Abstract
Background: Patients (pts) with polycythemia vera (PV) and essential thrombocythemia (ET) suffer from difficult MPN associated symptoms which impact quality of life (QoL). The impact of initial cytoreduction on ET/PV symptoms, resulting toxicity and QoL have not been prospectively studied.
Methods: MPD-RC 112 trial (NCT01258856) randomized 168 therapy-naive (hydroxyurea [HU] <3 mo) high risk ET/PV pts (<5 years of disease) to response adjusted pegylated interferon alpha 2a (PEG) or HU. MPN symptoms, in depth toxicity assessment, and QoL were measured by two symptom instruments (MPN-SAF, EORTC QLQ-C30: baseline,3, 6, 9, and 12 mo) and toxicity questions. Blinded response review by European Leukemia Net (ELN) hematological responses (HR) was conducted at 12 months. Individual time point and longitudinal group comparisons were based on mixed models adjusting for age.
Results:
Patients: Of 168 randomized pts (86 HU, 82 PEG), 164 (98%) completed the survey package at baseline and 151 (HU 83%; PEG 98%) completed at least 1 survey during treatment. Median age was 61 (range 18-87) with 70 (42%) females; 81 (48%) / 87 (52%) with ET / PV; 46 (27%) had a history of a thrombosis. 119 (71%) received prior HU (up to 3 months). Baseline characteristics were balanced between arms with the exception of age (median HU 63 vs. PEG 60 yrs; p=0.02).
BaselineSymptom Burden/QoL: Mean MPN-SAF Total Symptom Score (TSS), scale 0 [absent]-100 [worst imaginable] was 15.8 (SD 12.6; range 0-62.0) with means of 15.1 (SD 12.6) / 16.5 (SD 12.6) for ET / PV which were slightly lower than reported means of a previous cohort receiving any line of treatment (Scherber RM, JCO 2012). Median number of moderate (defined as score >=3) symptoms in ET patients was 3.0 and PV 4.0. Most common moderate symptoms were fatigue (65.9%), insomnia (37.2%), numbness (33.5%) , itching (29.9%), dizziness (27.4%), early satiety (26.8%) , headache (23.2%), sad mood (23.2%), inactivity (22%), concentration (20.7%). 50.6% of patients had one or more symptom with a score >=6. Mean QLQ-C30 global health status/QoL (GHS/QoL) was 70.8 (SD 21.9) - comparable to a general population (mean 71.2, SD 22.4) and better than a general cancer population (mean 61.3, SD 24.2; QLQ-C30 Reference Manual 2008). At baseline, TSS, symptoms, and QoL were similar between treatment arms. In patients with TSS >20 at baseline (highly symptomatic, n=50; ET:20, PV:30), median TSS was 28.0 (range 20.0 to 62.0); the highest scored symptom was fatigue (median=7.0), itching (median=4.5) and insomnia (median=4.0).
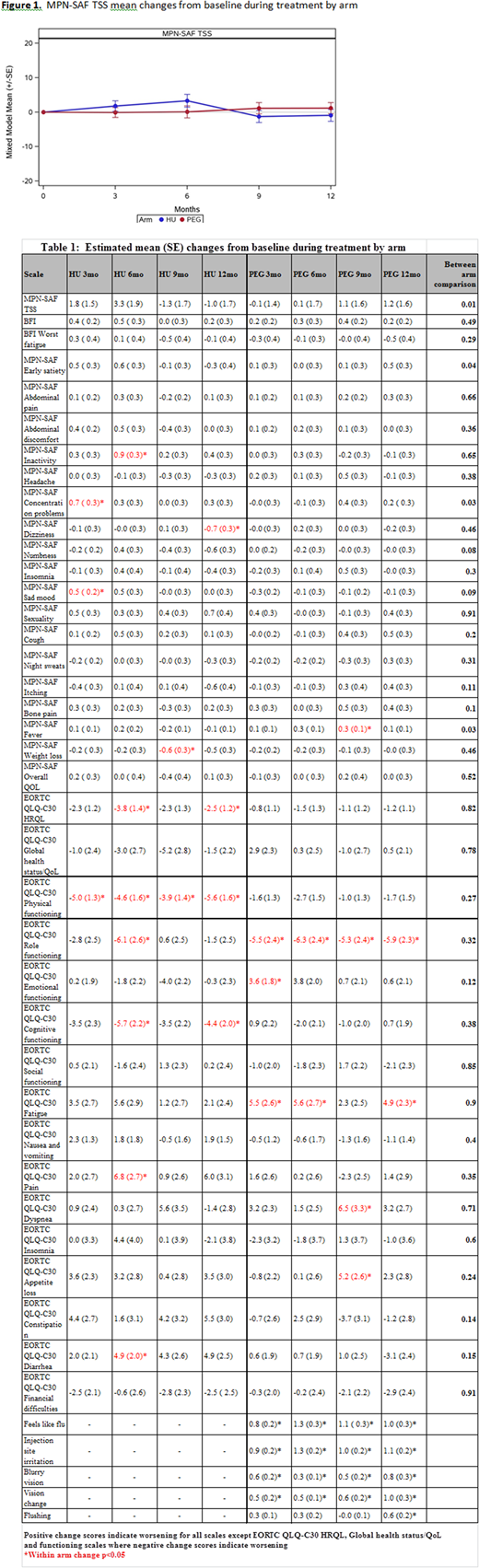
Impact of Therapy onSymptom Burden/QoL: On HU, pts experienced worsening QoL (physical, cognitive functioning, HRQoL) and some persistent or transient worsened symptoms (inactivity, concentration (p<0.05) (Table 1)). On PEG, pts experienced worsening of fever, dyspnea, appetite loss and PEG-related symptoms including flu-like symptoms, injection site irritation, blurry vision, and visual changes (all p<0.05), but not sad mood (not corrected for antidepressants). In comparing arms, change in TSS significantly differed (p=0.01) between arms with increasing symptoms on HU vs PEG at 3 and 6 mo, but lower symptom burden at 9 and 12 mos (Figure 1). In highly symptomatic pts (with a baseline TSS > 20, n=50), improvements in weight loss, abdominal discomfort, fatigue, itching, fever, early satiety (12 m.) were observed in pts on HU. For PEG, in highly symptomatic pts, improvements were observed for overall TSS score, fatigue, inactivity, night sweats, and itching (with similar toxicities to all PEG patients).
ELN Response and Symptom Burden/QoL: Among the 133 pts with 12-mo symptom data, complete hematologic response (CHR) rate was 43%. Inactivity and sad mood were worse in those patient achieving a CHR (inactivity: CHR worsening 0.68 vs PHR/NR better 0.26; p=0.03; sad mood: CHR worsening 0.67 vs PHR/NR better 0.67, p=0.002)). These latter changes were not related to higher doses utilized (in either arm), suggesting obtaining CHR may have negative effects on patient symptoms.
Conclusions:In pts with a significant baseline MPN symptom burden, cytoreductive therapy has both a beneficial impact on baseline MPN symptom burden but also leads to therapy associated toxicity, although different patterns of efficacy and toxicity between HU and PEG. Achievement of a CHR in PV/ET may be associated with higher rates of drug related toxicities, and the value of achieving CHR may need further validation.
Mesa:Incyte Corporation: Research Funding; CTI Biopharma: Research Funding; Gilead: Research Funding; NS Pharma: Research Funding; Promedior: Research Funding; Novartis: Consultancy; Celgene: Research Funding; UT Health San Antonio - Mays Cancer Center: Employment; Genentech: Research Funding; Pfizer: Research Funding. Mascarenhas:Merck: Research Funding; CTI Biopharma: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees; Roche: Research Funding; Promedior: Research Funding; Incyte: Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Research Funding; Janssen: Research Funding. Rambaldi:Omeros: Consultancy; Roche: Consultancy; Celgene: Consultancy; Pfizer: Consultancy; Novartis: Consultancy; Italfarmaco: Consultancy; Amgen Inc.: Consultancy. Yacoub:Novartis: Honoraria, Speakers Bureau; Cara Therapeutics: Equity Ownership; Ardelyx, INC.: Equity Ownership; Inycte: Honoraria, Speakers Bureau; Seattle Genetics: Honoraria, Speakers Bureau; Dynavax: Equity Ownership. Harrison:Gilead: Honoraria, Speakers Bureau; CTI BioPharma: Consultancy, Honoraria; Roche: Consultancy, Honoraria; Novartis: Consultancy, Honoraria, Research Funding, Speakers Bureau; Celgene: Consultancy, Honoraria, Speakers Bureau. Kiladjian:AOP Orphan: Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees. Mead:Elstar: Research Funding; Celgene: Research Funding; ARIAD: Consultancy; Cell Therapeutics: Consultancy; Evotek: Research Funding; Novartis: Consultancy, Honoraria, Research Funding, Speakers Bureau; Bristol-Myers Squibb: Consultancy. Kessler:Sangamo: Research Funding; Octapharma: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novo Nordisk: Honoraria, Research Funding; Dimension Advisory boards: Membership on an entity's Board of Directors or advisory committees; Bayer: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; DSMB: Membership on an entity's Board of Directors or advisory committees; Genentech: Research Funding; Biomarin: Research Funding. Kremyanskaya:Incyte: Research Funding. Rampal:Incyte: Honoraria, Research Funding; Constellation: Research Funding; Jazz: Consultancy, Honoraria; Stemline: Research Funding; Celgene: Honoraria. Hoffman:Incyte: Research Funding; Janssen: Research Funding; Merus: Research Funding; Formation Biologics: Research Funding; Summer Road: Research Funding. Dueck:Pfizer: Honoraria; Bayer: Employment; Phytogine: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal