Key Points
The TCP regimen showed promising efficacy and safety to treat newly diagnosed iMCD.
Although more research is needed, the TCP regimen is an important treatment option, particularly when siltuximab is not available.
Abstract
Idiopathic multicentric Castleman disease (iMCD) is a rare lymphoproliferative disorder. The anti–interleukin 6 (IL-6) therapy siltuximab is not available everywhere, and is not effective for over one-half of patients. Alternative treatment approaches are urgently needed. In the first iMCD clinical trial directed against a target other than IL-6 signaling, we investigated a thalidomide-cyclophosphamide-prednisone (TCP) regimen in newly diagnosed iMCD patients. This single-center, single-arm, phase 2 study enrolled 25 newly diagnosed iMCD patients between June 2015 and June 2018. The TCP regimen (thalidomide 100 mg daily for 2 years; oral cyclophosphamide 300 mg/m2 weekly for 1 year; prednisone 1 mg/kg twice a week for 1 year) was administered for 2 years or until treatment failure. The primary end point was durable tumor and symptomatic response for at least 24 weeks. Twelve patients (48%) achieved the primary end point with no relapse, 3 patients (12%) demonstrated stable disease, and 10 patients (40%) were evaluated as treatment failure. Even when considering all patients, there were significant (P < .05) improvements in median symptom score, IL-6 level, hemoglobin, erythrocyte sedimentation rate, albumin, and immunoglobulin G. Among responders, the median levels of all evaluated parameters significantly improved, to the normal range, after treatment. The regimen was well tolerated. One patient died of pulmonary infection and 1 patient had a grade 3 adverse event (rash); 2 patients died following disease progression. Estimated 1-year progression-free survival and overall survival were 60% and 88%, respectively. The TCP regimen is an effective and safe treatment of newly diagnosed iMCD patients, particularly when siltuximab is unavailable. This trial was registered at www.clinicaltrials.gov as #NCT03043105.
Introduction
Castleman disease (CD) describes a group of rare, heterogeneous lymphoproliferative disorders that share common histopathological features ranging from hyaline vascular (HV) to plasmacytic (PC). Unicentric CD involves a single region of enlarged lymph nodes with mild symptomatology, whereas multicentric CD (MCD) includes multiple regions of enlarged lymph nodes and systemic symptoms. MCD is further divided into human herpesvirus-8 (HHV-8)–associated MCD, which is caused by uncontrolled HHV-8 infection in HIV+ or otherwise immunocompromised individuals,1 and HHV-8− MCD, which is also called idiopathic MCD (iMCD) because the etiology is unknown.2
iMCD is characterized by systemic inflammatory symptoms, cytopenias, and life-threatening organ dysfunction due to a cytokine storm often including interleukin 6 (IL-6).2 The limited understanding of the etiology of iMCD has hindered the discovery of targeted therapies. Unlike unicentric CD, which is almost uniformly cured with surgical resection, treatment of iMCD patients is much more challenging and the 5-year mortality is 23% to 49%.3-5 A wide variety of medical therapies, including prednisone, conventional chemotherapy (eg, cyclophosphamide, hydroxydaunorubicin, vincristine, and prednisone [CHOP] regimen),6 immunomodulatory agents, and anti–IL-6 therapy7 have been reported in small series and case reports in iMCD.1 However, these therapies have not been systematically evaluated and have notable limitations. Prednisone monotherapy is rarely capable of inducing a complete or long-term response. Combination chemotherapies such as rituximab-CHOP are effective at inducing responses,8 but present significant toxicities and rarely provide long-term remissions. The anti–IL-6 receptor therapy, tocilizumab, improved symptoms and laboratory abnormalities in a large portion of patients in an open-label study.9 Although no overall response criteria were assessed in the study and a randomized controlled trial (RCT) was never performed, tocilizumab was approved in Japan for the treatment of iMCD. A phase 1 study and a phase 2 RCT demonstrating the efficacy and safety of siltuximab, an anti–IL-6–targeted treatment,7 led to its regulatory approval for iMCD in over 40 countries worldwide. However, siltuximab did not induce a response in over 50% of patients in the RCT. Furthermore, the high price and requirement for repeated IV administration of tocilizumab and siltuximab limit their utilization as first-line therapies for iMCD in low-income countries like China; tocilizumab and siltuximab are currently not available in the Chinese market for iMCD. Additional treatment options are urgently needed for siltuximab nonresponders and patients for whom siltuximab is not available.
Increased lymph node plasma cells, activated T cells, vascular endothelial growth factor (VEGF), and several other proinflammatory cytokines have been reported in iMCD and represent rational therapeutic targets.10-12 Thalidomide is a relatively inexpensive immunomodulatory drug that has been frequently used to treat multiple myeloma; polyneuropathy, organomegaly, endocrinopathy, monoclonal gammopathy, and skin changes (POEMS) syndrome13 ; and, in a few reported cases, iMCD.1,6,14,15 Thalidomide is known to have direct inhibitory effects on plasma cells and to suppress angiogenesis and inhibit production of cytokines, including tumor necrosis factor-α, VEGF, IL-1, IL-6, IL-12, and IL-10.16,17 Cyclophosphamide, an alkylating agent that induces cell death by impairing DNA replication and strongly inhibits T-cell proliferation, and prednisone, an anti-inflammatory glucocorticoid, have been reported to be effective in a limited number of case reports of iMCD but have never been evaluated in a prospective clinical trial.6,10,18 The side-effect profiles of these drugs are well characterized. Thalidomide is associated with peripheral neuropathy, birth defects, gastrointestinal (GI) complications, somnolence, and thrombotic events. Cyclophosphamide is associated with cytopenias, infections, infertility, and bladder toxicity. Prednisone is associated with dermatologic, neuropsychiatric, metabolic, and immunologic effects. These drugs are all available in oral preparations, which is convenient for patients. The thalidomide, oral cyclophosphamide, and prednisone (TCP) regimen is therefore a promising and economical treatment approach for iMCD that has been used previously for multiple myeloma. Therefore, we performed a phase 2 study to evaluate the efficacy and safety of the TCP regimen in patients with newly diagnosed iMCD.
Materials and methods
Study design and participants
This phase 2, single-center, single-arm trial was conducted at Peking Union Medical College Hospital (PUMCH; Beijing, China). Adult patients (≥18 years of age) who were newly diagnosed with iMCD2 and met the following criteria were considered as eligible candidates for this study: (1) histopathologic lymph node features consistent with iMCD (confirmed by 2 independent pathologists from PUMCH); (2) enlarged lymph nodes (≥1 cm in short-axis diameter) in at least 2 lymph node stations; (3) HHV-8− confirmed by blood polymerase chain reaction or latency-associated nuclear antigen 1 (LANA-1) staining by immunohistochemistry and HIV− confirmed by serology test; and (4) newly diagnosed and previously untreated (patients were allowed to have received oral prednisone for up to 1 week before enrollment), symptomatic iMCD patients.2 Symptomatic disease was defined by the presence of clinical symptoms with National Cancer Institute Common Toxicity Criteria for Adverse Events version 4.0 (NCI-CTCAE) grading ≥1 attributable to the disease and for which treatment is indicated. Exclusion criteria included known malignancies or other severe concurrent diseases, such as POEMS syndrome (M-spike on serum protein electrophoresis was exclusionary) and systemic lupus erythematosus (positive anti–double-stranded DNA or anti-extractable nuclear antigen antibody was exclusionary); known hypersensitivity to study agents; pregnancy or breastfeeding; and plans to become pregnant within 1 year after enrollment. Low-titer anti-nuclear antibody in the absence of a definitive autoimmune diagnosis was not exclusionary.
Patients were evaluated for the thrombocytopenia, anasarca, fever/elevated C-reactive protein (CRP), renal insufficiency/reticulin fibrosis, and organomegaly (TAFRO) syndrome subtype of iMCD for potential stratification during data analysis.19
All enrolled patients provided signed informed consent before study entry. The study was performed in accordance with the Declaration of Helsinki, with prior approval of the institutional review board and the ethics committee of the local hospital.
Procedures
The TCP regimen (thalidomide 100 mg daily at bedtime for year 1 and year 2; oral cyclophosphamide 300 mg/m2 weekly on days 1, 8, 15, and 22 of a 4-week cycle for year 1; prednisone 1 mg/kg twice a week on days 1 and 2, days 8 and 9, days 15 and 16, and days 22 and 23 of a 4-week cycle for year 1) was administered for 2 years or until treatment failure. Treatment failure7 was defined as sustained increase in grade ≥2 disease-related symptoms persisting ≥12 weeks, new disease-related grade ≥3 symptoms, sustained ≥2-point increase in Eastern Cooperative Oncology Group performance status (ECOG-PS) persisting for ≥12 weeks, radiological progression, initiation of another treatment of iMCD, or death. At treatment failure, patients received second-line therapy of either rituximab-based or bortezomib-based combination chemotherapy. Given the thrombotic risk of thalidomide, 100 mg of aspirin were given daily to all patients. Hematological and nonhematological toxicities requiring dose delays and/or modification were recorded. All patients were assessed for the response criteria every 3 months during the treatment phase and every 12 months after the first 2 years. Computerized tomography imaging was performed at baseline and at each response criteria evaluation.
Outcomes
The primary end point of the study was durable tumor and symptomatic response for at least 24 weeks, which was assessed by radiologic imaging and disease evaluations. The durable tumor and symptomatic response criteria were adopted as the primary outcome based on the phase 2 RCT of siltuximab.7 The definition of durable tumor and symptomatic response in our study was identical except for the time duration (18 weeks in the prior RCT and 24 weeks in our study), which was adjusted based on our follow-up schedule. Durable tumor and symptomatic response was defined as 1 of the following (complete response [CR] or partial response [PR]) sustained for at least 24 weeks: CR meant complete disappearance of all measurable and evaluable disease (eg, pleural effusion) and resolution of baseline symptoms attributed to iMCD; PR meant a ≥50% decrease in sum of the product of diameters of measurable lesion(s), with at least stable disease (SD) in all other evaluable disease in the absence of treatment failure. This end point was subdivided into tumor response and symptomatic response. Tumor response, which describes changes in the size of the enlarged lymph nodes in iMCD, was assessed by radiologists and hematologists using Cheson criteria. Complete disappearance of all measurable and evaluable disease was defined as tumor CR; a ≥50% decrease in sum of the product of diameters of measurable lesion(s), with at least SD in all other evaluable disease, was defined as tumor PR. Symptomatic response was assessed based on MCD-related overall symptom score, a published score calculated as the sum of the toxicity grades of the NCI-CTCAE terms. A total score of all symptoms (34 symptoms from 5 categories) was calculated as the sum of the toxicity grades.7 For symptomatic response, durable symptomatic response (PR and CR) was defined as a ≥50% reduction in overall MCD-related symptom score sustained for at least 24 weeks prior to treatment failure; durable complete symptomatic response was defined as a 100% reduction of symptom score for at least 24 weeks prior to treatment failure. Patients who did not achieve durable tumor and symptomatic response and did not meet the criteria of treatment failure were defined as SD. Patients who achieved the primary end point were defined as responders; patients with SD or treatment failure were defined as nonresponders.
The secondary end points of the study included the trend of biochemical parameters, progression-free survival (PFS), which was defined as the time to treatment failure or death, and overall survival (OS), which was defined as the time to patients’ death.
Safety data were collected until 30 days after the last dose of study drugs, except for secondary primary malignancies (which were assessed throughout the duration of follow-up). Secondary primary malignancy was defined as any malignancy observed after initiation of the TCP regimen. Adverse events were graded as per NCI-CTCAE (version 4.0).
Statistical analysis
Analyses were performed with SPSS 22 (SPSS, Inc, Chicago, IL). The independent samples Student t test (for parameters with normal distribution) and Mann-Whitney test (for parameters that were not normally distributed) were used for comparison of baseline characteristics between responders and nonresponders. The Wilcoxon signed-rank test was used to compare parameters before and after the TCP regimen. For patients who were evaluated as treatment failure, the time of treatment failure was considered as the end of treatment. OS and PFS were calculated from the date of treatment. For PFS analyses, death or treatment failure were considered as events. Survival curves were plotted with the Kaplan-Meier method. P < .05 was considered statistically significant. The final follow-up date was 1 June 2018.
Results
Patient characteristics
A total of 25 newly diagnosed iMCD patients participated in this phase 2 study from June 2015 to June 2018 and were followed for at least 24 weeks. All patients met the diagnostic criteria for iMCD, including exclusion of overlapping disorders. All 25 patients had a negative serum protein electrophoresis; 5 of 25 patients had an anti-nuclear antibody of 1:80, but negative anti–double-stranded DNA and anti–extractable nuclear antibody. Baseline demographic and disease characteristics are summarized in Table 1. The median age was 40 years (20-63 years), with a male-to-female ratio of 2.1:1. Two patients fulfilled the criteria for the TAFRO syndrome subtype of iMCD. Forty percent of patients demonstrated lymph node features consistent with the HV histopathological subtype, 44% were classified as the PC histopathological subtype, and 16% were considered mixed. The median baseline symptom score was 14 (6-41) and median baseline IL-6 level was 21.3 pg/mL (7.4-865; normal range, 0-5.9 pg/mL). Median hemoglobin, platelet count, serum creatinine, CRP, erythrocyte sedimentation rate (ESR), serum albumin, and immunoglobin G (IgG) levels were 100 g/L (73-141 g/L), 297 × 109/L (64 × 109/L-725 × 109/L), 55 μmol/L (25-182 μmol/L), 73.9 mg/L (0.24-185.6 mg/L), 97.5 mm/h (2-140 mm/h), 32 g/L (24-43 g/L), and 25.9 g/L (5.3-85.1 g/L), respectively.
Baseline characteristics of iMCD patients treated with the TCP regimen (n = 25)
| Characteristics . | Normal range . | All patients, n = 25 . | Responders, n = 12 . | Nonresponders, n = 13 . | P . |
|---|---|---|---|---|---|
| Age, median (range), y | 40 (20-63) | 38 (20-63) | 41 (29-61) | .660 | |
| Sex, male, n (%) | 17 (68) | 9 (75) | 8 (61.5) | .574 | |
| Histology | .152 | ||||
| Hyaline vascular | 10 (40%) | 6 | 4 | ||
| Plasmacytic | 11 (44%) | 6 | 5 | ||
| Mixed | 4 (16%) | 0 | 4 | ||
| ECOG-PS | .852 | ||||
| 0 | 11 (44%) | 6 | 5 | ||
| 1 | 11 (44%) | 4 | 7 | ||
| 2 | 3 (12%) | 2 | 1 | ||
| Symptom score, median (range) | 14 (6-41) | 16 (7-38) | 13 (6-41) | .666 | |
| Fever, n (%) | 14 (56) | 10 (83.3) | 4 (30.8) | .008 | |
| Weight loss, n (%) | 19 (76) | 10 (83.3) | 9 (69.2) | .645 | |
| Peripheral lymphadenopathy, n (%) | 23 (92) | 12 (100) | 11 (84.6) | .480 | |
| Splenomegaly, n (%) | 9 (36) | 3 (25) | 6 (46.2) | .411 | |
| Skin involvement,* n (%) | 8 (32) | 3 (25) | 5 (38.5) | .673 | |
| Pulmonary involvement, n (%) | 5 (20) | 0 (0) | 5 (38.5) | .039 | |
| Anasarca, n (%) | 6 (24) | 3 (25) | 3 (23.1) | 1.000 | |
| IL-6, median (range), pg/mL | <5.9 | 21.3 (7.4-865) | 28.55 (7.4-865) | 20.7 (7.7-61.9) | .437 |
| Hemoglobin, median (range), g/L | Male, 120-160; female, 110-150 | 100 (73-141) | 98 (76-133) | 102.5 (73-141) | .931 |
| Platelet count, median (range), 109/L | 100-350 | 297 (64-725) | 167 (64-688) | 322.5 (64-725) | .689 |
| Serum creatinine, median (range), μmol/L | Male, 59-104; female, 45-84 | 55 (25-182) | 55 (42-182) | 58.5 (25-138) | .782 |
| CRP, median (range), mg/L | 0-8 | 73.9 (0.2-185.6) | 41.625 (0.2-185.6) | 85.685 (1.6-119.2) | .453 |
| ESR, median (range), mm/h | Male, 0-15; female, 0-20 | 97.5 (2-140) | 90 (6-115) | 102 (2-140) | .178 |
| Albumin, median (range), g/L | 35-52 | 32 (24-43) | 32 (26-43) | 31 (24-43) | .623 |
| IgG, median (range), g/L | 7.00-17.00 | 25.9 (5.3-85.1) | 21.74 (14.4-43.8) | 36.475 (5.3-85.1) | .221 |
| Characteristics . | Normal range . | All patients, n = 25 . | Responders, n = 12 . | Nonresponders, n = 13 . | P . |
|---|---|---|---|---|---|
| Age, median (range), y | 40 (20-63) | 38 (20-63) | 41 (29-61) | .660 | |
| Sex, male, n (%) | 17 (68) | 9 (75) | 8 (61.5) | .574 | |
| Histology | .152 | ||||
| Hyaline vascular | 10 (40%) | 6 | 4 | ||
| Plasmacytic | 11 (44%) | 6 | 5 | ||
| Mixed | 4 (16%) | 0 | 4 | ||
| ECOG-PS | .852 | ||||
| 0 | 11 (44%) | 6 | 5 | ||
| 1 | 11 (44%) | 4 | 7 | ||
| 2 | 3 (12%) | 2 | 1 | ||
| Symptom score, median (range) | 14 (6-41) | 16 (7-38) | 13 (6-41) | .666 | |
| Fever, n (%) | 14 (56) | 10 (83.3) | 4 (30.8) | .008 | |
| Weight loss, n (%) | 19 (76) | 10 (83.3) | 9 (69.2) | .645 | |
| Peripheral lymphadenopathy, n (%) | 23 (92) | 12 (100) | 11 (84.6) | .480 | |
| Splenomegaly, n (%) | 9 (36) | 3 (25) | 6 (46.2) | .411 | |
| Skin involvement,* n (%) | 8 (32) | 3 (25) | 5 (38.5) | .673 | |
| Pulmonary involvement, n (%) | 5 (20) | 0 (0) | 5 (38.5) | .039 | |
| Anasarca, n (%) | 6 (24) | 3 (25) | 3 (23.1) | 1.000 | |
| IL-6, median (range), pg/mL | <5.9 | 21.3 (7.4-865) | 28.55 (7.4-865) | 20.7 (7.7-61.9) | .437 |
| Hemoglobin, median (range), g/L | Male, 120-160; female, 110-150 | 100 (73-141) | 98 (76-133) | 102.5 (73-141) | .931 |
| Platelet count, median (range), 109/L | 100-350 | 297 (64-725) | 167 (64-688) | 322.5 (64-725) | .689 |
| Serum creatinine, median (range), μmol/L | Male, 59-104; female, 45-84 | 55 (25-182) | 55 (42-182) | 58.5 (25-138) | .782 |
| CRP, median (range), mg/L | 0-8 | 73.9 (0.2-185.6) | 41.625 (0.2-185.6) | 85.685 (1.6-119.2) | .453 |
| ESR, median (range), mm/h | Male, 0-15; female, 0-20 | 97.5 (2-140) | 90 (6-115) | 102 (2-140) | .178 |
| Albumin, median (range), g/L | 35-52 | 32 (24-43) | 32 (26-43) | 31 (24-43) | .623 |
| IgG, median (range), g/L | 7.00-17.00 | 25.9 (5.3-85.1) | 21.74 (14.4-43.8) | 36.475 (5.3-85.1) | .221 |
P values shown in bold represent statistically significant (P < .05) differences between responders and nonresponders.
One patient had pemphigus.
Response
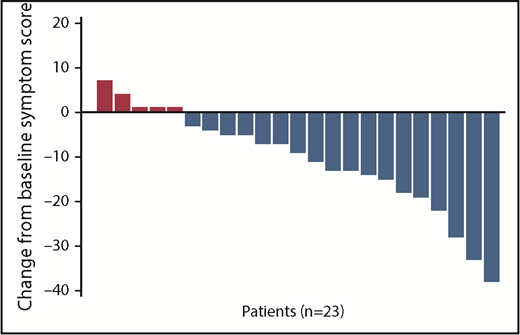
After administration of the TCP regimen, 12 patients (48%; including 1 of the 2 patients with the TAFRO syndrome subtype) achieved durable tumor and symptomatic response (CR plus PR) for at least 24 weeks. Of them, 1 patient (4%) achieved durable complete tumor and symptomatic response (CR). Twelve patients achieved tumor response (1 tumor CR and 11 tumor PR). Twelve patients achieved durable symptomatic response; of them, 7 achieved durable complete symptomatic response. Comparison of baseline parameters between the 12 responders and the 13 nonresponders demonstrated that responders were more likely to have fever and less likely to have pulmonary involvement (Table 1). When looking across all 25 patients who received the TCP regimen, the median symptom score declined significantly from 14 (6-41) at baseline to 3 (0-20) at the time of last follow-up after treatment (P < .001). Figure 1 presents the absolute change in symptom score from baseline for individual patients, excluding the 2 patients who died before the first follow-up. Even when combining responders and nonresponders in the analysis, there were significant improvements in the median IL-6 level (P = .019), hemoglobin level (P = .007), ESR (P = .004), albumin level (P < .001), and IgG level (P < .001) at the time of last follow-up. The median CRP level trended toward a significant improvement (P = .064) (Table 2; comparisons between baseline and week 24 are also provided in Table 2).
Change in absolute symptom score from baseline for each individual patient. n = 23, excluding 2 patients who died before first follow-up.
Change in absolute symptom score from baseline for each individual patient. n = 23, excluding 2 patients who died before first follow-up.
Characteristics at baseline, at week 24, and at last follow-up for all patients (n = 25)
| Characteristics . | Baseline, median (range) . | Week 24, median (range) . | P* . | Last follow-up, median (range) . | P* . |
|---|---|---|---|---|---|
| Symptom score | 14 (6-41) | 2.5 (0-13) | .001 | 3 (0-20) | <.001 |
| IL-6, pg/mL | 21.3 (7.4-865) | 10.6 (2-42.8) | .140 | 9.7 (2-70.3) | .019 |
| Hemoglobin, g/L | 100 (73-141) | 124 (67-150) | .079 | 124 (67-177) | .007 |
| CRP, mg/L | 73.9 (0.2-185.6) | 53.8 (0.8-112.2) | .433 | 23.2 (0.7-176.0) | .064 |
| ESR, mm/h | 97.5 (2-140) | 60.5 (5-140) | .030 | 27 (1-140) | .004 |
| Albumin, g/L | 32 (24-43) | 37 (31-56) | .002 | 38 (27-56) | <.001 |
| IgG, g/L | 25.9 (5.3-85.1) | 18.9 (7.3-44.1) | .001 | 15.4 (5.3-35.9) | <.001 |
| Characteristics . | Baseline, median (range) . | Week 24, median (range) . | P* . | Last follow-up, median (range) . | P* . |
|---|---|---|---|---|---|
| Symptom score | 14 (6-41) | 2.5 (0-13) | .001 | 3 (0-20) | <.001 |
| IL-6, pg/mL | 21.3 (7.4-865) | 10.6 (2-42.8) | .140 | 9.7 (2-70.3) | .019 |
| Hemoglobin, g/L | 100 (73-141) | 124 (67-150) | .079 | 124 (67-177) | .007 |
| CRP, mg/L | 73.9 (0.2-185.6) | 53.8 (0.8-112.2) | .433 | 23.2 (0.7-176.0) | .064 |
| ESR, mm/h | 97.5 (2-140) | 60.5 (5-140) | .030 | 27 (1-140) | .004 |
| Albumin, g/L | 32 (24-43) | 37 (31-56) | .002 | 38 (27-56) | <.001 |
| IgG, g/L | 25.9 (5.3-85.1) | 18.9 (7.3-44.1) | .001 | 15.4 (5.3-35.9) | <.001 |
Compared with baseline.
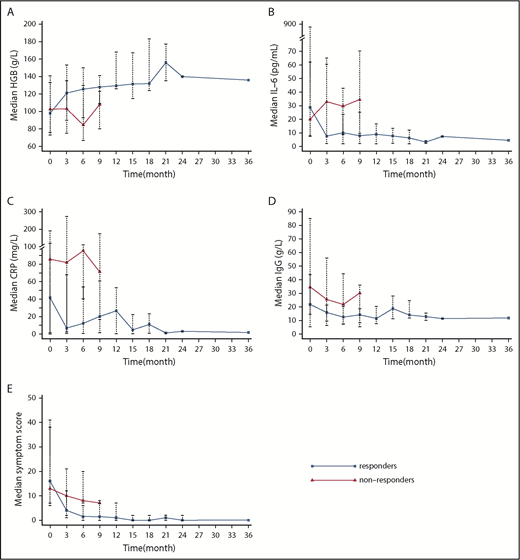
Among the 12 patients who experienced a durable tumor and symptomatic response (responders), all evaluated parameters showed statistically significant improvement after treatment (at the time of last follow-up) with median levels returning to the normal range (Table 3; comparisons between baseline and week 24 are also provided in Table 3). The median hemoglobin level increased from 98 g/L (baseline) to 132 g/L (P = .013) (Figure 2A), median IL-6 level decreased from 28.55 pg/mL to 3.7 pg/mL (P = .002) (Figure 2B), median CRP level declined from 41.625 mg/L to 3.165 mg/L (P = .005) (Figure 2C), median IgG level decreased from 21.74 g/L to 14.50 g/L (P = .002) (Figure 2D), and median symptom score decreased from 16 to 0.5 (Figure 2E).
Characteristics at baseline, at week 24, and at last follow-up for responders (n = 12)
| Characteristics . | Baseline, median (range) . | Week 24, median (range) . | P* . | Last follow-up, median (range) . | P* . |
|---|---|---|---|---|---|
| Symptom score | 16 (7-38) | 1 (0-6) | .003 | 0.5 (0-6) | .002 |
| IL-6, pg/mL | 28.6 (7.4-865) | 10.0 (2-23.5) | .012 | 3.7 (2-25.1) | .002 |
| Hemoglobin, g/L | 98 (76-133) | 125.5 (93-150) | .093 | 132 (93-177) | .013 |
| CRP, mg/L | 41.6 (0.2-185.6) | 12.4 (0.8-54.8) | .028 | 3.2 (0.7-53.0) | .005 |
| ESR, mm/h | 90 (6-115) | 31 (5-125) | .042 | 16 (1-125) | .013 |
| Albumin, g/L | 32 (26-43) | 37 (36.7-56) | .018 | 39.5 (36-56) | .007 |
| IgG, g/L | 21.7 (14.4-43.8) | 12.4 (7.3-20.3) | .012 | 14.5 (7.9-24.6) | .002 |
| Characteristics . | Baseline, median (range) . | Week 24, median (range) . | P* . | Last follow-up, median (range) . | P* . |
|---|---|---|---|---|---|
| Symptom score | 16 (7-38) | 1 (0-6) | .003 | 0.5 (0-6) | .002 |
| IL-6, pg/mL | 28.6 (7.4-865) | 10.0 (2-23.5) | .012 | 3.7 (2-25.1) | .002 |
| Hemoglobin, g/L | 98 (76-133) | 125.5 (93-150) | .093 | 132 (93-177) | .013 |
| CRP, mg/L | 41.6 (0.2-185.6) | 12.4 (0.8-54.8) | .028 | 3.2 (0.7-53.0) | .005 |
| ESR, mm/h | 90 (6-115) | 31 (5-125) | .042 | 16 (1-125) | .013 |
| Albumin, g/L | 32 (26-43) | 37 (36.7-56) | .018 | 39.5 (36-56) | .007 |
| IgG, g/L | 21.7 (14.4-43.8) | 12.4 (7.3-20.3) | .012 | 14.5 (7.9-24.6) | .002 |
Compared with baseline.
The trend of biochemical parameters and symptom score after treatment. Change in median hemoglobin level (A), IL-6 (B), CRP (C), IgG (D), and symptom score (E) over time in responders (blue line) and nonresponders (red line). Median and range are labeled with dots and dashed lines except for month 36 (only 1 patient was followed to month 36, so no range is available).
The trend of biochemical parameters and symptom score after treatment. Change in median hemoglobin level (A), IL-6 (B), CRP (C), IgG (D), and symptom score (E) over time in responders (blue line) and nonresponders (red line). Median and range are labeled with dots and dashed lines except for month 36 (only 1 patient was followed to month 36, so no range is available).
Three patients (12%) experienced SD, and 10 patients were evaluated as treatment failure. The median time to treatment failure was 18 weeks (4-24 weeks) for these patients. During the treatment period, 2 deaths occurred. One patient, who had the TAFRO syndrome subtype, died of disease progression (severe anemia) at week 12; another patient died due to pulmonary infection at week 4. A third patient, who failed treatment and discontinued the study regimen at month 3, died due to pulmonary infection at month 6 while on lenalidomide-dexamethasone; this event was not attributed to the TCP regimen. The change in clinically relevant parameters (baseline vs last follow-up) for the 13 nonresponders is listed in Table 4 (comparisons between baseline and week 24 are also provided in Table 4). Despite not achieving response criteria, these patients still experienced a significant improvement in median albumin level and IgG level with treatment; none of the other parameters improved significantly. The changes over time in hemoglobin (Figure 2A), IL-6 (Figure 2B), CRP (Figure 2C), IgG (Figure 2D), and symptom score (Figure 2E) in responders and nonresponders are provided in Figure 2.
Characteristics at baseline, at week 24, and at last follow-up for nonresponders (n = 13)
| Characteristics . | Baseline, median (range) . | Week 24, median (range) . | P* . | Last follow-up, median (range) . | P* . |
|---|---|---|---|---|---|
| Symptom score | 13 (6-41) | 7 (3-13) | .109 | 8 (3-20) | .212 |
| IL-6, pg/mL | 20.7 (7.7-61.9) | 29.5 (8.8-42.8) | .735 | 29.7 (2.1-70.3) | 1.000 |
| Hemoglobin, g/L | 102.5 (73-141) | 86 (67-133) | .753 | 109 (67-135) | .374 |
| CRP, mg/L | 85.7 (1.6-119.2) | 93.0 (40.5-112.2) | .463 | 73.3 (2.6-176.0) | .779 |
| ESR, mm/h | 102 (2-140) | 102.5 (27-140) | .500 | 90 (11-140) | .161 |
| Albumin, g/L | 31 (24-43) | 33 (31-41) | .043 | 35.5 (27-46) | .018 |
| IgG, g/L | 36.5 (5.3-85.1) | 21.9 (7.5-44.1) | .028 | 21.7 (5.3-35.9) | .008 |
| Characteristics . | Baseline, median (range) . | Week 24, median (range) . | P* . | Last follow-up, median (range) . | P* . |
|---|---|---|---|---|---|
| Symptom score | 13 (6-41) | 7 (3-13) | .109 | 8 (3-20) | .212 |
| IL-6, pg/mL | 20.7 (7.7-61.9) | 29.5 (8.8-42.8) | .735 | 29.7 (2.1-70.3) | 1.000 |
| Hemoglobin, g/L | 102.5 (73-141) | 86 (67-133) | .753 | 109 (67-135) | .374 |
| CRP, mg/L | 85.7 (1.6-119.2) | 93.0 (40.5-112.2) | .463 | 73.3 (2.6-176.0) | .779 |
| ESR, mm/h | 102 (2-140) | 102.5 (27-140) | .500 | 90 (11-140) | .161 |
| Albumin, g/L | 31 (24-43) | 33 (31-41) | .043 | 35.5 (27-46) | .018 |
| IgG, g/L | 36.5 (5.3-85.1) | 21.9 (7.5-44.1) | .028 | 21.7 (5.3-35.9) | .008 |
Compared with baseline.
Safety
With regards to safety, 1 patient died of pulmonary infection, as described in the previous section, and 1 patient suffered from a grade 3 rash. No other patients experienced grade 3 or higher adverse events. Grade 1 or 2 constipation (40%), pruritus (20%), rash (16%), peripheral sensory neuropathy (16%), and nausea (16%) were the most common adverse events. Other documented adverse events included irregular menses (12.5% among female patients), glucose intolerance (12%), alanine aminotransferase elevation (12%), pneumonitis (12%), decreased libido (12%), leukopenia (8%), peripheral neuropathy (motor) (8%), erectile dysfunction (5.8% among male patients), neutropenia (4%), and hepatitis B virus reactivation (4%). The summary of adverse events following initiation of the TCP regimen are listed in Table 5. No thrombotic events were observed during follow-up and no bleeding events were documented.
Summary of adverse events
| Adverse events . | N (%) . |
|---|---|
| Hematological toxicity | |
| Leukopenia | |
| Grade 1 | 2 (8) |
| Neutropenia | |
| Grade 1 | 1 (4) |
| Nonhematological toxicity | |
| Rash | |
| Grade 1 | 3 (12) |
| Grade 3 | 1 (4) |
| Pruritus | |
| Grade 1 | 5 (20) |
| Constipation | |
| Grade 1 | 8 (32) |
| Grade 2 | 2 (8) |
| Irregular menses* | |
| Grade 1 | 1 (12.5) |
| Grade 2 | 1 (12.5) |
| Decreased libido | |
| Grade 1 | 3 (12) |
| Erectile dysfunction† | |
| Grade 1 | 1 (5.8) |
| Pneumonitis | |
| Grade 1 | 1 (4) |
| Grade 2 | 1 (4) |
| Grade 5 | 1 (4) |
| Neuropathy: motor | |
| Grade 1 | 2 (8) |
| Neuropathy: sensory | |
| Grade 1 | 2 (8) |
| Grade 2 | 2 (8) |
| HBV reactivation | 1 (4) |
| Nausea | |
| Grade 1 | 4 (16) |
| Glucose intolerance | |
| Grade 1 | 2 (8) |
| Grade 2 | 1 (4) |
| ALT elevation | |
| Grade 1 | 3 (12) |
| Adverse events . | N (%) . |
|---|---|
| Hematological toxicity | |
| Leukopenia | |
| Grade 1 | 2 (8) |
| Neutropenia | |
| Grade 1 | 1 (4) |
| Nonhematological toxicity | |
| Rash | |
| Grade 1 | 3 (12) |
| Grade 3 | 1 (4) |
| Pruritus | |
| Grade 1 | 5 (20) |
| Constipation | |
| Grade 1 | 8 (32) |
| Grade 2 | 2 (8) |
| Irregular menses* | |
| Grade 1 | 1 (12.5) |
| Grade 2 | 1 (12.5) |
| Decreased libido | |
| Grade 1 | 3 (12) |
| Erectile dysfunction† | |
| Grade 1 | 1 (5.8) |
| Pneumonitis | |
| Grade 1 | 1 (4) |
| Grade 2 | 1 (4) |
| Grade 5 | 1 (4) |
| Neuropathy: motor | |
| Grade 1 | 2 (8) |
| Neuropathy: sensory | |
| Grade 1 | 2 (8) |
| Grade 2 | 2 (8) |
| HBV reactivation | 1 (4) |
| Nausea | |
| Grade 1 | 4 (16) |
| Glucose intolerance | |
| Grade 1 | 2 (8) |
| Grade 2 | 1 (4) |
| ALT elevation | |
| Grade 1 | 3 (12) |
ALT, alanine aminotransferase; HBV, hepatitis B virus.
Among 8 female patients.
Among 17 male patients.
Survival
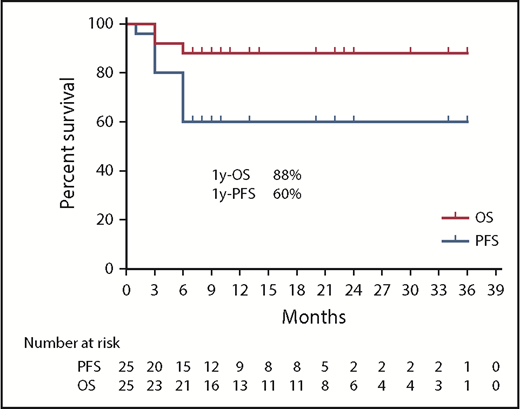
The median duration of follow-up was 14 months (6-36 months) from the start of treatment. For patients who achieved durable tumor and symptomatic response, no relapses of disease were observed during follow-up. Median OS and PFS were not reached, with an estimated 1-year PFS and OS of 60.0% and 88.0%, respectively (Figure 3).
Discussion
We report the results of the first phase 2 trial for the treatment of newly diagnosed iMCD patients with an oral treatment regimen. This is also the first iMCD clinical trial performed of agents directed against targets other than the IL-6–signaling pathway. The TCP regimen induced durable tumor and symptomatic responses in 48% of patients for at least 24 weeks, significantly reduced symptoms, and significantly improved laboratory parameters.
Considering that a durable tumor and symptomatic response in 34% of patients in the phase 2 RCT of siltuximab led to regulatory approvals worldwide, the 48% response rate observed with the TCP regimen is a meaningful clinical benefit. Furthermore, 0% of patients in the placebo arm of the phase 2 RCT of siltuximab achieved this response criteria, suggesting that the 48% overall response rate observed in this study is likely due to treatment and not placebo effect. The first-ever expert consensus iMCD treatment guidelines20 recommend siltuximab as first-line therapy for all iMCD patients based on the phase 1 and phase 2 RCT data as well as real-world evidence.21 Importantly, the TCP regimen is less expensive, an oral alternative, and potentially more accessible than siltuximab in certain regions of the world. The oral treatment modality could also have an important benefit of convenience over the frequent infusions needed with other iMCD treatments. Moreover, for patients who responded on the TCP regimen, we observed a lasting effect (Figure 2), which was reflected by the improvement of symptom score and laboratory parameters over time; no responders went on to relapse. Additional follow-up is needed to assess whether 2 years of the TCP regimen is sufficient to induce a continued response after the regimen is discontinued. Even when combining the entire study population (including patients with treatment failure and SD) for analysis, the median symptom score, IL-6 level, and other key laboratory parameters improved significantly.
Another key finding of this study is that a large proportion (6 of 10) of patients with the HV histopathological subtype of iMCD responded to the TCP regimen. As 0% (0 of 18) of the iMCD patients with HV histopathology responded to siltuximab in the phase 2 RCT,7 this observation (though based on a small sample size) may suggest that the TCP regimen is an important treatment option for these patients. However, 5 of 18 patients with HV histopathology met different response criteria in the phase 1 study of siltuximab,22 thus the influence of histopathology on anti–IL-6 response rate is unclear. Thalidomide’s antiangiogenic effect via VEGF inhibition may play an important role in these HV patients whose lymph nodes are highly vascularized.23 The TCP regimen was also effective in iMCD patients with PC and mixed histopathology, possibly related to plasma cell targeting with thalidomide and T-cell targeting with cyclophosphamide.
The TCP regimen was also well tolerated. Several anticipated side effects, such as GI disturbances, dermatologic issues, glucose intolerance, and peripheral neuropathy were observed at low grades. Importantly, no thrombotic events, bleeding events, somnolence, or unintended pregnancies were reported. Less than 10% of patients experienced cytopenias or infections.
Morra et al recently demonstrated that response to siltuximab in the phase 2 RCT was strongly associated with abnormal laboratory parameters indicative of an inflammatory response, such as CRP, fibrinogen, and albumin.24 In this study, response to the TCP regimen was associated with fever (another parameter of inflammation) and absence of pulmonary involvement. The TCP regimen may therefore represent a treatment approach with applicability in iMCD patients with a hyperinflammatory state and without pulmonary involvement.
Recent studies have demonstrated that IL-6 is not highly elevated nor likely to be the disease driver in all iMCD patients.25 Thus, treatments directed at targets other than IL-6 need to be investigated. The TAFRO syndrome is a newly recognized clinical subtype of iMCD with inferior survival, where IL-6 does not appear to be a major pathogenic driver in all cases.19,26,27 We tried the TCP regimen in 2 TAFRO-iMCD patients. One patient died of progression of disease and the other achieved durable tumor and symptomatic response. Investigation of the TCP regimen is needed in more iMCD cases with TAFRO syndrome as well as cases who fail to respond to anti–IL-6 therapy.
This study has several limitations. First, this is a single-center trial with a small number of patients from a single ethnic group. Biases from the investigators may exist with regards to pathological review and diagnosis, which could affect subject enrollment. However, cases were rereviewed in light of the recently published consensus diagnostic criteria and found to be consistent. Also, considering the rarity of the target disease and previous clinical studies in iMCD, the sample size is sufficient to assess the efficacy and safety of the TCP regimen in iMCD. Second, no control arm was included. As siltuximab is not available in China, placebo would be unethical given that there are already approved treatments for iMCD, and as iMCD’s clinical course is quite severe, an optimal control arm was not available. Because we used the same response criteria as the phase 2 RCT of siltuximab, the 0% response rate in the placebo arm of the RCT is likely to represent what we would have observed. Third, though an international working group recently agreed on consensus response criteria,20 no consensus response criteria existed when designing this study. Thus, we used the criteria from the phase 2 registrational study of siltuximab and presented a number of biochemical parameters as secondary end points. Lastly, the observation period is relatively short. Thus, long-term sequalae of exposure to the TCP regimen in iMCD are not known. As such, cyclophosphamide, an alkylating agent that can increase the risk of myelodysplastic syndrome and/or acute myeloid leukemia,28 should be used with caution. We will continue to collect data and extend the time of follow-up to determine the long-term survival benefit and risks of this regimen, particularly after treatment is discontinued.
In conclusion, the TCP regimen, an inexpensive oral therapy, is an effective and safe treatment option for newly diagnosed iMCD patients, particularly when siltuximab is not available.
Data sharing statement: Individual participant data will not be available. Study protocol will be available beginning 9 months and ending 36 months following article publication at pumczhanglu@126.com.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the patients and their families.
This work was supported by institutional research funding provided by the Fundamental Research Funds for the Central Universities (grant no. 3332018036) (L.Z.), the National Natural Science Foundation of China (grant no. 81570195) (J.L.), the Beijing Natural Science Foundation (grant no. 7182128) (J.L.), the Foundation for Distinguished Young Physician of Peking Union Medical College Hospital (grant nos. JQ201501 [J.L.] and JQ201508 [L.Z.]), the CAMS Innovation Fund for Medical Sciences (grant no. 2016-12M-1-002) (J.L.), and the National Key Research and Development Program of China (grant no. 2016YFC0901503) (J.L.).
Authorship
Contribution: L.Z., J.L., and D.-b.Z. designed the study; L.Z., M.-h.D., X.-x.C., J.F., and J.L. recruited patients; A.-l.Z. and L.Z. collected the data and performed the analyses; Z.-y.L. and D.-r.Z. reviewed the pathology; L.Z., A.-l.Z., J.L., and D.C.F. interpreted the data and wrote the manuscript; and all authors had access to primary clinical trial data and gave final approval to submit for publication.
Conflict-of-interest disclosure: D.C.F. receives research funding from Janssen Pharmaceuticals. The remaining authors declare no competing financial interests.
Correspondence: Jian Li, Department of Hematology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100730, China; e-mail: lijian@pumch.cn.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal