Key Points
Recipient macrophages persist in hematopoietic tissues and self-repopulate via in situ proliferation after syngeneic transplantation.
Targeted depletion of recipient CD169+ macrophages after transplant impaired long-term bone marrow engraftment of hematopoietic stem cells.
Abstract
Distinct subsets of resident tissue macrophages are important in hematopoietic stem cell niche homeostasis and erythropoiesis. We used a myeloid reporter gene (Csf1r-eGFP) to dissect the persistence of bone marrow and splenic macrophage subsets following lethal irradiation and autologous hematopoietic stem cell transplantation in a mouse model. Multiple recipient bone marrow and splenic macrophage subsets survived after autologous hematopoietic stem cell transplantation with organ-specific persistence kinetics. Short-term persistence (5 weeks) of recipient resident macrophages in spleen paralleled the duration of extramedullary hematopoiesis. In bone marrow, radiation-resistant recipient CD169+ resident macrophages and erythroid-island macrophages self-repopulated long-term after transplantation via autonomous cell division. Posttransplant peak expansion of recipient CD169+ resident macrophage number in bone marrow aligned with the persistent engraftment of phenotypic long-term reconstituting hematopoietic stem cells within bone marrow. Selective depletion of recipient CD169+ macrophages significantly compromised the engraftment of phenotypic long-term reconstituting hematopoietic stem cells and consequently impaired hematopoietic reconstitution. Recipient bone marrow resident macrophages are essential for optimal hematopoietic stem cell transplantation outcomes and could be an important consideration in the development of pretransplant conditioning therapies and/or chemoresistance approaches.
Introduction
Successful hematopoietic stem cell (HSC) transplantation requires effective homing and lodgment of HSCs within bone marrow (BM) and subsequent sustained engraftment of cells to drive hematopoietic reconstitution (active HSC pool) and sustain lifelong hematopoiesis (quiescent HSC pool).1 The BM environment contributes to successful HSC transplantation outcomes.2 HSCs at steady state reside in specialized niches3 that influence HSC homeostasis and fate decisions.3 Within hematopoietic niches, macrophages (Mφs) interact directly with HSCs4 and other nonhematopoietic niche cells5-8 to regulate niche homeostasis. BM Mφs are heterogeneous in location, function, and phenotype. Distinction of Mφ subpopulations, as well as segregation from monocytes and other myeloid cells, requires multiplexed strategies. Erythroid island Mφs (EIMs),9 osteal Mφs,10 and HSC niche Mφs7 are distinct BM Mφ populations. The specific identity of HSC niche Mφs remains ambiguous (reviewed in Kaur et al11 ).
HSC transplantation involves ablation of recipient hematopoietic tissue using radiation and/or chemotherapeutic agents. In mice and humans, resident/stromal Mφs in many tissues and organs are resistant to radiation12-14 and myeloablative doses of cyclophosphamide.15 There is a correlation between resident Mφ replenishment and HSC restoration after a single dose of 5-fluorouracil in mice.4 These observations suggest that some Mφs persist after myeloablative conditioning and contribute to HSC niche reestablishment after BM injury, including in BM transplantation.
The current study aimed to characterize HSC niche Mφs, their resilience and capacity for self-repopulation after autologous HSC transplantation, and their contribution to successful persistent engraftment of long-term reconstituting donor HSCs.
Material and methods
Mice
Procedures complied with the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes and were approved by The University of Queensland Health Sciences Ethics Committee. Mixed-sex 8- to 9-week-old Csf1r-eGFP (MacGreen) mice,16 B6.SJL Ptprca mice, ubiquitin C promoter (UBC)–GFP mice and heterozygous CD169-DTR (diphtheria toxin receptor) mice (CD169DTR/+, sourced from Riken Bio Resource Centre17 ) were supplied from in-house breeding colonies. C57BL/6 mice were sourced from the Australian Resources Centre (Western Australia, Australia).
Isolation of lineage−Sca-1+Kit+ (LSK) hematopoietic stem and progenitor cells
BM was harvested and enriched for c-Kit+ (CD117) cells using magnetic-activated cell sorting (Miltenyi Biotec) as previously described.18 Staining antibody cocktails similar to those previously described19 were used to gate on immune LSK cells, which were sorted using a MoFlo Astrios cell sorter (Beckman Coulter, Brea, CA).
Irradiation, transplantation, and cell and tissue collection
All recipient mice were lethally irradiated using a 137Cs γ Cell 40 Exactor Irradiator (Nordion, Canada) with 11.5 Gy in 2 split doses (4 hours apart). MacGreen mice were transplanted (via IV injection) with 1000 sorted LSK cells plus 2 × 105 irradiated (15 Gy) blood cells from syngeneic B6.SJL Ptprca donor mice. CD169-DTR and C57BL/6 mice were transplanted with 1000 sorted LSK plus 2 × 105 irradiated blood cells from UBC-GFP mice.
Cells and tissue were collected from transplanted and age-matched naive mice as indicated in the figures. BM was flushed to generate BM single-cell suspensions. Spleens were dissected and weighed, and a portion of each spleen was dissociated using a gentle MACS dissociator (Miltenyi Biotec, Cologne, Germany).20 For in situ analysis, left hind-limbs were fixed using 4% PFA for 24 hours, decalcified using 14% EDTA for at least 2 weeks, and then processed using a Shandon Pathcentre Processor (Thermo Electron Corporation, Waltham, MA) and paraffin embedded. Four-micrometer serial sections were cut using a microtome (Leica Biosystems, Wetzlar, Germany) and then deparaffinized and rehydrated.
Selective in vivo depletion of recipient Mφs in chimeric CD169-DTR mice and competitive reconstitution assays
Chimeric CD169-DTR or C57Bl/6 male primary recipient mice were treated with diphtheria toxin (DT; 10 µg/kg) or saline via intraperitoneal injection (total of 4 injections, 3 days apart) initiated 3 weeks posttransplant. Tissues and cells were collected at 5 weeks posttransplant. BM was harvested from chimeric saline- or DT-treated CD169-DTR and C57Bl/6 primary recipient or naive C57Bl/6 mice as described previously.19 BM cells (2 × 105) from individual primary recipient mice were mixed with 2 × 105 BM cells from naive C57Bl/6 donors to generate a competitive secondary graft. The secondary grafts were injected IV into lethally irradiated 8- to 10-week-old C57Bl/6 secondary recipient mice. Blood was collected at 8, 12, and 16 weeks posttransplant to quantify chimerism.
Flow cytometry
Flow cytometry was performed on BM, spleens, and blood cell suspensions. All antibodies were purchased from BioLegend (San Diego, CA) or BD Biosciences (Franklin Lakes, NJ) except anti-ER-HR3 biotin, which was purchased from Novus biologicals (Littleton, CO). For myeloid lineage phenotyping, 106 BM or spleen cells were resuspended in CD16/CD32 hybridoma 2.4G2 supernatant. Cells were then stained with the following antibody cocktail: anti-CD11b brilliant violet 605, anti-F4/80 Alexa Fluor 647, anti-Ly6G-PECy7, anti-VCAM-1 Pacific blue, anti-ER-HR3 biotin and anti-CD169 phycoerythrin primary antibodies, with respective isotope stain performed in parallel, for 40 min at 4°C. Hematopoietic stem and progenitor cell (HSPC) phenotyping was performed similar to previously described with only minor fluorophore modifications.19 Prior to analysis, 5 μg/mL 7-amino actinomycin D (Life Technologies) was added to all samples. Analysis was performed on Beckman Coulter’s CyA ADP Analyzer (Beckman Coulter, Brea, CA). Data analysis was performed on singlet events and with dead cells excluded using FlowJo software version 8.8.7 (Tree Star Data Analysis Software; Tree Star, Ashland, OR). Gated population percent frequency conversion to absolute cell number was achieved using a standard approach. Cell cycle assessment was performed using a flow cytometry approach as described previously.21
Immunohistochemistry and in vivo imaging
Serial sections from paraffin-embedded bones were stained with rat anti-F4/80 (Abcam, Cambridge, United Kingdom), rat anti-ER-HR3 (Novus Biologicals), or rabbit anti-Ki-67 (Abcam) monoclonal antibodies or rabbit anti-GFP (Invitrogen) polyclonal antibody. Antigen retrieval was performed using 0.3% trypsin digestion (anti-ER-HR3), citrate buffer heat retrieval (anti-F4/80 and anti-Ki-67), or 0.05 μg/mL proteinase K digestion (anti-GFP). Primary antibodies were detected using biotinylated anti-rat or anti-rabbit secondary antibody followed by horseradish peroxidase-conjugated streptavidin (Dako, Glostrup, Denmark) and detected using diaminobenzidine chromogen (Dako). Sections were hematoxylin counterstained, mounted, and analyzed using an Olympus Bx50 microscope and Cell Sens standard software 7.1 (Olympus, Tokyo, Japan).
Quantification and statistical analysis
Statistically significance was determined using either an unpaired Student t test or 1-way analysis of variance (ANOVA) Tukey’s multiple comparison test with PRISM 6 (GraphPad Software version 6.04; GraphPad, La Jolla, CA). P < .05 was considered significantly different. In all cases, data are presented as mean ± standard deviation (SD).
Results
Recipient BM resident Mφs persist after lethal irradiation and expand in a model of autologous transplantation
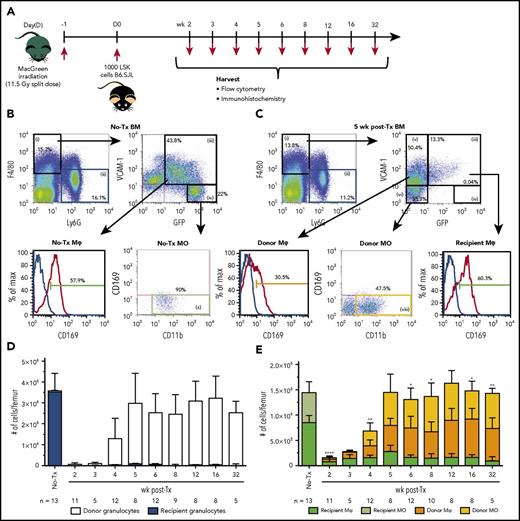
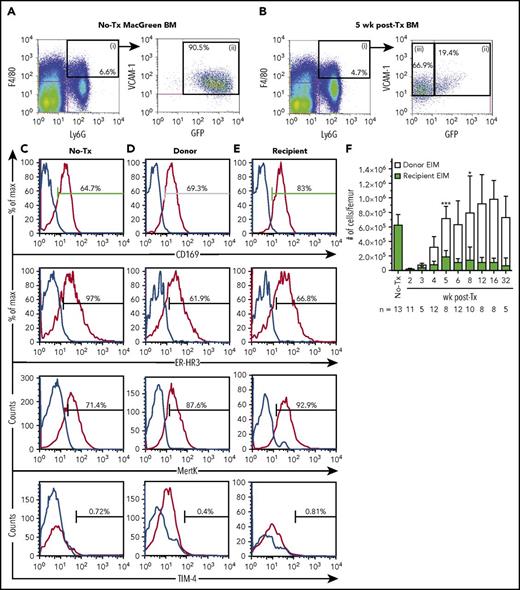
The Csf1r-eGFP reporter transgene (MacGreen mice), which labels all myeloid cells,16 was used to distinguish recipient and donor Mφs in a BM transplantation model (Figure 1A). Mature myeloid cells within BM of transplanted MacGreen mice were segregated based upon cell surface marker profiles as summarized in Figures 1 and 3 as well as Table 1, with successful delineation of mature myeloid cells including segregation of a BM resident Mφ subset that was distinct from EIMs.22 Granulocytes are radiation sensitive,23 have a short half-life,24 and express GFP in naive MacGreen mice25 (Figure 1B, F4/80negLy6G+ gate ii; Figure 1D). Therefore, F4/80negLy6G+ granulocyte (Figure 1B-C, gate ii) chimerism was used to evaluate myeloablation efficiency and consequently the validity of GFP expression to accurately segregate myeloid cell origin. Only transplanted MacGreen mice with ≥99% GFPneg donor-derived granulocyte chimerism in BM (Figure 1D) were included in the study. Importantly, recipient-derived GFP+ granulocytes did not reemerge in BM up to 32 weeks posttransplantation (Figure 1D). Donor GFPneg granulocytes were reliably detected in BM starting 4 weeks posttransplant and were restored to pretransplant levels by 5 weeks (Figure 1D).
Recipient BM Mφs persist and expand posttransplantation. (A) Schematic of the experimental BM transplantation model. Lethally irradiated MacGreen recipient mice were transplanted with 1000 syngeneic B6.SJL LSK cells and harvested at indicated time points posttransplant. (B-C) Representative flow cytometry analysis of naive (B; no-Tx) and posttransplant (C; 5 weeks shown) recipient MacGreen BM (see supplemental Figure 2A-C for representative isotype control staining). F4/80+Ly6Gneg cells (gate i) were subsequently gated into VCAM-1+GFP+ cells (gate iii) that were predominantly CD169+ BM Mφ (dark green gate), VCAM-1negGFPhi cells (gate iv) consisted of CD169neg recipient monocytes (light green gate x), VCAM-1+GFPneg cells (gate v) consisted of donor BM CD169+ Mφs (orange gate), and VCAM-1negGFPneg cells (gate vi) contained CD169neg donor monocytes (yellow gate viii). Granulocytes were gated as F4/80negLy6G+ cells (gate ii), which in BM were uniformly GFP+ or GFPneg in no-Tx and posttransplant samples, respectively (not depicted). (D) Number of recipient (blue bars) and donor (white bars) granulocytes (gate ii) per femur across the transplantation time course. (E) Total number of recipient (dark green bars) and donor (orange bars) Mφs and recipient (light green bars) and donor (yellow bars) monocytes per femur across the transplantation time course. In panels D and E, data are presented as mean ± SD. Statistical analysis of recipient Mφs was performed using 1-way ANOVA Tukey’s multiple comparison test. In panel E, asterisks represent significant differences in recipient Mφ number compared with 5 weeks posttransplant (*P < .05, **P < .01, and ****P < .0001). Flow cytometry plots and histograms as well as associated gated cell percent frequencies are from a representative animal, and the percentage of positive cells is based on the preceding parent populations. Data are representative of 5 to 13 individual animal samples per time point from either 1 (weeks 3 and 32), 2 (week 5), or 3 (all other time points) biological replicate experiments. 300 000 events were collected from each experiment sample by flow cytometry. MO, monocyte; Tx, transplant.
Recipient BM Mφs persist and expand posttransplantation. (A) Schematic of the experimental BM transplantation model. Lethally irradiated MacGreen recipient mice were transplanted with 1000 syngeneic B6.SJL LSK cells and harvested at indicated time points posttransplant. (B-C) Representative flow cytometry analysis of naive (B; no-Tx) and posttransplant (C; 5 weeks shown) recipient MacGreen BM (see supplemental Figure 2A-C for representative isotype control staining). F4/80+Ly6Gneg cells (gate i) were subsequently gated into VCAM-1+GFP+ cells (gate iii) that were predominantly CD169+ BM Mφ (dark green gate), VCAM-1negGFPhi cells (gate iv) consisted of CD169neg recipient monocytes (light green gate x), VCAM-1+GFPneg cells (gate v) consisted of donor BM CD169+ Mφs (orange gate), and VCAM-1negGFPneg cells (gate vi) contained CD169neg donor monocytes (yellow gate viii). Granulocytes were gated as F4/80negLy6G+ cells (gate ii), which in BM were uniformly GFP+ or GFPneg in no-Tx and posttransplant samples, respectively (not depicted). (D) Number of recipient (blue bars) and donor (white bars) granulocytes (gate ii) per femur across the transplantation time course. (E) Total number of recipient (dark green bars) and donor (orange bars) Mφs and recipient (light green bars) and donor (yellow bars) monocytes per femur across the transplantation time course. In panels D and E, data are presented as mean ± SD. Statistical analysis of recipient Mφs was performed using 1-way ANOVA Tukey’s multiple comparison test. In panel E, asterisks represent significant differences in recipient Mφ number compared with 5 weeks posttransplant (*P < .05, **P < .01, and ****P < .0001). Flow cytometry plots and histograms as well as associated gated cell percent frequencies are from a representative animal, and the percentage of positive cells is based on the preceding parent populations. Data are representative of 5 to 13 individual animal samples per time point from either 1 (weeks 3 and 32), 2 (week 5), or 3 (all other time points) biological replicate experiments. 300 000 events were collected from each experiment sample by flow cytometry. MO, monocyte; Tx, transplant.
Detail phenotype of BM myeloid cells in naive MacGreen mice
| Antigens . | BM resident Mφs . | EIMs . | BM monocytes . | Granulocytes . |
|---|---|---|---|---|
| F4/80 | + | + | + | − |
| Ly6G | − | + | − | + |
| VCAM-1 | + | + | − | − |
| GFP | + | ++ | ++ | + |
| CD169 | + | + | − | − |
| ER-HR3 | + | + | − | − |
| Mer-TK | + | + | − | − |
| TIM-4 | + | − | − | − |
| Frequency in total BM, % | 3.78 | 3.04 | 2.5 | 16.4 |
| Antigens . | BM resident Mφs . | EIMs . | BM monocytes . | Granulocytes . |
|---|---|---|---|---|
| F4/80 | + | + | + | − |
| Ly6G | − | + | − | + |
| VCAM-1 | + | + | − | − |
| GFP | + | ++ | ++ | + |
| CD169 | + | + | − | − |
| ER-HR3 | + | + | − | − |
| Mer-TK | + | + | − | − |
| TIM-4 | + | − | − | − |
| Frequency in total BM, % | 3.78 | 3.04 | 2.5 | 16.4 |
As shown in Figure 1B-C, within the F4/80+Ly6Gneg BM population, monocytes were gated as VCAM-1negCD11b+CD169neg (gating path iv and x). All BM monocytes in naive MacGreen mice were GFP+ (Figure 1B, gate iv). Similar to recipient granulocytes, recipient monocytes were ablated following autologous HSC transplant (Figure 1C, gate iv; Figure 1E, light green gate x). GFPneg donor monocytes paralleled donor granulocyte reconstitution in BM and reached steady-state levels at 5 weeks posttransplant (Figure 1C, yellow gate viii; Figure 1E, yellow bars).
Nontransplanted BM resident Mφs were gated as F4/80+Ly6GnegVCAM+GFP+CD169+ cells (Figure 1B-C). In contrast to granulocyte and monocytes, 9% of recipient BM resident Mφs persisted at 2 weeks posttransplant (Figure 1C, F4/80+Ly6GnegVCAM+GFP+CD169+ cells; Figure 1E, dark green bars). Unexpectedly, the small number of residual GFP+ recipient Mφs expanded within the first 5 weeks posttransplant (Figure 1E; 32.5% of naive BM Mφs) and were still evident 32 weeks posttransplant (Figure 1E). Donor GFPneg resident Mφs (Figure 1C, F4/80+Ly6GnegVCAM1+GFPnegCD169+ cells; Figure 1E, orange bars) were detected in BM at 2 weeks posttransplant, before the appearance of donor GFPneg BM granulocytes (Figure 1D) or GFPneg monocytes (Figure 1E). These donor BM resident Mφs expanded over the next 3 weeks (Figure 1E) so that by 5 weeks, the total BM resident Mφ pool (recipient plus donor) was restored to pretransplant levels (Figure 1E).
To validate evidence of persistence of recipient Mφs in a different transplantation model, we lethally irradiated and transplanted UBC-GFP mice, in which enhanced GFP is ubiquitously expressed, with syngeneic LSK HSPCs from wild-type B6.SJL mice. Grafted UBC-GFP mice were harvested at 8 weeks posttransplant (supplemental Figure 1, available on the Blood Web site). Flow cytometry analysis of BM mononuclear cells confirmed persistence of recipient resident Mφs (gated similarly to Table 1, with the only variation being reduced GFP transgene specificity; see supplemental Figure 1A-B).
Recipient splenic resident Mφs expand rapidly but transiently after transplantation
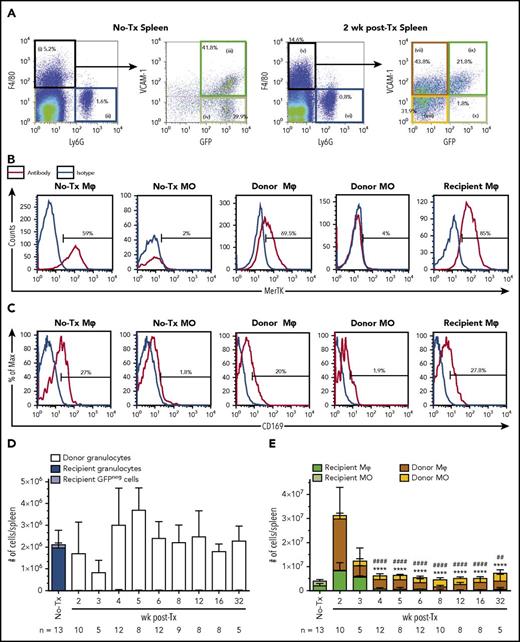
The spleen supports extramedullary hematopoiesis during BM stress.26 F4/80+Ly6Gneg resident Mφs in the spleen of naive MacGreen mice were VCAM-1+GFP+MerTK+ (Figure 2A, gate iii; Figure 2B), but unlike BM, only 25% to 30% expressed CD169 (Figure 2C). All other gating of splenic myeloid cells (Figure 2A-C) matched the strategy used in BM as summarized in Table 1. Splenic recipient granulocytes and monocytes were radiosensitive and remained undetectable posttransplant (Figure 2D-E, respectively). Donor-derived GFPneg granulocyte reconstitution was more rapid in spleen (Figure 2D) than in BM (Figure 1D).
Characterization of Mφs and monocytes in spleens of naive and 2 week posttransplant MacGreen mice. (A) Representative flow cytometry analysis (n = 5-13) of naive (no-Tx) and 2 week posttransplant MacGreen splenocytes demonstrating extended characterization of gated Mφ and monocyte populations as summarized in Table 1. Specifically, monocyte-Mφs were first enriched by gating F4/80+Ly6Gneg cells (gate i) and further subdivided into VCAM-1+GFP+ naive/recipient Mφs (gate iii and ix), VCAM-1negGFP+ naive/recipient monocytes (gate iv or x), VCAM-1+GFPneg donor Mφs (gate vii), and VCAM-1negGFPneg donor monocytes (gate viii). Granulocytes were gated as F4/80negLy6G+ cells (gate ii or vi). B and C) Representative histograms for MerTK (B) and CD169 (C) for all populations of interest as indicated by histogram titles. Antibody expression is shown as red lines, and appropriate isotype staining is shown as blue lines. (D) Number of recipient (blue bars) and donor (white bars) granulocytes per spleen across the transplantation time course. (E) Total number of recipient (dark green bars) and donor (brown bars) Mφ, recipient (light green bars) and donor (yellow bars) monocytes per spleen across the transplantation time course. Data are presented as mean ± SD. Statistical analysis of recipient Mφs was performed using 1-way ANOVA Tukey’s multiple comparison test. In panel E, asterisks and crosshatches indicate significant differences in recipient Mφ number compared with 2 and 3 weeks posttransplant, respectively (##P < .01 and ****/####P < .0001). Data represent 5 to 13 samples per time point. Flow cytometry plots and histograms as well as associated gated cell percent frequencies are from a representative animal, and the percentage of positive cells is based on the preceding parent populations. Data are representative of 5 to 13 individual animal samples per time point from either 1 (weeks 3 and 32), 2 (week 5), or 3 (all other time points) biological replicate experiments. 300 000 events were collected from each experiment sample by flow cytometry.
Characterization of Mφs and monocytes in spleens of naive and 2 week posttransplant MacGreen mice. (A) Representative flow cytometry analysis (n = 5-13) of naive (no-Tx) and 2 week posttransplant MacGreen splenocytes demonstrating extended characterization of gated Mφ and monocyte populations as summarized in Table 1. Specifically, monocyte-Mφs were first enriched by gating F4/80+Ly6Gneg cells (gate i) and further subdivided into VCAM-1+GFP+ naive/recipient Mφs (gate iii and ix), VCAM-1negGFP+ naive/recipient monocytes (gate iv or x), VCAM-1+GFPneg donor Mφs (gate vii), and VCAM-1negGFPneg donor monocytes (gate viii). Granulocytes were gated as F4/80negLy6G+ cells (gate ii or vi). B and C) Representative histograms for MerTK (B) and CD169 (C) for all populations of interest as indicated by histogram titles. Antibody expression is shown as red lines, and appropriate isotype staining is shown as blue lines. (D) Number of recipient (blue bars) and donor (white bars) granulocytes per spleen across the transplantation time course. (E) Total number of recipient (dark green bars) and donor (brown bars) Mφ, recipient (light green bars) and donor (yellow bars) monocytes per spleen across the transplantation time course. Data are presented as mean ± SD. Statistical analysis of recipient Mφs was performed using 1-way ANOVA Tukey’s multiple comparison test. In panel E, asterisks and crosshatches indicate significant differences in recipient Mφ number compared with 2 and 3 weeks posttransplant, respectively (##P < .01 and ****/####P < .0001). Data represent 5 to 13 samples per time point. Flow cytometry plots and histograms as well as associated gated cell percent frequencies are from a representative animal, and the percentage of positive cells is based on the preceding parent populations. Data are representative of 5 to 13 individual animal samples per time point from either 1 (weeks 3 and 32), 2 (week 5), or 3 (all other time points) biological replicate experiments. 300 000 events were collected from each experiment sample by flow cytometry.
Recipient splenic resident Mφ (Figure 2A, gate ix; Figure 2E) expanded fourfold within 2 weeks posttransplant (Figure 2E; P < .001) but then declined rapidly (Figure 2E). Repopulation of donor monocytes (Figure 2A, gate viii) and Mφs (Figure 2A, gate vii) also occurred more rapidly within spleen (Figure 2E) than in BM (Figure 1E). Donor Mφ numbers surpassed basal splenic Mφ numbers within the first 2 weeks (Figure 2E) but contracted to pretransplant levels by 3 weeks (Figure 2E). These observations were validated using an alternative autologous transplant model with UBC-GFP recipient mice and C57BL/6 LSK cell graft (supplemental Figure 1C) and are consistent with a transient role for the spleen in myeloid reconstitution.
Recipient and donor resident Mφ phenotype varied in posttransplantation BM and spleen
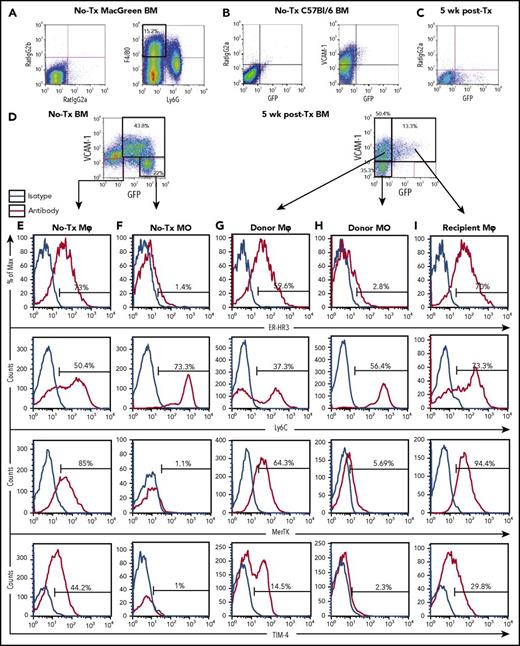
Variation in the expression of molecules known to contribute to Mφ function were assessed between recipient and donor Mφs in BM and spleen. BM phenotype was assessed at 5 weeks posttransplant as total myeloid population frequencies had reached steady state (Figure 1D-E). Naive and recipient origin F4/80+Ly6GnegVCAM-1+GFP+ cells expressed Mφ markers, including CD169 (Figure 1B), ER-HR3, MerTK, and TIM-4 (Figure 3E and 3I, respectively). Conversely, F4/80+Ly6GnegVCAM-1+GFPneg donor cell CD169 expression was variable, with most negative or weakly positive for the antigen (Figure 1C), a phenotype more resembling naive splenic resident Mφs (Figure 2C). TIM-4 expression on donor Mφs was significantly reduced compared with recipient BM Mφ (14.57 ± 2.13 vs 29.77 ± 2.275 respectively, P = .0082, Figure 3G). CD169 has been reported to be an HSC niche Mφ marker,5 and TIM-4 is a mature Mφ marker27 potentially involved in hematopoiesis.28 Accordingly, the results indicate that (1) recipient BM resident Mφ expression of the functional makers assessed was minimally affected by lethal radiation and transplantation, and (2) complete donor BM resident Mφ maturation has not occurred in the immediate (5 week) posttransplant period.
Extended flow cytometry characterization of Mφs and monocytes in the BM of naive and 5 week posttransplant MacGreen mice. Representative flow cytometry analysis of BM myeloid populations, extending data provided in Figure 1. (A) Naive (no-Tx) MacGreen BM stained with anti-F4/80 and anti-Ly6G antibodies (right plot) demonstrating specificity of staining and gating of F4/80+Ly6Gneg monocyte-Mφ–enriched cells. (B) Naive C57BL/6 BM stained with appropriate isotype control (left plot) or anti-VCAM-1 antibody (right plot) demonstrating specificity of staining and lower limit of detection for GFP transgene expression. (C-I) Representative flow cytometry analysis (n = 3-13) of BM from no transplant and 5 weeks posttransplant MacGreen mice demonstrating isotype control staining in 5 weeks posttransplant BM (C), VCAM-1 and GFP staining in no-Tx and 5 weeks posttransplant (D), and extended ER-HR3, Ly6C, MerTK, and TIM-4 expression in no-transplant Mφs (E), no-transplant monocyte (F) , donor Mφ (G), donor monocyte (H), and recipient Mφ (I) populations as summarized in Table 1. The histograms show antibody staining (red lines) compared with appropriate isotype staining (blue lines). Flow cytometry plots and histograms as well as associated gated cell percent frequencies are from a representative animal, and the percentage of positive cells is based on the preceding parent populations. Data were collected from a single experiment with n = 3 mice, and 300 000 events were collected by flow cytometry.
Extended flow cytometry characterization of Mφs and monocytes in the BM of naive and 5 week posttransplant MacGreen mice. Representative flow cytometry analysis of BM myeloid populations, extending data provided in Figure 1. (A) Naive (no-Tx) MacGreen BM stained with anti-F4/80 and anti-Ly6G antibodies (right plot) demonstrating specificity of staining and gating of F4/80+Ly6Gneg monocyte-Mφ–enriched cells. (B) Naive C57BL/6 BM stained with appropriate isotype control (left plot) or anti-VCAM-1 antibody (right plot) demonstrating specificity of staining and lower limit of detection for GFP transgene expression. (C-I) Representative flow cytometry analysis (n = 3-13) of BM from no transplant and 5 weeks posttransplant MacGreen mice demonstrating isotype control staining in 5 weeks posttransplant BM (C), VCAM-1 and GFP staining in no-Tx and 5 weeks posttransplant (D), and extended ER-HR3, Ly6C, MerTK, and TIM-4 expression in no-transplant Mφs (E), no-transplant monocyte (F) , donor Mφ (G), donor monocyte (H), and recipient Mφ (I) populations as summarized in Table 1. The histograms show antibody staining (red lines) compared with appropriate isotype staining (blue lines). Flow cytometry plots and histograms as well as associated gated cell percent frequencies are from a representative animal, and the percentage of positive cells is based on the preceding parent populations. Data were collected from a single experiment with n = 3 mice, and 300 000 events were collected by flow cytometry.
Recipient EIMs persist in hematopoietic organs after transplantation
As shown in Figure 4A-E, EIMs have a unique profile among resident marrow Mφ in their expression of Ly6G+ plus ER-HR3+.22 These Mφs also express F4/80, CD169, the Csf1r-eGFP transgene (Figure 4A-E), and MerTK (Figure 4C-E), as previously reported,29 but lack TIM-4 (Figure 4C-E). The surface-marker phenotypes of recipient and donor EIMs were indistinguishable (Figure 4A-E). Although EIM numbers in BM were greatly reduced early posttransplant, both recipient (green bars) and donor (white bars) EIMs expanded rapidly between 3 and 5 weeks to reach steady state (Figure 4F). As expected, EIMs were rare in spleen,22 and this did not alter as a consequence of irradiation and transplantation (data not shown).
Recipient EIMs persist and expand after transplantation. (A-E) Representative flow cytometry of EIMs in naive (no-Tx) and posttransplant (5 weeks) MacGreen BM. (A and B) F4/80+Ly6G+ EIMs (gate i) were further segregated into naive and posttransplant recipient VCAM-1+GFP+ cells (gate ii) or posttransplant donor VCAM-1+GFPneg cells (gate iii). (C-E) Representative flow histograms showing CD169, ER-HR3, MerTK, and TIM-4 expression in no-transplant (C), donor (D), and recipient (E) EIMs. Isotype control staining is represented by the blue line, and specific staining represented as the red line. (F) Total number of recipient (green bars) and donor EIMs (white bars) per femur across the transplantation time course (n = 5-13 mice/time point). Data are presented as mean ± SD. Statistical analysis was performed using a 1-way ANOVA Tukey’s multiple comparison test on recipient EIMs in transplanted groups, where asterisks indicate significant differences when compared with 2 weeks posttransplant (*P < .05 and *** P < .001). Flow cytometry plots and histograms as well as associated gated cell percent frequencies are from a representative animal, and the percentage of positive cells is based on the preceding parent populations. Data are representative of 5 to 13 individual animal samples per time point from either 1 (weeks 3 and 32), 2 (week 5), or 3 (all other time points) biological replicate experiments. 300 000 events were collected from each experiment sample by flow cytometry.
Recipient EIMs persist and expand after transplantation. (A-E) Representative flow cytometry of EIMs in naive (no-Tx) and posttransplant (5 weeks) MacGreen BM. (A and B) F4/80+Ly6G+ EIMs (gate i) were further segregated into naive and posttransplant recipient VCAM-1+GFP+ cells (gate ii) or posttransplant donor VCAM-1+GFPneg cells (gate iii). (C-E) Representative flow histograms showing CD169, ER-HR3, MerTK, and TIM-4 expression in no-transplant (C), donor (D), and recipient (E) EIMs. Isotype control staining is represented by the blue line, and specific staining represented as the red line. (F) Total number of recipient (green bars) and donor EIMs (white bars) per femur across the transplantation time course (n = 5-13 mice/time point). Data are presented as mean ± SD. Statistical analysis was performed using a 1-way ANOVA Tukey’s multiple comparison test on recipient EIMs in transplanted groups, where asterisks indicate significant differences when compared with 2 weeks posttransplant (*P < .05 and *** P < .001). Flow cytometry plots and histograms as well as associated gated cell percent frequencies are from a representative animal, and the percentage of positive cells is based on the preceding parent populations. Data are representative of 5 to 13 individual animal samples per time point from either 1 (weeks 3 and 32), 2 (week 5), or 3 (all other time points) biological replicate experiments. 300 000 events were collected from each experiment sample by flow cytometry.
Recipient BM Mφs are located in HSC niche–enriched medullary regions
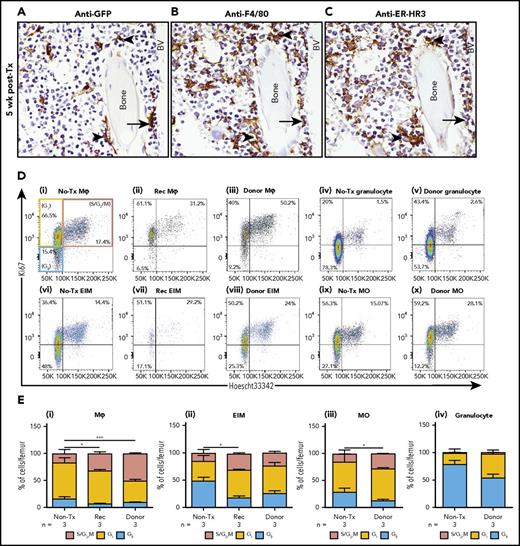
HSCs reside in perivascular niches that are enriched in endosteal regions of the BM.30-32 In naive MacGreen mice, GFP+ cells were distributed throughout the BM (supplemental Figure 2A-B). Two weeks posttransplant, there was clear morphological evidence of BM injury and myeloablation (supplemental Figure 2C) that had recovered by 5 weeks posttransplant (supplemental Figure 2D) with GFP+ cells evident in the medulla at both time points. At 5 and 16 weeks posttransplant, the reconstituting hematopoietic tissue was interspersed with GFP+ recipient Mφs in perivascular and endosteal regions (Figure 5A; supplemental Figure 2E-J). The GFP+ cells were predominantly of ramified Mφ-like morphology (Figure 5A; supplemental Figure 2) and also expressed F4/80 and ER-HR3 (Figure 5A-C; supplemental Figure 2E-G). These observations place at least some radiation-resistant recipient Mφs at locations known to harbor HSC niches.
Resilient recipient Mφs are located within perivascular and endosteal regions of the BM and proliferate autonomously after irradiation and transplantation. (A-C) Representative immunohistochemistry staining using anti-GFP (A), anti-F4/80 (B), and anti-ER-HR3 antibodies (C) in serial femoral BM sections from 5 week posttransplant recipient MacGreen mice. GFP+ recipient Mφs expressing F4/80 and ER-HR3 in HSC-enriched endosteal (arrowheads) and perivascular (arrows) regions (A-C, original magnification ×60). All sections were counterstained with hematoxylin (blue). (D) Representative flow cytometry analysis of BM myeloid populations gated as per Table 1 showing cell cycle gating using Ki67 and Hoechst 33342 (Ho) staining. Ki-67negHolow cells (blue gate) represent cells in G0 phase, Ki-67+Holow cells (yellow gate) are in G1 phase, and Ki-67+Ho+ cells (pink gate) are in S/G2/M phase of the cell cycle. Representative dot plots show naive (no-Tx) MacGreen Mφs (i), 4 week posttransplant recipient (Rec Mφs; ii) and donor Mφs (iii), no-Tx MacGreen granulocytes (iv), 4 week posttransplant donor granulocytes (v), no-Tx MacGreen EIMs (vi), 4 week posttransplant Rec (vii) and donor (viii) EIMs, no-Tx MacGreen monocytes (ix), and 4 week posttransplant donor monocytes (x). (E) Percentage of no-Tx MacGreen, 4 week posttransplant recipient (Rec) and donor (donor) BM resident Mφs (i), EIMs (ii), monocytes (iii), and granulocytes (iv) in phase G0 (blue bar), G1 (yellow bars), or S/G2/M (pink bars). Data represent mean ± SD of n = 3 mice. Flow cytometry plots and histograms are from a representative animal, and the percentage of positive cells is based on the proceeding parent population. Statistical analysis was performed using 1-way ANOVA and a Tukey’s multiple comparison test on S/G1/M phase, where asterisks indicate significant differences from nontransplanted mice (*P < .05 and ***P < .001). Data were collected from a single experiment with 3 animals per group, and 300 000 events were collected by flow cytometry.
Resilient recipient Mφs are located within perivascular and endosteal regions of the BM and proliferate autonomously after irradiation and transplantation. (A-C) Representative immunohistochemistry staining using anti-GFP (A), anti-F4/80 (B), and anti-ER-HR3 antibodies (C) in serial femoral BM sections from 5 week posttransplant recipient MacGreen mice. GFP+ recipient Mφs expressing F4/80 and ER-HR3 in HSC-enriched endosteal (arrowheads) and perivascular (arrows) regions (A-C, original magnification ×60). All sections were counterstained with hematoxylin (blue). (D) Representative flow cytometry analysis of BM myeloid populations gated as per Table 1 showing cell cycle gating using Ki67 and Hoechst 33342 (Ho) staining. Ki-67negHolow cells (blue gate) represent cells in G0 phase, Ki-67+Holow cells (yellow gate) are in G1 phase, and Ki-67+Ho+ cells (pink gate) are in S/G2/M phase of the cell cycle. Representative dot plots show naive (no-Tx) MacGreen Mφs (i), 4 week posttransplant recipient (Rec Mφs; ii) and donor Mφs (iii), no-Tx MacGreen granulocytes (iv), 4 week posttransplant donor granulocytes (v), no-Tx MacGreen EIMs (vi), 4 week posttransplant Rec (vii) and donor (viii) EIMs, no-Tx MacGreen monocytes (ix), and 4 week posttransplant donor monocytes (x). (E) Percentage of no-Tx MacGreen, 4 week posttransplant recipient (Rec) and donor (donor) BM resident Mφs (i), EIMs (ii), monocytes (iii), and granulocytes (iv) in phase G0 (blue bar), G1 (yellow bars), or S/G2/M (pink bars). Data represent mean ± SD of n = 3 mice. Flow cytometry plots and histograms are from a representative animal, and the percentage of positive cells is based on the proceeding parent population. Statistical analysis was performed using 1-way ANOVA and a Tukey’s multiple comparison test on S/G1/M phase, where asterisks indicate significant differences from nontransplanted mice (*P < .05 and ***P < .001). Data were collected from a single experiment with 3 animals per group, and 300 000 events were collected by flow cytometry.
Recipient BM resident Mφs proliferate autonomously after transplantation
Some Mφ subsets can divide autonomously in situ.14,33 Cell cycle analysis based on DNA content (Hoechst 33342) and Ki-67 expression21,34 was performed on BM from MacGreen mice before and 4 weeks after transplantation. In the steady state, 17% of BM resident Mφs (Figure 5Di,Ei), 14% of EIM (Figure 5Dvi,Eii) and 15% of monocytes (Figure 5Dix,Eiii) were in S/G2/M phase. In contrast, few granulocytes were in this cell cycle phase (Figure 5Div,Dv,Eiv). Following transplantation, a significantly greater proportion of donor monocytes (Figure 5Dx,Eiii) and donor and recipient BM resident Mφs were in S/G2/M (Figure 5Ei) compared with nontransplanted controls. By contrast, the proportion of donor EIMs in S/G2/M phase was similar to naive and recipient EIMs (Figure 5Eii). The proportion of recipient BM resident Mφs (Figure 5Ei) and recipient EIMs (Figure 5Eii) in S/G2/M phase was greater than in nontransplanted mice at 5 weeks posttransplantation. Expression of the cell cycling marker Ki-67 in a portion of GFP+ recipient Mφs was also confirmed by immunohistochemistry in BM posttransplant (supplemental Figure 2K-L).
Recipient BM resident Mφ expansion is synchronous with the persistent engraftment of phenotypic long-term reconstituting HSCs in the BM
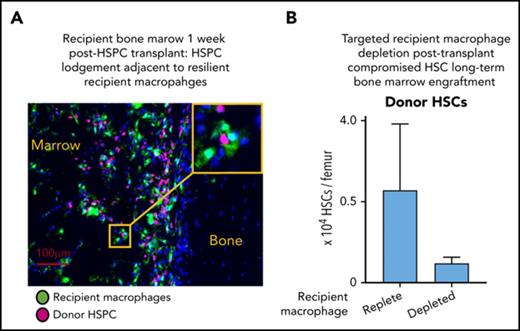
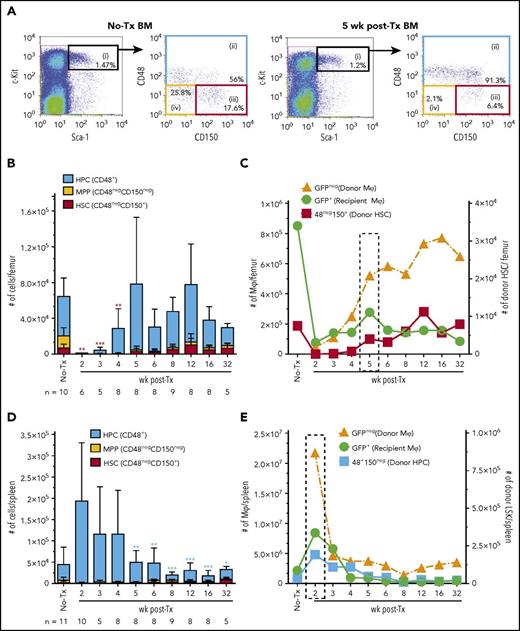
Kinetics of donor LSK HSPC persistent engraftment was monitored using multiplexed flow cytometry analysis and phenotypically segregating LSK HSPCs into enriched populations of hematopoietic progenitor cells (HPCs; blue gate), multipotent progenitors (MPPs; yellow gate), and long-term repopulating HSCs (red gate) using CD48 and CD150 expression (Figure 6A).35 In MacGreen naive and transplanted recipient mice, HSPCs were GFPneg (data not shown). Phenotypic LSK HSPC, as assessed by ex vivo flow cytometry, were only consistently detected in BM from 3 weeks posttransplant onward (Figure 6B). In situ imaging (supplemental Figure 3) indicated this apparent lack of early HSPC BM homing and lodgment reflected a combination of low HSPC lodgment up to 1 week posttransplant and prolonged downregulation of c-Kit expression, as has been previously reported after total body irradiation conditioning and transplantation.36 Notably, within the first 3 weeks posttransplant, donor HSPCs that had homed to the BM were frequently lodged adjacent to recipient Mφs (supplemental Figure 3B-D), and emerging donor hematopoietic islands were also interspersed with recipient Mφs (supplemental Figure 3C-D). Therefore, while donor HSPCs homed and lodged in the BM within the first 3 weeks posttransplant, sustained engraftment within a niche capable of supporting HSPC homeostasis as indicated by phenotype normalization did not occur until at least 3 weeks posttransplant. Few if any phenotypic MPPs were detected at any stage posttransplant (Figure 6B). Phenotypic HSCs were detected in the BM from 5 weeks and peaked at 12 weeks posttransplant (Figure 6B). Posttransplant BM sustained engraftment of phenotypic donor HSCs coincided with peak expansion of recipient Mφs and a period of rapid expansion of donor-derived Mφs (Figure 6C).
Autonomous repopulation of recipient BM Mφs synchronizes with BM engraftment of phenotypic donor HSCs. (A) Representative flow cytometry analysis of naive (no-Tx) and 5 week posttransplant MacGreen recipient BM. LSK cells (black, gate i) consist of CD48+ HPCs (blue, gate ii), CD48negCD150neg MPP (yellow, gate iv), and CD48negCD150+ HSCs (red, gate iii). (B) Enumeration of total number of HPCs (blue bars), MPPs (yellow bars), and HSCs (red bars) in the BM (n = 5-12 mice/time point). Data are presented as mean ± SD. Statistical analysis used a 1-way ANOVA Tukey’s multiple comparison test on donor HSCs (red bars) in transplanted groups, and asterisks represent significant differences in HSC numbers compared with 12 weeks posttransplant. (C) Overlay of results from Figure 1F-G with Figure 6B to align recovery kinetics of posttransplant Mφ numbers (#, left y-axis) with donor HSC numbers (right y-axis) in the BM. Dotted boxed area indicates recipient Mφ population number peak. (D) Enumeration of total number of HPCs (blue bars), MPPs (yellow bars), and HSCs (red bars) in the spleen. Data are presented as mean ± SD. Statistical analysis used a 1-way ANOVA Tukey’s multiple comparison test on donor HPCs (blue bars) in transplanted groups, and asterisks represent significant differences in HPC numbers compared with 2 weeks posttransplant. (E) Overlay of results from Figure 2E with panel D to align recovery kinetics of posttransplant Mφ numbers (#, left y-axis) with donor HPC numbers (right y-axis) in spleen. Dotted boxed area indicates recipient Mφ population number peak. Significance values are designated as *P < .05, **P < .01, and ***P < .001. Flow cytometry plots and histograms are from a representative animal, and the percentage of positive cells is based on the proceeding parent populations. Data are representative of 5 to 13 individual animal samples per time point from either 1 (week 3 and 32), 2 (week 5), or 3 (all other time points) biological replicate experiments. 1 × 106 events were collected from each experiment sample by flow cytometry.
Autonomous repopulation of recipient BM Mφs synchronizes with BM engraftment of phenotypic donor HSCs. (A) Representative flow cytometry analysis of naive (no-Tx) and 5 week posttransplant MacGreen recipient BM. LSK cells (black, gate i) consist of CD48+ HPCs (blue, gate ii), CD48negCD150neg MPP (yellow, gate iv), and CD48negCD150+ HSCs (red, gate iii). (B) Enumeration of total number of HPCs (blue bars), MPPs (yellow bars), and HSCs (red bars) in the BM (n = 5-12 mice/time point). Data are presented as mean ± SD. Statistical analysis used a 1-way ANOVA Tukey’s multiple comparison test on donor HSCs (red bars) in transplanted groups, and asterisks represent significant differences in HSC numbers compared with 12 weeks posttransplant. (C) Overlay of results from Figure 1F-G with Figure 6B to align recovery kinetics of posttransplant Mφ numbers (#, left y-axis) with donor HSC numbers (right y-axis) in the BM. Dotted boxed area indicates recipient Mφ population number peak. (D) Enumeration of total number of HPCs (blue bars), MPPs (yellow bars), and HSCs (red bars) in the spleen. Data are presented as mean ± SD. Statistical analysis used a 1-way ANOVA Tukey’s multiple comparison test on donor HPCs (blue bars) in transplanted groups, and asterisks represent significant differences in HPC numbers compared with 2 weeks posttransplant. (E) Overlay of results from Figure 2E with panel D to align recovery kinetics of posttransplant Mφ numbers (#, left y-axis) with donor HPC numbers (right y-axis) in spleen. Dotted boxed area indicates recipient Mφ population number peak. Significance values are designated as *P < .05, **P < .01, and ***P < .001. Flow cytometry plots and histograms are from a representative animal, and the percentage of positive cells is based on the proceeding parent populations. Data are representative of 5 to 13 individual animal samples per time point from either 1 (week 3 and 32), 2 (week 5), or 3 (all other time points) biological replicate experiments. 1 × 106 events were collected from each experiment sample by flow cytometry.
In control MacGreen spleen, there were few if any phenotypic HSCs (Figure 6D), a small but consistent population of MPPs (Figure 6D), and variable numbers of HPCs (Figure 6D). In spleens of transplanted MacGreen mice, there were low numbers of phenotypic HSCs detected from 6 weeks onward (Figure 6D) but large numbers of phenotypic HPCs until 4 weeks (Figure 6D). Macroscopic evidence of extramedullary hematopoiesis was observed at 2 weeks posttransplant, including splenic enlargement (0.278 ± 0.011 g vs 0.093 ± 0.026 g in transplanted versus naive mice, respectively; P < .0001). Overlaying the splenic resident Mφ (Figure 1G) and HPC (Figure 6D) repopulation/reconstitution dynamics suggests that Mφs contribute to the support of extramedullary hematopoiesis (Figure 6E).
Depletion of CD169+ recipient BM resident Mφ abates the engraftment and reconstitution capability of donor HSCs
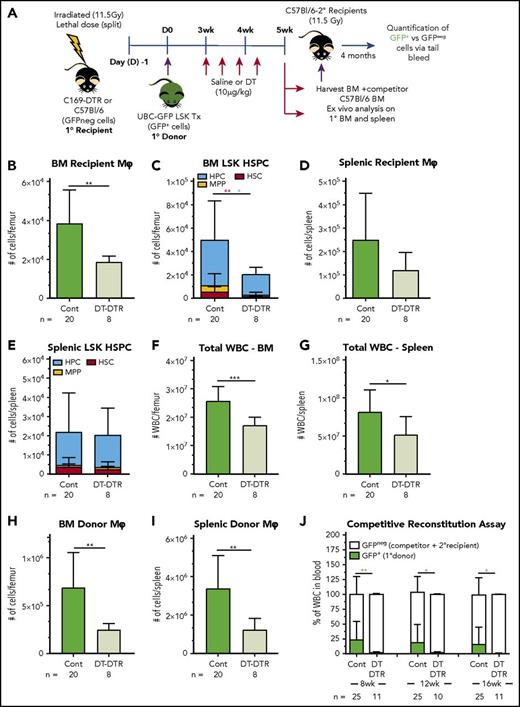
Since radiation-resistant recipient BMs Mφ express CD169, we used chimeric CD169-DTR knockin mice to specifically deplete recipient-derived resident Mφs (Figure 7A) and determine their contribution to sustained HSC engraftment and BM recovery. Specific recipient resident Mφ depletion in CD169-DTR chimeric mice was achieved by DT treatment (Figure 7A), the start of which coincided with initiation of phenotypic donor HSPC sustained engraftment in BM (3 weeks, as per Figure 6B). Based on previous data,22 depletion of recipient BM resident Mφ would be triggered within 24 to 48 hours of the first DT injection, and depletion was sustained at the 5-week harvest time point in DT-treated CD169-DTR recipient mice (Figure 7B). CD169+ recipient BM resident Mφ depletion was associated with a significant reduction in the sustained engraftment of donor HPCs and HSCs within the BM (Figure 7C). DT treatment had no significant impact on recipient resident Mφs in the spleen (Figure 7D), reflecting reduced CD169 expression in these Mφ (Figure 2), or HSPC number in the spleen (Figure 7E). Reduced donor total white blood cell counts (Figure 7F-G) and donor Mφ numbers (Figure 7H-I) in both BM and spleen were also observed in DT-treated mice, with data collectively supporting that these reductions are downstream secondary impacts on hematopoietic reconstitution resulting from comprised HSC and HPC BM sustained engraftment.
Depletion of recipient CD169+Mφs reduces engraftment of donor HSCs posttransplantation. (A) Schematic of the transplantation model using lethally irradiated CD169-DTR or C57BL/6 primary (1°) recipients and congenic UBC-GFP LSK cells as the 1° donor graft. BM from 1° recipients was collected at 5 weeks posttransplant, mixed with C57BL/6 competitor BM cells, and transplanted into secondary (2°) lethally irradiated C57BL/6 recipients. (B-F) As no significant differences were observed in any of the outcome measures between saline-treated CD169-DTR and DT-treated C57BL/6 1° recipient mice, these groups were pooled as the control (Cont). (B) Number of recipient resident Mφs in the BM of 1° recipients at 5 weeks posttransplant. (C) Number of 1° donor HPCs (blue bars), MPPs (yellow bars), and HSCs (red bars) in the BM of 1° recipients at 5 weeks posttransplant. (D) Number of recipient resident Mφs in spleen of 1° recipients at 5 weeks posttransplant. (E) Number of 1° donor HPCs (blue bars), MPPs (yellow bars), and HSCs (red bars) in the spleen of 1° recipients at 5 weeks posttransplant. (F-G) Number of leukocytes (total white blood cells) in the BM (F) and spleen (G) of 1° recipients at 5 weeks posttransplant. (H and I) Number of donor Mφ in BM (H) or spleen (I) of 1° recipients at 5 weeks posttransplant. Data are presented as mean ± SD (n = 8-20 1° recipients/group). (J) Blood GFP+ chimerism of 2° recipients (n = 10-25 mice/group) Statistical analysis was performed using unpaired Student t test, with colored asterisks matching relevant cell populations where appropriate (*P < .05 and **P < .01). Data were pooled from 3 independent biological replicate experiments, and 300 000 events were collected by flow cytometer to analyze myeloid population; 1 × 106 events were collected to analyze LSK populations, and 100 000 events were collected to analyze tail bleeds. ***P = .0002. WBC, white blood cell.
Depletion of recipient CD169+Mφs reduces engraftment of donor HSCs posttransplantation. (A) Schematic of the transplantation model using lethally irradiated CD169-DTR or C57BL/6 primary (1°) recipients and congenic UBC-GFP LSK cells as the 1° donor graft. BM from 1° recipients was collected at 5 weeks posttransplant, mixed with C57BL/6 competitor BM cells, and transplanted into secondary (2°) lethally irradiated C57BL/6 recipients. (B-F) As no significant differences were observed in any of the outcome measures between saline-treated CD169-DTR and DT-treated C57BL/6 1° recipient mice, these groups were pooled as the control (Cont). (B) Number of recipient resident Mφs in the BM of 1° recipients at 5 weeks posttransplant. (C) Number of 1° donor HPCs (blue bars), MPPs (yellow bars), and HSCs (red bars) in the BM of 1° recipients at 5 weeks posttransplant. (D) Number of recipient resident Mφs in spleen of 1° recipients at 5 weeks posttransplant. (E) Number of 1° donor HPCs (blue bars), MPPs (yellow bars), and HSCs (red bars) in the spleen of 1° recipients at 5 weeks posttransplant. (F-G) Number of leukocytes (total white blood cells) in the BM (F) and spleen (G) of 1° recipients at 5 weeks posttransplant. (H and I) Number of donor Mφ in BM (H) or spleen (I) of 1° recipients at 5 weeks posttransplant. Data are presented as mean ± SD (n = 8-20 1° recipients/group). (J) Blood GFP+ chimerism of 2° recipients (n = 10-25 mice/group) Statistical analysis was performed using unpaired Student t test, with colored asterisks matching relevant cell populations where appropriate (*P < .05 and **P < .01). Data were pooled from 3 independent biological replicate experiments, and 300 000 events were collected by flow cytometer to analyze myeloid population; 1 × 106 events were collected to analyze LSK populations, and 100 000 events were collected to analyze tail bleeds. ***P = .0002. WBC, white blood cell.
The long-term repopulation potential of donor HSCs in primary recipient BM was assessed using competitive transplantation assays (Figure 7A). BM from control primary recipient mice consistently resulted in robust reconstitution of GFP+ donor blood leukocytes in secondary recipients (Figure 7J). However, GFP+ donor blood leukocytes were not detected in secondary recipients that received BM grafts from DT-treated primary CD169-DTR recipients (Figure 7J), demonstrating that host-derived CD169+ Mφs and their associated mediators are necessary to maintain donor-derived HSC reconstitution potential.
Discussion
Mφs are required for the maintenance and function of HSC niches.4-7,11 Myeloablative conditioning is generally assumed to eradicate all recipient leukocytes, including Mφs, but evidence of Mφ resilience to such treatments is mounting12-15 . This study used a Csf1r-eGFP transgenic transplantation model combined with robust myeloid phenotype segregation (Table 1) to show that both EIMs and resident Mφs (Table 1), 2 phenotypically and functionally distinct Mφ subsets, were radiation resistant in the BM and spleen. These findings quantitatively extend upon earlier observations in which separate Mφ subsets were not resolved.13-15 We demonstrate that EIM and resident Mφ persistence involved evasion of total body irradiation cytotoxicity by a small portion of each Mφ subset, followed by in situ autonomous proliferation (Figure 4B). This supports previous reports that Mφs can self-repopulate throughout adult life both in steady state and after genotoxic insult.14,33,37
The contribution of in situ self-renewal vs monocyte differentiation to tissue resident Mφ replacement varies between organs. In the absence of trauma or other challenge, microglial cells in the brain38 and Kupffer cells in the liver39 replenish primarily via self-renewal, while intestinal Mφs are continually renewed from BM-derived monocytes.40-42 In the current transplant model restoration of the total BM resident Mφ population density to preconditioning levels was achieved by 5 weeks posttransplantation through a contribution of both recipient and donor-derived Mφs. In situ proliferative capacity of either BM resident Mφs or EIMs has not been previously reported. Similar to Mφ emergence prior to definitive hematopoiesis during embryonic development,43,44 donor-derived BM EIMs and resident recipient Mφ repopulation were initiated prior to the appearance of donor BM monocytes. Thus, monocytes are not a necessary intermediate for tissue seeding of these resident Mφ.
Depletion of CD169+ Mφs was previously shown to induce HSC mobilization into blood.5 In the current study, depletion of recipient CD169+ BM resident Mφs posttransplantation profoundly diminished maintenance of HSC acceptance within the BM (Figure 7C) and compromised associated hematopoietic reconstitution (Figure 7F), the 2 essential components of HSC “engraftment.” A more detailed assessment of the early posttransplant (1- to 3-week) period that circumvents the challenges in phenotypic long-term HSC identification based on c-Kit expression will be needed to understand resident macrophage contributions to HSC homing, lodgment, and early engraftment events. The expression of VCAM-1 by radiation-resistant BM resident Mφs adds weight to the view that they directly contribute to the maintenance of the niche for donor HSCs. VCAM-1 is critical for maintaining HSPCs within the BM26,45-47 and spleen during extramedullary hematopoiesis.26 Direct contact with CD234+ Mφs also promotes HSC quiescence,4 suggesting a direct influence of Mφs on HSCs and their niche. These observations favor a more specific role for recipient BM resident Mφs in supporting HSC and recovery of quiescent HSC niches posttransplant.
The inability of donor BM resident Mφs to compensate for loss of recipient BM resident Mφs in supporting HSC engraftment (Figure 5) is consistent with a previous murine study in which recipient BM Mφs compensated for the absence of donor BM Mφs in the setting of csf1r−/− grafts.14 Phenotypic differences between recipient and donor BM Mφs were suggestive of inadequate donor Mφ functional maturation, and this was validated by their inability to support HSC niche engraftment after depletion of recipient BM Mφs.
The requirement of recipient Mφ for sustained engraftment of long-term HSCs in the BM suggests that an approach to expand recipient Mφs before or immediately after transplant may improve transplantation outcomes. Recent experimental models13,48 and older clinical trials49,50 using the Mφ growth factor CSF1 support this proposal. One previous study suggested utility of CSF1 in allotransplantation, even though the dose of recombinant CSF1 used was only marginally active in mice.51 HSC engraftment was not directly assessed.13 The clinical safety of CSF1 as a hematopoietic growth factor in BM transplantation or during recovery from myeloablative therapies has been confirmed,50 and clinical benefit was observed when administered after transplant50 or after myeloablative therapy.49 However, because of the limited number of preclinical and clinical studies testing the benefits of Mφ-targeting approaches, the use of CSF1 in this setting has not been adopted.52 There is a potential risk in expanding pathogenic donor Mφs and exacerbating graft-versus-host disease.53 However, despite this risk and in light of the data presented here, further investigation into the potential benefit of CSF1 in BM transplantation and whether this is due to BM macrophage impacts is warranted.
Our results highlight that transplantation chimera models need to be used cautiously to distinguish between hematopoietic (particularly Mφ) and nonhematopoietic stromal contributions to HSC biology and pathology. This is particularly important when the gene of interest is expressed by both Mφs and nonhematopoietic stromal cells. The knowledge or paradigms attained by this approach require careful reassessment.
In overview, we have shown that in the BM and spleen, resilience and in situ self-repopulation of both recipient EIMs and recipient resident Mφs are associated with successful posttransplantation repopulation of the hematopoietic system. Splenic resident Mφ of both recipient and donor origin rapidly expanded to achieve temporary supraphysiologic Mφ density that occurred concomitantly with initiation of emergency extramedullary hematopoiesis. The maintenance of recipient BM resident Mφs was clearly necessary for subsequent reestablishment of the BM as a suitable environment for HSC engraftment and subsequently reestablishment of BM hematopoiesis. Our study has revealed a key targetable mechanism to potentially improve the condition of a patient’s BM environment not only after HSPC transplantation but also in cases of radiation and chemotherapy cytotoxicity.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
RFP mice breeding stock were generously provided by Patrick Tam (Children’s Medical Research Institute, University of Sydney, Westmead, Australia) and Susie Nilsson (Commonwealth Scientific and Industrial Research Organization and Monash University, Clayton, Australia). Technical support was provided by the Translational Research Institute flow cytometry, histology, and microscopy core facilities, The University of Queensland Biological Resource’s Research Facility and the Queensland Institute or Medical Research–Berghofer Histology Service processed tissues.
This work was supported by the National Health and Medical Research Council (project grants APP1102964, APP1108352, and APP1033736), Cancer Council Queensland (APP1066051), the Mater Foundation, The University of Queensland International Scholarship (S.K.), an Australian and New Zealand Bone and Mineral Society Gap Fellowship, an Australian Research Council Future Fellowship (ARP FT150100335), and a National Health and Medical Research Council Senior Research Fellowship (APP1044091) (J.-P.L.).
Authorship
Contribution: S.K. designed and performed experiments, analyzed data, prepared figures, and prepared the manuscript; L.J.R. and J.-P.L. designed and performed experiments, analyzed data, and prepared the manuscript; A.C.W. and L.B. performed experiments and approved the manuscript; S.M.M. performed experiments, analyzed data and approved manuscript; R.N.J. and K.P.M. designed experiments and approved the manuscript; D.A.H., I.G.W., and A.C.P. interpreted data and edited the manuscript; and A.R.P. designed, led, and coordinated the project, performed experiments, analyzed data, and prepared and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Allison R. Pettit, Mater Research Institute–The University of Queensland, Translational Research Institute, 37 Kent St, Woolloongabba, QLD 4102, Australia; e-mail: allison.pettit@mater.uq.edu.au.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal