In this issue of Blood, Khasabova et al demonstrate the analgesic efficacy of R-flurbiprofen, an inhibitor of cyclooxygenase-2 (COX-2)-mediated 2-arachidonoylglycerol (2-AG) oxidation, and MRS2578, a P2Y6 receptor antagonist, in the Berkeley transgenic mouse model of sickle cell disease (SCD).1
Identification of these therapeutic targets increases the relatively small pool of non-opioid–based analgesics available for SCD pain management. Transgenic mouse models and patients with SCD regularly experience both acute and chronic pain; in fact, severe pain is the leading reason for emergency department visits among patients with SCD.2 Although acute SCD pain is traditionally thought to arise from peripheral vaso-occlusion, the causes of chronic SCD pain, like that which was studied in this manuscript, are less defined, but may include chronic inflammation and aberrant peripheral and central nervous system activity. As a result, broad-spectrum analgesics like opioids are commonly prescribed for chronic SCD pain. However, a recent descriptive study did not find chronic opioid therapy effective in alleviating SCD pain; in contrast, patients on chronic opioid therapy reported higher clinical pain scores, more frequent pain crises, and higher depression scores.3 Compounded with the often baseless provider assumptions about drug-seeking and addiction status in patients with SCD,4 there is an urgent and unmet need for non-opioid–based analgesics in chronic SCD pain management.
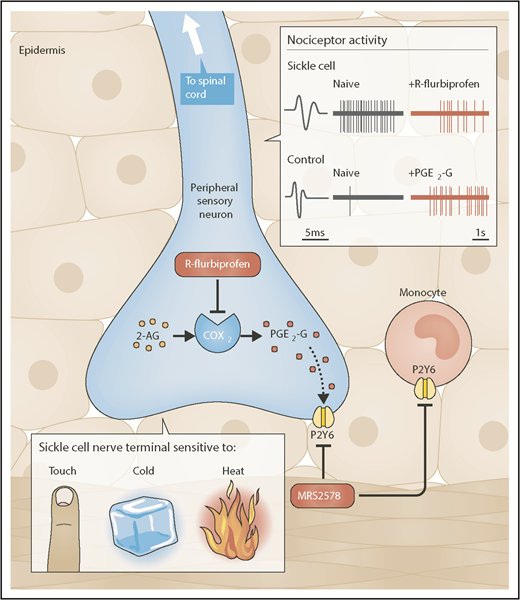
R-flurbiprofen and MRS2578 are 2 novel analgesics for chronic SCD pain. SCD mice exhibit chronic behavioral and neuronal hypersensitivity to mechanical, cold, and heat stimuli. Peripheral administration of the COX-2 inhibitor R-flurbiprofen decreases cold and mechanical behavioral hypersensitivity in SCD mice as well as spontaneous and mechanically-induced nociceptor firing in in vivo recording preparations from SCD mice. In control mice, peripheral application of PGE2-G, a byproduct of COX-2-mediated 2-AG oxidation, increases behavioral mechanical hypersensitivity, induces nociceptor firing, and increases nociceptor responsiveness to mechanical, cold and heat stimuli. Administration of the P2Y6 antagonist MRS2578 decreases the PGE2-G-induced behavioral hypersensitivity in control mice and the naturally-occurring chronic behavioral hypersensitivity observed in SCD mice. Schematic shows the peripheral terminal of the cutaneous nociceptive sensory neuron and that R-flurbiprofen inhibits the COX2-mediated production of PGE2-G which activates the P2Y6 receptor. Alternatively, MRS2578, blocks P2Y6 receptors on either peripheral nerve terminals or monocytes. Inset shows a C fiber nociceptor from SCD mice (top) and control mice (bottom). The SCD C fiber shows significant spontaneous action potential firing (naïve) compared to the control (naïve). R-flurbiprofen reduces the spontaneous action potential firing in the SCD C fiber. The bottom shows that PGE2-G induces action potential firing in the control C fiber. Professional illustration by Neil Smith.
R-flurbiprofen and MRS2578 are 2 novel analgesics for chronic SCD pain. SCD mice exhibit chronic behavioral and neuronal hypersensitivity to mechanical, cold, and heat stimuli. Peripheral administration of the COX-2 inhibitor R-flurbiprofen decreases cold and mechanical behavioral hypersensitivity in SCD mice as well as spontaneous and mechanically-induced nociceptor firing in in vivo recording preparations from SCD mice. In control mice, peripheral application of PGE2-G, a byproduct of COX-2-mediated 2-AG oxidation, increases behavioral mechanical hypersensitivity, induces nociceptor firing, and increases nociceptor responsiveness to mechanical, cold and heat stimuli. Administration of the P2Y6 antagonist MRS2578 decreases the PGE2-G-induced behavioral hypersensitivity in control mice and the naturally-occurring chronic behavioral hypersensitivity observed in SCD mice. Schematic shows the peripheral terminal of the cutaneous nociceptive sensory neuron and that R-flurbiprofen inhibits the COX2-mediated production of PGE2-G which activates the P2Y6 receptor. Alternatively, MRS2578, blocks P2Y6 receptors on either peripheral nerve terminals or monocytes. Inset shows a C fiber nociceptor from SCD mice (top) and control mice (bottom). The SCD C fiber shows significant spontaneous action potential firing (naïve) compared to the control (naïve). R-flurbiprofen reduces the spontaneous action potential firing in the SCD C fiber. The bottom shows that PGE2-G induces action potential firing in the control C fiber. Professional illustration by Neil Smith.
In their manuscript, Khasabova et al identify 2 new analgesic targets by exploring the causative relationship between persistent inflammation and neuronal activity in SCD mice (see figure). Increased levels of COX-2 enzyme expression, activity, and oxidation products were detected in the dorsal root ganglia of SCD mice. Traditionally, COX-2 oxidation and prostaglandin (PG) synthesis are inhibited with nonsteroidal anti-inflammatory drugs (NSAIDs) including ibuprofen and aspirin. In this manuscript, R-enantiomers of the NSAID flurbiprofen were used to specifically limit the oxidation of 2-AG, an endocannabinoid with antihyperalgesic effects, to the PG glycerol ester PGE2-glycerol (PGE2-G). When R-flurbiprofen was administered to SCD mice, both the behavioral and neuronal sensitivity to mechanical and thermal stimuli decreased. MRS2578-mediated inhibition of the purinergic receptor P2Y6 also decreased the mechanical and cold hypersensitivity in SCD mice and PGE2-G–induced hypersensitivity in control mice. Based on these results, R-flurbiprofen and MRS2578 may be effective analgesics for evoked chronic SCD pain. Notably, the analgesic effects of these compounds have not yet been tested with regard to nonevoked SCD pain, the alleviation of which could have an even greater impact on patient quality of life. “Spontaneous” nociceptor activity and resulting pain behaviors have been previously reported in SCD mice.5,6 Although not traditional vaso-occlusive crisis (VOC) pain, we use the word “spontaneous” gingerly because we believe that there may be many circulating factors in SCD that actively drive this neuronal activity and subsequent behavior. Regardless, in this manuscript, “spontaneous” SCD nociceptor activity was normalized following application of R-flurbiprofen. However, the effects of this compound on corresponding “spontaneous” pain-like behaviors in SCD mice were not assessed. To measure nonevoked pain in rodents, facial grimace, paw/limb attending, or home cage behavioral assessments are often used in addition to classical conditioning paradigms, such as analgesic conditioned place preference. R-flurbiprofen and MRS2578 efficacy in these behavioral assays should be evaluated and compared with that of opioid analgesics. Additionally, it will be important to assess the potential abuse risk of these compounds vs that of opioids using classical conditioning paradigms in control and SCD mice.
Another outstanding and clinically relevant question arising from this work is whether R-flurbiprofen or MRS2578 will prove effective for acute VOC pain in SCD. Circulating PGs are elevated in patients with SCD both during and in between VOCs when compared with race-matched controls.7 It is unknown whether PGE2-G is specifically elevated during a VOC, but if it is, then both R-flurbiprofen and MRS2578 might effectively decrease VOC pain intensity and duration. SCD mice can be used for initial assessments of drug efficacy in this context because VOCs can be induced in SCD mice by altering environmental oxygen concentrations.
We appreciate how this manuscript explains SCD pain from both an inflammatory and neuronal perspective; substantial evidence suggests that both are critical for the maintenance of chronic SCD pain. However, one of the largest unanswered questions remaining in SCD pain research is how patients develop chronic pain in the first place. Following the sharp decrease in fetal hemoglobin that occurs during the first ∼12 months of life, patients with SCD can begin to experience acute VOCs that are accompanied by excruciating pain. Chronic pain is reported in pediatric SCD patients, but the prevalence of this condition increases with age.8 Would chronic R-flurbiprofen treatment prevent or delay the development of chronic SCD pain? Or would NSAID-related tissue toxicities outweigh the analgesic benefits of these drugs?9 Would long-term use of R-flurbiprofen or MRS2578 be sufficient to decrease ongoing nociceptor activity and thereby prevent the development of central sensitization?10 Longitudinal studies across the developmental timespan in both mouse models and human patients will be critical for understanding the inflammatory and neuronal changes that occur during disease progression and, ultimately, the development of non-opioid–based therapies for chronic SCD pain.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal