |
| Median OS, mo | 51.3 | 26.5 | 48.8 | 43.2 | Not reached | 44.5 |
| HR for Kd56 vs Vd (95% CI) | 0.53 (0.272-1.029) | 0.88 (0.577-1.353) | 0.77 (0.491-1.203) | |||
| 2-3 prior lines | 46 | 56 | 90 | 92 | 97 | 88 |
| Median OS, mo | 28.6 | 21.7 | 36.1 | 28.2 | Not reached | 32.9 |
| HR for Kd56 vs Vd (95% CI) | 0.79 (0.475-1.298) | 0.81 (0.552-1.186) | 0.70 (0.469-1.059) | |||
CR, complete response; DOR, duration of response; VGPR, very good partial response.
Data are from the primary analysis data cut.
Kaplan-Meier PFS curves for Kd56 and Vd by renal impairment subgroup. CrCL ≥15 to <50 (A), 50 to < 80 (B), and ≥ 80 mL/min (C). Kaplan-Meier curves were displayed until there were ≤10 patients (Kd56 and Vd combined) at risk.
Kaplan-Meier PFS curves for Kd56 and Vd by renal impairment subgroup. CrCL ≥15 to <50 (A), 50 to < 80 (B), and ≥ 80 mL/min (C). Kaplan-Meier curves were displayed until there were ≤10 patients (Kd56 and Vd combined) at risk.
Kaplan-Meier OS curves for Kd56 and Vd by renal impairment subgroup. CrCL ≥15 to <50 (A), 50 to <80 (B), and ≥80 mL/min (C).
Kaplan-Meier OS curves for Kd56 and Vd by renal impairment subgroup. CrCL ≥15 to <50 (A), 50 to <80 (B), and ≥80 mL/min (C).
Within each renal subgroup, there was also a consistent PFS and OS benefit with Kd56 compared with Vd regardless of the number of prior lines of therapy (1 or 2-3 lines; Table 2). Compared with the Vd group, the Kd56 group had higher overall response rates (ORRs) and longer median duration of response in each renal subgroup (Table 2). In addition, a greater percentage of patients in the Kd56 arm compared with the Vd arm had a complete response or better and very good partial response or better, regardless of baseline renal impairment. In the Kd56 group, 13.5% of patients with baseline CrCL ≥50 mL/min had a complete response or better, compared with 8.2% of patients with baseline CrCL <50 mL/min (odds ratio, 1.733; 95% CI, 0.757-3.964). In the Vd group, these values were 6.8% and 4.0%, respectively (odds ratio, 1.741; 95% CI, 0.591-5.126).
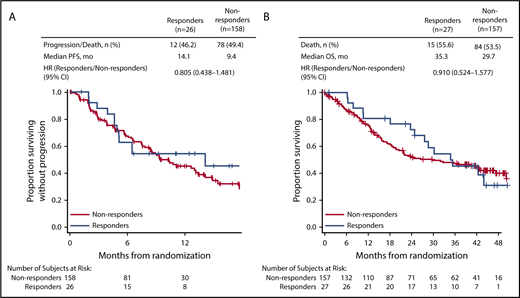
In patients with CrCL ≥15 to <50 mL/min, complete renal response rates were comparable between the 2 treatment arms (Kd56, 15.3%; Vd, 14.1%). Among patients achieving a complete renal response in the CrCL ≥15 to <50 mL/min group, median time to complete renal response was rapid in both treatment arms (Kd56, 1.9 months; range, 0.4-7.2; Vd, 1.5 months; range, 0.1-4.7). In a combined analysis of patients treated with Kd56 and Vd, median PFS was longer in the complete renal responder group compared with the nonresponder group among patients with CrCL ≥15 to <50 mL/min (14.1 vs 9.4 months; HR, 0.805; 95% CI, 0.438-1.481). Median OS was also longer among complete renal responders compared with renal nonresponders (35.3 vs 29.7 months; HR, 0.91; 95% CI, 0.524-1.577; Figure 3). Results from multivariate logistic models assessing the association of patient and disease characteristics with achievement of a complete renal response in the CrCL ≥15 to <50 mL/min group are shown in Table 3. These analyses were conducted in the combined population of patients treated with Kd56 or Vd. There was a trend for several factors to predict for achievement of renal response, including age <75 years, male sex, ISS stage 1, lactate dehydrogenase <300 U/L, BCCA ≥10.5 mg/dL, no presence of light chain only, 2 to 3 prior lines of treatment, and response to treatment (yes).
Association between renal response and individual variables in multivariate analysis
| Variable . | Exact odds ratio (95% CI) . |
|---|---|
| Age: <75 vs ≥75 y | 3.947 (1.259-15.076) |
| Sex: female vs male | 0.570 (0.201-1.567) |
| ISS stage: 1 vs 2-3 | 1.881 (0.502-6.506) |
| Prior lines of treatment: 1 vs 2-3 | 0.568 (0.200-1.527) |
| Response to treatment*: no vs yes | 0.485 (0.176-1.307) |
| LDH: <300 vs ≥300 U/L | 8.511 (1.005-428.903) |
| BCCA <10.5 vs ≥10.5 mg/dL | 0.263 (0.071-0.975) |
| Light chain only: no vs yes | 4.791 (1.287-23.839) |
| Variable . | Exact odds ratio (95% CI) . |
|---|---|
| Age: <75 vs ≥75 y | 3.947 (1.259-15.076) |
| Sex: female vs male | 0.570 (0.201-1.567) |
| ISS stage: 1 vs 2-3 | 1.881 (0.502-6.506) |
| Prior lines of treatment: 1 vs 2-3 | 0.568 (0.200-1.527) |
| Response to treatment*: no vs yes | 0.485 (0.176-1.307) |
| LDH: <300 vs ≥300 U/L | 8.511 (1.005-428.903) |
| BCCA <10.5 vs ≥10.5 mg/dL | 0.263 (0.071-0.975) |
| Light chain only: no vs yes | 4.791 (1.287-23.839) |
Fitted with all variables in 1 logistic regression model.
BCCA, baseline corrected calcium; LDH, lactate dehydrogenase.
Very good partial response or better at PFS primary analysis: no vs yes.
Median duration of treatment was longer and median number of cycles received was higher with Kd56 vs Vd across all CrCL subgroups (Table 4). Grade ≥3 AE rates for Kd56 vs Vd were 87.1% vs 79.4% (CrCL ≥15 to <50 mL/min), 84.4% vs 71.8% (CrCL 50 to <80 mL/min), and 77.1% vs 65.9% (CrCL ≥80 mL/min; supplemental Table 1). Across renal impairment groups, the most common grade ≥3 AEs were anemia, pneumonia, hypertension, and thrombocytopenia. Rates of grade ≥3 acute kidney injury, hypertension, cardiac failure, and dyspnea were generally higher with Kd56 compared with Vd across renal subgroups, whereas grade ≥3 peripheral neuropathy rates were lower in the Kd56 arm compared with the Vd arm across renal subgroups (Table 4). Among patients with CrCL ≥15 to <50 mL/min, the percentage of those with AEs leading to drug discontinuation was higher in the Kd56 arm (31.8%) compared with the Vd arm (23.7%).
Safety outcomes by renal impairment subgroup
| . | N (%) . | |||||
|---|---|---|---|---|---|---|
| CrCL, mL/min . | ||||||
| ≥15 to <50 . | 50 to <80 . | ≥80 . | ||||
| Kd56 (n = 85) . | Vd (n = 97) . | Kd56 (n = 186) . | Vd (n = 174) . | Kd56 (n = 192) . | Vd (n = 185) . | |
| Median duration of treatment, wk | 36 | 21 | 50 | 27 | 52 | 31 |
| IQR | 18-68 | 12-34 | 25-87 | 15-45 | 26-103 | 17-60 |
| Median n of cycles received | 9 | 7 | 12 | 8 | 13 | 10 |
| IQR | 4-17 | 4-11 | 6-21 | 5-13 | 7-25 | 6-19 |
| Patients with AEs leading to carfilzomib or bortezomib discontinuation | 27 (31.8) | 23 (23.7) | 47 (25.3) | 41 (23.6) | 46 (24.0) | 38 (20.5) |
| Treatment-emergent grade ≥3 AEs of interest* | ||||||
| Hypertension | 12 (14.1) | 3 (3.1) | 27 (14.5) | 7 (4.0) | 30 (15.6) | 5 (2.7) |
| Peripheral neuropathy | 0 | 4 (4.1) | 5 (2.7) | 16 (9.2) | 1 (0.5) | 8 (4.3) |
| Dyspnea | 8 (9.4) | 2 (2.1) | 12 (6.5) | 2 (1.1) | 9 (4.7) | 6 (3.2) |
| Cardiac failure | 4 (4.7) | 0 | 7 (3.8) | 1 (0.6) | 3 (1.6) | 2 (1.1) |
| Acute kidney injury | 3 (3.5) | 2 (2.1) | 4 (2.2) | 2 (1.1) | 5 (2.6) | 3 (1.6) |
| Grade ≥2 peripheral neuropathy* | 0 | 14 (14.4) | 13 (7.0) | 36 (20.7) | 5 (2.6) | 34 (18.4) |
| . | N (%) . | |||||
|---|---|---|---|---|---|---|
| CrCL, mL/min . | ||||||
| ≥15 to <50 . | 50 to <80 . | ≥80 . | ||||
| Kd56 (n = 85) . | Vd (n = 97) . | Kd56 (n = 186) . | Vd (n = 174) . | Kd56 (n = 192) . | Vd (n = 185) . | |
| Median duration of treatment, wk | 36 | 21 | 50 | 27 | 52 | 31 |
| IQR | 18-68 | 12-34 | 25-87 | 15-45 | 26-103 | 17-60 |
| Median n of cycles received | 9 | 7 | 12 | 8 | 13 | 10 |
| IQR | 4-17 | 4-11 | 6-21 | 5-13 | 7-25 | 6-19 |
| Patients with AEs leading to carfilzomib or bortezomib discontinuation | 27 (31.8) | 23 (23.7) | 47 (25.3) | 41 (23.6) | 46 (24.0) | 38 (20.5) |
| Treatment-emergent grade ≥3 AEs of interest* | ||||||
| Hypertension | 12 (14.1) | 3 (3.1) | 27 (14.5) | 7 (4.0) | 30 (15.6) | 5 (2.7) |
| Peripheral neuropathy | 0 | 4 (4.1) | 5 (2.7) | 16 (9.2) | 1 (0.5) | 8 (4.3) |
| Dyspnea | 8 (9.4) | 2 (2.1) | 12 (6.5) | 2 (1.1) | 9 (4.7) | 6 (3.2) |
| Cardiac failure | 4 (4.7) | 0 | 7 (3.8) | 1 (0.6) | 3 (1.6) | 2 (1.1) |
| Acute kidney injury | 3 (3.5) | 2 (2.1) | 4 (2.2) | 2 (1.1) | 5 (2.6) | 3 (1.6) |
| Grade ≥2 peripheral neuropathy* | 0 | 14 (14.4) | 13 (7.0) | 36 (20.7) | 5 (2.6) | 34 (18.4) |
AEs were not adjusted for exposure.
IQR, interquartile range.
Preferred term.
Discussion
ENDEAVOR is the largest randomized trial in RRMM to include patients with impaired baseline renal function. In this post hoc exploratory analysis from ENDEAVOR of RRMM patients with varying degrees of renal impairment, PFS and OS improvements were observed with Kd56 compared with Vd (Table 2). These results are consistent with those observed in the overall ENDEAVOR population.28,29 Patients with impaired baseline renal function (CrCL ≥15 to <50 mL/min) treated with Kd56 had an 8.4-month improvement in median PFS and an 18.4-month improvement in median OS compared with Vd. Median OS in the Kd56 group was 42.1 months, which is higher than expected for this patient population.33 These results suggest that Kd56 may overcome the poor prognosis of baseline advanced renal impairment. Among patients with CrCL ≥15 to <50 mL/min, the proportion of patients with high-risk cytogenetics was higher in the Vd group (26.3%) than in the Kd56 group (12.9%); however, it should also be noted that the proportion of patients with unknown/missing cytogenetics was higher in the Kd56 group (22.4%) than in the Vd group (12.1%). Consistent with the CrCL ≥15 to <50 mL/min subgroup, improvements in PFS with Kd56 vs Vd were observed in both the CrCL 50 to <80 mL/min and ≥80 mL/min subgroups. A consistent trend for numerical improvements in OS with Kd56 vs Vd was also observed across all 3 subgroups. The 95% CIs for the OS HRs did not cross unity for the CrCL ≥15 to <50 mL/min subgroup but did cross unity for the other 2 subgroups.
In our analysis, complete renal response rates were 15.3% (95% CI, 8.4-24.7) in the Kd56 group and 14.1% (95% CI, 8.0-22.6) in the Vd group, and median time to complete renal response was rapid (Kd56, 1.9 months; range, 0.4-7.2; Vd, 1.5 months; range, 0.1-4.7) in both treatment groups, suggesting a potential for recovery of renal function in patients treated with these regimens. Patients with complete renal response had superior survival outcomes compared with renal nonresponders across treatment groups (median PFS, 14.1 vs 9.4 months; HR, 0.805; 95% CI, 0.438-1.481; median OS, 35.3 vs 29.7 months; HR, 0.91; 95% CI, 0.524-1.577), highlighting the association between improved renal function and greater survival rates. Median PFS was longer by 4.7 months and median OS by 5.6 months for responders compared with nonresponders. A multivariate logistic regression model demonstrated that there was a trend for age <75 years, ISS stage 1, lactate dehydrogenase <300 U/L, male sex, 2 to 3 prior lines of treatment, response to treatment (yes), lack of presence of light chain only, and BCCA ≥10.5 mg/dL to predict for achievement of a renal response. Because of the small sample size of responders, these results should be interpreted with caution. Consistent with our study, younger age has been previously associated with higher renal impairment reversal rates in a study of MM patients with moderately impaired renal function (n = 677).34 In a study of patients (n = 46) with MM and renal impairment who received bortezomib-based therapies, patients who were previously untreated or who had light chain only myeloma had a higher probability of achieving a renal response.9
The renal responder analysis results from ENDEAVOR are in line with findings from previous studies showing that achievement of renal responses is associated with improved clinical efficacy outcomes in patients with MM. In a single-center analysis of newly diagnosed MM patients with renal impairment at diagnosis, median OS was greater in patients who achieved renal response compared with those who did not (56 vs 33 months; P = .006).8 An analysis of MM patients by Dimopoulos et al9 found that patients with a renal response had a greater 1-year survival rate compared with renal nonresponders (82% vs 54%; P = .025). In a study of patients with newly diagnosed MM, clinical response rates were higher in patients with reversible vs irreversible renal impairment.34 Collectively, and similar to our study, these studies reported an association between renal response and improved clinical outcomes.8,9,34,35
ORRs were higher in the Kd56 group compared with the Vd group for all renal impairment subgroups examined. These ORR results are consistent with the data from the overall ENDEAVOR population.28 Furthermore, patients in the Kd56 arm had deeper responses compared with the Vd arm, regardless of baseline renal impairment (Table 2). These results support findings from other studies showing activity of carfilzomib in patients with renal impairment.26,27
When patients were grouped by number of prior lines of therapy, median PFS and OS were longer with Kd56 vs Vd in almost all renal impairment subgroups examined, including the CrCL ≥15 to <50 mL/min subgroup. Importantly, Kd56 was more effective than Vd at first relapse, as well as after 2 to 3 lines of prior therapy. It is important to choose an effective therapy early in the course of MM to optimize treatment outcomes, because shorter response duration and increased treatment resistance are associated with successive lines of therapy.36,37
Previous studies have shown varying degrees of efficacy of anti-MM drugs, including proteasome inhibitors, immunomodulatory agents, and monoclonal antibodies, in patients with newly diagnosed MM, relapsed MM, or RRMM and renal impairment.12-15,20 The present analysis is the first head-to-head comparison of Kd56 vs Vd with regard to renal response and survival outcomes in MM patients with renal impairment. Current International Myeloma Working Group guidelines recommend bortezomib-based regimens for the management of MM-related renal impairment and note that carfilzomib can also be administered because it needs no dose modification and produces similar results in patients with and without renal impairment.22 ENDEAVOR, the largest phase 3 head-to-head RRMM trial to include patients with renal impairment, also demonstrates that carfilzomib is effective in this setting. The results we report here suggest that Kd56 is more effective than Vd in patients with varying degrees of renal impairment.
In general, a greater percentage of patients in the CrCL ≥15 to <50 mL group reported grade ≥3 AEs compared with the other groups. In a study of patients with relapsed MM, a greater percentage of patients with ESRD had treatment-related grade ≥3 AEs compared with patients with normal renal function (55% vs 47%).27 Rates of grade ≥3 acute kidney injury, hypertension, cardiac failure, and dyspnea were higher with Kd56 vs Vd across renal subgroups, which was consistent with results from the overall population.28,29 Despite a higher frequency of grade ≥3 acute kidney injury, outcomes were improved with Kd56 vs Vd. The longer duration of treatment observed with Kd56 vs Vd might have contributed to the higher frequency of grade ≥3 AEs. These renal and cardiac AEs have been previously reported in phase 2 and 3 carfilzomib trials30,38,39 and are generally manageable with appropriate pretreatment assessment and monitoring during treatment.40-42 The safety profile was consistent with the findings from the previous interim analysis.28
There were several limitations associated with this analysis. This study was a post hoc analysis; therefore, it was limited to the study population in the primary ENDEAVOR trial. Because of the patient enrollment criteria of the ENDEAVOR study, this analysis did not include RRMM patients with ESRD on dialysis and therefore could not assess the potential benefit of carfilzomib in patients with ESRD. Additional analyses are needed to compare the efficacy and safety of carfilzomib vs bortezomib in patients with CrCL <15 mL/min or on dialysis. The analysis reported here was conducted using the C-G equation, although the Chronic Kidney Disease Epidemiology Collaboration formula is generally preferred when evaluating renal function in MM patients.22 However, the Chronic Kidney Disease Epidemiology Collaboration equation requires data on race and therefore would not have supported the analysis of the entire ENDEAVOR patient population because of missing data on race. The C-G equation, which does not require data on race, allowed all patient data to be used.
Renal impairment is a common complication of MM and is associated with poor outcomes. The introduction of new classes of drugs, such as proteasome inhibitors, has improved survival outcomes in MM patients. It is important to evaluate the efficacy and safety of these agents in MM patients with renal impairment, particularly in those with advanced renal impairment, because they are often excluded from clinical trials, and treatment options may be limited. In this study, carfilzomib demonstrated improved PFS and OS compared with bortezomib in patients with varying CrCL levels, including those with severe renal impairment (CrCL ≥15 to <50 mL/min; supplemental Figure 2). Patients achieving complete renal response had superior PFS and OS outcomes compared with nonresponders across treatment groups. These results confirm that improved renal response is associated with better survival outcomes in patients with baseline renal impairment. Overall, these data suggest that Kd56 has a favorable benefit-risk profile and should be considered a standard of care in patients with RRMM, regardless of baseline renal function.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The ENDEAVOR study was supported by Amgen, Inc. Medical writing and editorial assistance was provided by Sachi Yim and Andrew Gomes of BlueMomentum, an Ashfield Company, part of UDG Healthcare PLC, and funded by Amgen, Inc.
Authorship
Contribution: Z.Y., A.S.K., K.S.I., and K.M. analyzed the data; M.D., D.S., D.J.W., R.B., H.L., and R.N. helped interpret the data; and all authors contributed to the study design, collected data, participated in the analysis and interpretation of data, participated in drafting and revising the manuscript, and approved the final version before submission.
Conflict-of-interest disclosure: M.D. reports consulting or advisory role fees from Celgene, Janssen, Amgen, Novartis, and Takeda, research funding fees from Genesis Pharma, and honoraria fees from Celgene, Janssen, Amgen, Novartis, and Takeda; D.S. reports speakers’ bureau and honoraria fees from Amgen, Celgene, Takeda, Novartis, Bristol-Myers Squibb, and Janssen; D.J.W. reports grants and personal fees from Celgene, Amgen, Janssen, and Takeda, outside the submitted work; R.B. served on advisory boards for Pfizer Inc. and Sandoz; Z.Y., A.S.K., K.S.I., and K.M. are employees and stock/equity owners of Amgen; H.L. served as a consultant/advisory for Takeda, Amgen, and Janssen, served on the speakers’ bureau for Celgene, Takeda, Amgen, Bristol-Myers Squibb, and Janssen, and received research funding from Takeda and Amgen; and R.N. reports consulting from Celgene, Amgen, Takeda, Janssen, and Bristol-Myers Squibb.
Correspondence: Meletios Dimopoulos, National and Kapodistrian University of Athens 157 72, Athens, Greece; e-mail: mdimop@med.uoa.gr.