TO THE EDITOR:
Successful engraftment of hematopoietic stem cells (HSCs) involves overcoming nonimmunologic barriers that hinder access to the HSC niches in the bone marrow (BM).1,2 Cytotoxic chemotherapy and/or radiation are currently used to reduce these barriers in clinical hematopoietic cell transplantation (HCT).1,2 Targeted and safer methods to eliminate endogenous HSCs to achieve niche clearance would substantially reduce the morbidity and mortality of an HCT.3 We previously showed that an anti-mouse monoclonal antibody targeting CD117, a cytokine receptor tyrosine kinase expressed on HSCs, depletes endogenous HSCs and allows donor HSC engraftment in immunodeficient mice.4-6 Signals transmitted through CD117 after interaction with its ligand, stem cell factor, are critical for HSC survival, proliferation, and differentiation.4,7
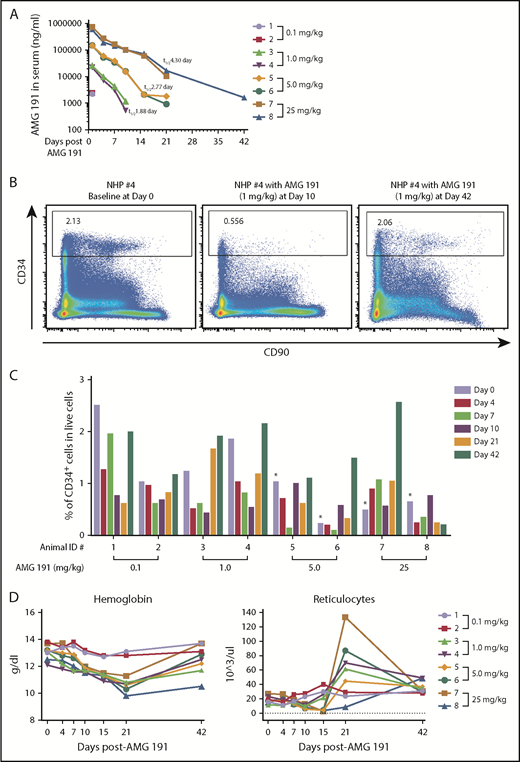
We identified a humanized anti-human CD117 immunoglobulin G1 monoclonal antibody, AMG 191, as a candidate therapeutic antibody with the potential to achieve BM HSC niche clearance for use as conditioning for clinical HCT. Prior studies showed that AMG1 191 and its parent clone, SR-1, from which AMG 191 was derived block stem cell factor binding to CD117 thereby interrupting the signals transmitted through this receptor.8,9 We assessed AMG 191 for its ability to safely target and deplete hematopoietic stem and progenitor cells (HSPCs) in the BM of nonhuman primates (NHPs) and human HSPCs xenografted into immune-deficient mice. We first confirmed that AMG 191 binds to human and NHP HSCs by immunophenotypic analysis and that AMG 191 impairs hematopoiesis of human and NHP HSCs in in vitro studies (supplemental Figure 1, available on the Blood Web site). The HSPC-depletive in vivo activity of AMG 191 was then tested in NHPs (Figure 1). Immunocompetent juvenile cynomolgus macaques received a single IV infusion of AMG 191 (0.1, 1, 5, or 25 mg/kg). All animals survived to the end of study and there were no adverse clinical observations attributable to treatment with AMG 191 (data not shown). The pharmacokinetic (PK) clearance of AMG 191 in the serum was dose dependent in a nonlinear fashion (Figure 1A). Phenotypic CD34+ HSPCs were depleted in the BM of all animals that received AMG 191 except NHP #7 (Figure 1B-C). Of note, at the time of infusion, NHP #7 had emesis, markedly elevated neutrophils, and white blood cells, suggesting that it was ill (supplemental Figure 2), which may have influenced this animal’s physiologic state. HSPC depletion lasted up to 21 days in most animals and >42 days in NHP #8, which received the highest dose. All animals except NHP #8 recovered normal range HSPC frequency by day 42, suggesting that high doses of AMG 191 can cause delayed HSPC recovery.
Effects of in vivo administration of AMG 191 on CD34+ HSPCs and peripheral blood of cynomolgus macaques. (A) AMG 191 is cleared in the serum of NHPs in a dose-dependent manner. The highest levels of AMG 191 in serum of all animals were observed at the first time point collection (5 minutes after dose administration on day 1). Antibody levels measured on day 4 in the 0.1 mg/kg group fell below the assay detection limit of 50 ng/mL. The level of AMG 191 in serum was analyzed and terminal elimination half-life (t1/2) was determined. The averages of half-life (t1/2) obtained from 2 animals of each group are presented. (B) Representative fluorescence-activated cell sorter (FACS) plot showing CD34+ cells in BM of NHP #4 on days 0 (before AMG 191 treatment), 10, and 42 postadministration of 1.0 mg/kg AMG 191. CD34+ cells were gated from live cells. (C) Frequency of CD34+ cells among live cells in BM aspirates obtained from each NHP. BM aspirates were collected from individual NHPs (2 animals for each dose group) prior to the administration of AMG 191 (baseline, designated as day 0), and on days 4, 7, 10, 21, and 42 postadministration. AMG 191 was infused into animals on day 1. *Note: There was a technical inconsistency with the staining of the baseline cell samples collected from NHPs #5 to #8 (day 0). Red cell lysis was performed after the cells were stained, leading to a lower baseline of the CD34+ frequency of the stained populations. In all subsequent analyses, red cell lysis was performed before staining with the marker antibodies. Colors correspond to days relative to infusion. Animal identification (#1-#8) and dose level are shown on the x-axis. (D) RBC parameters affected by AMG 191 treatment. Hemoglobin and absolute reticulocyte count from individual NHPs taken before treatment (day 0) and postinfusion of AMG 191. Colored lines correspond to individual NHPs with doses as shown in the legend on the right. NHPs #3 to #8 had statistically significant decreases in hemoglobin from baseline compared with day 21 (P = .001; paired Student t test).
Effects of in vivo administration of AMG 191 on CD34+ HSPCs and peripheral blood of cynomolgus macaques. (A) AMG 191 is cleared in the serum of NHPs in a dose-dependent manner. The highest levels of AMG 191 in serum of all animals were observed at the first time point collection (5 minutes after dose administration on day 1). Antibody levels measured on day 4 in the 0.1 mg/kg group fell below the assay detection limit of 50 ng/mL. The level of AMG 191 in serum was analyzed and terminal elimination half-life (t1/2) was determined. The averages of half-life (t1/2) obtained from 2 animals of each group are presented. (B) Representative fluorescence-activated cell sorter (FACS) plot showing CD34+ cells in BM of NHP #4 on days 0 (before AMG 191 treatment), 10, and 42 postadministration of 1.0 mg/kg AMG 191. CD34+ cells were gated from live cells. (C) Frequency of CD34+ cells among live cells in BM aspirates obtained from each NHP. BM aspirates were collected from individual NHPs (2 animals for each dose group) prior to the administration of AMG 191 (baseline, designated as day 0), and on days 4, 7, 10, 21, and 42 postadministration. AMG 191 was infused into animals on day 1. *Note: There was a technical inconsistency with the staining of the baseline cell samples collected from NHPs #5 to #8 (day 0). Red cell lysis was performed after the cells were stained, leading to a lower baseline of the CD34+ frequency of the stained populations. In all subsequent analyses, red cell lysis was performed before staining with the marker antibodies. Colors correspond to days relative to infusion. Animal identification (#1-#8) and dose level are shown on the x-axis. (D) RBC parameters affected by AMG 191 treatment. Hemoglobin and absolute reticulocyte count from individual NHPs taken before treatment (day 0) and postinfusion of AMG 191. Colored lines correspond to individual NHPs with doses as shown in the legend on the right. NHPs #3 to #8 had statistically significant decreases in hemoglobin from baseline compared with day 21 (P = .001; paired Student t test).
Depletion of HSPCs in NHP BM as assessed by phenotype studies correlated with functional studies that showed reduced progenitor colony formation (supplemental Figure 3). CD117 is highly expressed on HSPCs, as well as early myeloid progenitor cells and downstream erythroid lineage cells. Its expression is also restricted to a small subset of natural killer and early T-cell precursors.10-15 Peripheral blood measurements showed that, as expected based on the expression pattern, the red blood cell (RBC) lineage was most impacted by AMG 191.11-13 In all animals except NHP #1 and #2 that received the lowest dose, significant reductions in hemoglobins were noted, which nadired at day 21 and then trended upward with most animals recovering to baseline by day 42 (Figure 1D). Similarly, reticulocyte counts in peripheral blood, a measure of red cell production,16 were decreased in NHPs #3 to #8. Interestingly, at day 21, the reticulocyte counts rebounded to levels above baseline in most animals receiving the higher AMG 191 doses (NHP #8 excepted) and returned to baseline by day 42. Because hematopoiesis is sensitive to RBC loss,17,18 we interpret this transient reticulocytosis to reflect endogenous erythropoietic response elicited by the AMG 191–induced transient anemia. The effect on white blood cells and neutrophils was more varied among animals (supplemental Figure 2). Taken together, AMG 191 transiently depletes HSPCs in BM of NHPs, suggesting it may effectively clear human HSPCs and be useful as a novel conditioning agent for HCT.
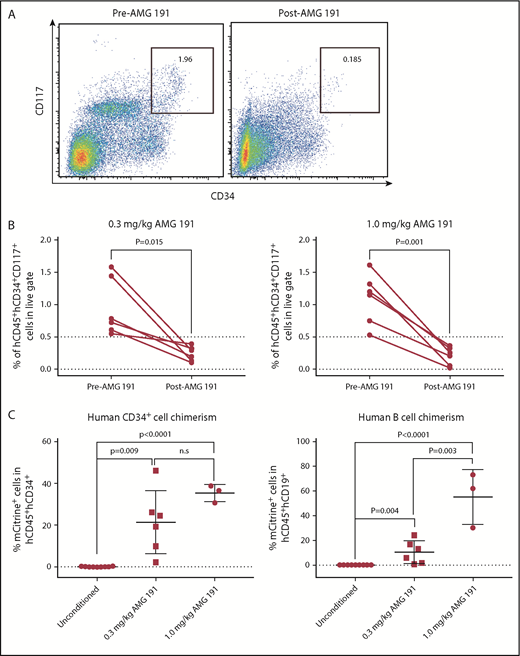
We next tested whether AMG 191 can deplete human HSPCs in immune-deficient mice xenografted with human hematopoietic cells.19,20 Treatment of xenografted mice with AMG 191 resulted in depletion of human HSPCs in all animals as assessed 2 weeks postantibody injection; however, the levels of depletion varied among mice (Figure 2A-B). The ratios of human CD45+/mouse CD45+ cells decreased in most mice, consistent with preferential depletion of engrafted human cells by AMG 191 (supplemental Figure 4A; supplemental Table 1). Among the remaining human cells, the frequency of myeloid cells and T cells were decreased and the frequency of B cells appeared marginally increased (supplemental Figure 4B-D).
AMG 191 depletes human HSPCs engrafted in NSG mice and second human donor engraftment level correlates with AMG 191 dose administered. (A) Representative FACS plots showing hCD34+hCD117+ cell population gated on hCD45+ cells present in BM aspirates obtained from humanized NSG mice before (Pre-AMG 191) and 2 weeks posttreatment of AMG 191 (1.0 mg/kg; Post-AMG 191). (B) Frequency of human hematopoietic progenitor cells (hCD45+hCD34+hCD117+) among all live cells present in BM aspirates obtained from humanized NSG mice treated with 0.3 mg/kg (left panel) and 1.0 mg/kg AMG 191 (right panel). P values were obtained using the paired Student t test. (C) A second human donor cell engraftment is facilitated by AMG 191 conditioning. Pooled cord blood CD34+ cells transduced with mCitrine-expressing lentivirus were transplanted into unconditioned and AMG 191–treated humanized NSG mice (0.3 and 1.0 mg/kg, see supplemental Materials and methods for details). Second donor grafts were infused on days 21 and 25 post–AMG 191 administration, days when the PK level was predicted to fall below 2000 ng/mL (supplemental Figure 5). The choice of this threshold level was based on PK studies in mice that showed that serum levels <2200 ng/mL of the anti-mouse CD117 antibody had no effect on donor mouse HSC engraftment (supplemental Figure 6). Six weeks after transplantation, chimerism was accessed in BM of transplanted humanized NSG mice. Secondary engraftment was measured by the frequency of mCitrine-expressing cells in each cell subset. Cell frequency was analyzed by FACS and FlowJo software. P values were obtained using the unpaired Student t test and Prism software. Data and error bars in panels B and C represent the mean plus or minus standard error of the mean (sem).
AMG 191 depletes human HSPCs engrafted in NSG mice and second human donor engraftment level correlates with AMG 191 dose administered. (A) Representative FACS plots showing hCD34+hCD117+ cell population gated on hCD45+ cells present in BM aspirates obtained from humanized NSG mice before (Pre-AMG 191) and 2 weeks posttreatment of AMG 191 (1.0 mg/kg; Post-AMG 191). (B) Frequency of human hematopoietic progenitor cells (hCD45+hCD34+hCD117+) among all live cells present in BM aspirates obtained from humanized NSG mice treated with 0.3 mg/kg (left panel) and 1.0 mg/kg AMG 191 (right panel). P values were obtained using the paired Student t test. (C) A second human donor cell engraftment is facilitated by AMG 191 conditioning. Pooled cord blood CD34+ cells transduced with mCitrine-expressing lentivirus were transplanted into unconditioned and AMG 191–treated humanized NSG mice (0.3 and 1.0 mg/kg, see supplemental Materials and methods for details). Second donor grafts were infused on days 21 and 25 post–AMG 191 administration, days when the PK level was predicted to fall below 2000 ng/mL (supplemental Figure 5). The choice of this threshold level was based on PK studies in mice that showed that serum levels <2200 ng/mL of the anti-mouse CD117 antibody had no effect on donor mouse HSC engraftment (supplemental Figure 6). Six weeks after transplantation, chimerism was accessed in BM of transplanted humanized NSG mice. Secondary engraftment was measured by the frequency of mCitrine-expressing cells in each cell subset. Cell frequency was analyzed by FACS and FlowJo software. P values were obtained using the unpaired Student t test and Prism software. Data and error bars in panels B and C represent the mean plus or minus standard error of the mean (sem).
We then examined in stably human-xenografted mice whether HSPC depletion by AMG 191 could permit engraftment of a second donor human HSC graft, thereby modeling an allogeneic transplantation. Human HSCs expressing mCitrine generated as previously described21 were transplanted into humanized NSG mice treated with AMG 191. Gene-marked second human donor cells were observed among hCD45+hCD34+ and hCD45+hCD19+ cells in all mice treated with AMG 191, whereas no evidence of second donor chimerism was found in unconditioned mice, suggesting that AMG 191 conditioning permitted second donor HSPC engraftment (Figure 2C). Donor chimerism was higher in mice treated with 1.0 mg/kg AMG 191 compared with the 0.3 mg/kg dose, indicating a beneficial effect of higher dose AMG 191 on second donor engraftment (Figure 2C). No myeloid or T-cell chimerism from the second donor was observed in any animal (data not shown). These data demonstrate that AMG 191 successfully depletes human HSPCs and that this depletion permitted engraftment of second human allografts in humanized mice.
The present study provides proof of concept that AMG 191 can be used as a BM niche-clearing agent to deplete endogenous HSPCs and supports its use as a nontoxic agent that will permit HSPC engraftment. Indeed, based on these NHP data, a human clinical trial now under way shows early promising engraftment results in children with severe combined immunodeficiency using AMG 191 as the only agent to facilitate BM niche clearance before infusion of CD34+-enriched grafts (clinicaltrials.gov NCT02963064). The observation that anti-mouse CD117 antibody works most effectively to permit HSC engraftment in lymphocyte-deficient mice6 served as the rationale for initiating AMG 191 conditioning in this target population. However, data presented here suggest that AMG 191 may be applicable to conditioning hosts with intact immune function. In immunocompetent NHP, AMG 191 achieved measurable HSPC depletion, even at low doses. The cell-depletive function of AMG 191 can also be enhanced if needed. Combination therapies of AMG 191 with other biologic reagents targeting the CD47-calreticulin axis,22 or with low-dose radiation,23 show promise in facilitating hematopoietic engraftment in immunocompetent hosts. In our view, use of AMG 191 alone or in combination will be applicable to a wide spectrum of disorders, including those cured by allogeneic HCT as well as those treatable by gene therapy–modified autologous HSCs.3,24 As the use of AMG 191 is extended to conditioning of other disorders in which grafts replete with T cells are used, it will be important to determine whether HSPC niche clearance by this class of antibody might positively or negatively impact development of graft-versus-host disease. We recently showed that anti-human CD117 antibody can successfully deplete clonally abnormal HSCs present in mice engrafted with cells from patients with myelodysplastic syndrome.25 In conclusion, we view AMG 191 as a first-of-its-kind therapy that has the potential to vastly improve the safety of transplantation for the many thousands of patients who undergo this procedure yearly.
The online version of this article contains a data supplement.
Acknowledgments
The authors thank members of the Shizuru and Weissman laboratories for helpful advice, critical discussion, and technical assistance. The authors thank Randall Armstrong for technical assistance in generating fluorescein isothiocyanate–conjugated AMG 191 antibody. The authors thank the Stanford Shared FACS Facility, Eurofins Pharma Bioanalytics Services US Inc, and SRI International. The authors thank the AMG 191 team at Amgen Inc for their important insights on study design, review of the manuscript, supply of AMG 191, and continued support for these studies.
This work was supported by the Virginia and D. K. Ludwig Fund for Cancer Research (J.A.S. and I.L.W.); California Institute for Regenerative Medicine grants DR2A-05365 (J.A.S.) and RT3-07683 (I.L.W.); National Institutes of Health, National Cancer Institute grant R01 CA86065 (I.L.W.) and National Institutes of Health, National Heart, Lung, and Blood Institute grant R01 HL058770 (I.L.W.); the Gunn/Olivier Research Fund (J.A.S. and I.L.W.); the H.L. Snyder Medical Foundation (J.A.S.); and the Stinehart-Reed Foundation (J.A.S).
Authorship
Contribution: H.-S.K., A.C.L., A. Chhabra, W.W.P., K.T., A.L., J.P., R.H., and B.V.K. performed experiments; H.-S.K., A.C.L., and A. Chhabra analyzed results and made the figures; A. Czechowicz, D.B.K., I.L.W., and S.S.P. gave advice and discussion on the design of the experiments, and edited the manuscript; and H.-S.K., A.C.L., and J.A.S. designed the research and wrote the paper.
Conflict-of-interest disclosure: I.L.W. and A. Czechowicz are inventors on patents that include the use of anti-CD117 antibodies in HCT conditioning, and I.L.W., J.A.S., and A. Chhabra are inventors on patents that pair anti-CD47 agents and anti-CD117 antibodies for HCT conditioning. I.L.W. is a cofounder, stockholder, and Director of Forty Seven, Inc, which has licensed these patents from Stanford University. J.A.S., A. Chhabra, and S.S.P. have equity ownership in Forty Seven, Inc. A. Czechowicz has equity ownership in Forty Seven, Inc; Magenta Therapeutics; Beam Therapeutics; Editas Medicines; and Global Blood Therapeutics. The remaining authors declare no competing financial interests.
Correspondence: Judith A. Shizuru, School of Medicine, Stanford University, 300 Pasteur Dr, Room H0101, MC: 5623, Stanford, CA 94305; e-mail: jshizuru@stanford.edu.
REFERENCES
Author notes
A.C.L. and A. Chhabra contributed equally.