Key Points
Transfusion-dependent Medicare patients with leukemia had a 51% shorter duration of hospice stay, indicating a barrier to timely referral.
Use of hospice in leukemia has increased and is associated with improved quality measures, regardless of transfusion dependence.
Abstract
Hospice provides high-quality end-of-life care, but patients with leukemias use hospice services less frequently than those with solid tumors. Transfusion dependence (TD) may hinder or delay enrollment, because hospice organizations typically disallow transfusions. We examined the association between TD and end-of-life outcomes among Medicare beneficiaries with leukemia. From the Surveillance, Epidemiology, and End Results-Medicare database, we selected beneficiaries with acute and chronic leukemias who died in 2001-2011. We defined TD as ≥2 transfusions within 30 days before death or hospice enrollment. End points included hospice enrollment and length of stay, reporting relative risk (RR) adjusted for key covariates. Among 21 033 patients with a median age of 79 years, 20% were transfusion dependent before death/hospice enrollment. Use of hospice increased from 35% in 2001 to 49% in 2011. Median time on hospice was 9 days and was shorter for transfusion-dependent patients (6 vs 11 days; P < .001). Adjusting for baseline characteristics, TD was associated with a higher use of hospice services (RR, 1.08; 95% confidence interval [CI], 1.04-1.12) but also with 51% shorter hospice length of stay (RR, 0.49; 95% CI, 0.44-0.54). Hospice enrollees had a lower likelihood of inpatient death and chemotherapy use and lower median Medicare spending at end-of-life, regardless of TD status. In conclusion, relatively increased hospice use combined with a markedly shorter length of stay among transfusion-dependent patients suggests that they have a high and incompletely met need for hospice services and that they experience a barrier to timely referral. Policy solutions supporting palliative transfusions may maximize the benefits of hospice for leukemia patients.
Introduction
Hospice care represents the gold standard in high-quality end-of-life (EOL) care for people with advanced cancer. Today, more than half of people who die of cancer in the United States receive hospice care services and, overall, hospice use has been increasing.1-3 However, patients with hematologic malignancies are less likely to access hospice care than are those with solid tumors.4 Furthermore, their average length of stay is shorter, whereas meaningful benefits from hospice care are maximized when patients enroll months or weeks, rather than days, before death.5,6 The discrepancy in hospice use among patients with hematologic malignancies remains poorly understood, but 3 primary hypotheses have emerged.
First, hospice organizations generally require patients to forego disease-modifying treatments, yet hematologists contend that it is difficult to recognize when patients with leukemia have exhausted options that might yield meaningful survival benefits.7-9 This identification is easier in patients with solid tumors, where progressive pain, malnutrition, and worsening performance status are in direct correspondence with the cancer bulk. Second, hematologic oncologists have different attitudes and beliefs about the appropriateness of aggressive therapy when a small chance of achieving a remission exists.10 Lastly, surveys of practitioners have suggested the inability to provide transfusion support by hospice agencies as a potential barrier to referral.11,12 However, the question of whether transfusion dependence (TD) is associated with receipt of hospice care has not been answered in the context of the clinically heterogeneous population of patients with leukemia. Acute and chronic leukemias differ markedly in the indication for immediate or deferred therapy, treatment goals (complete remission vs disease control), as well as patients’ and physicians’ expectations regarding the disease course and expected survival.
Patients with leukemias are among the most frequent users of transfusion services and represent ∼24 500 deaths per year in the United States.13 Therefore, elucidating the role of TD in the use of hospice services could have important policy implications and may be critical in the quest to improve the quality of EOL care in leukemia.14-16 Our objective was to examine the association between TD and the use of hospice services or duration of hospice length of stay in a population-based sample of older patients with acute and chronic leukemias. We hypothesized that TD would be associated with less frequent use of hospice and shorter hospice stays and that the association would be similar for acute and chronic leukemias, despite their clinical dissimilarity. A strong association would support aligning policies governing the hospice benefit with the needs of patients with leukemia. Furthermore, if the association between TD and end points were the same in acute and chronic leukemia (as evidenced by nonsignificant interaction terms in a statistical model), it would favor an effect of uniform policy rather than of disease-specific clinical factors.
Methods
Data source and study population
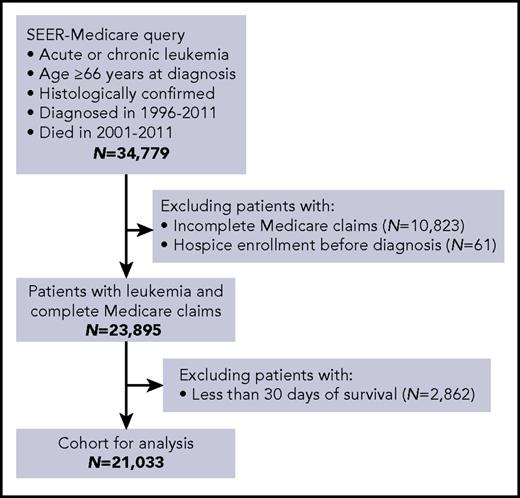
This study was approved by the Institutional Review Board at Rhode Island Hospital. We used the Surveillance, Epidemiology, and End Results (SEER)-Medicare dataset, which contains cancer registry data from areas covering ∼28% of the United States population, linked to administrative claims for Medicare enrollees.17 Using the World Health Organization’s histology codes, we selected Medicare beneficiaries who died in 2001-2011 after having been diagnosed with an acute (acute myeloid leukemia [AML] or acute lymphoblastic leukemia [ALL]) or chronic (chronic lymphocytic leukemia [CLL], chronic myeloid leukemia [CML], or chronic myelomonocytic leukemia [CMML]) leukemia in 1996-2011 (Figure 1). We allowed a longer timeframe for diagnoses to adequately capture subjects with chronic leukemias, who may survive many years from diagnosis. We included patients regardless of the recorded cause of death, because of known inaccuracies in cause-of-death attributions in cancer registries and because TD could affect the use of hospice, even in patients dying from conditions not directly related to leukemia. However, we also conducted a sensitivity analysis limited to patients with leukemia recorded as the cause of death, to confirm our observations. To assure complete Medicare records of health services, eligible beneficiaries were age ≥66 years at diagnosis and continuously enrolled in Medicare Parts A and B (excluding managed-care plans) from 12 months before diagnosis onward. To avoid misclassification when assigning TD, we excluded patients who died within 30 days of diagnosis, because they could not reliably meet TD criteria as defined below.
Cohort selection from the SEER-Medicare database. Inclusion and exclusion criteria are indicated.
Cohort selection from the SEER-Medicare database. Inclusion and exclusion criteria are indicated.
Variables and end points
The main exposure of interest was TD, ascertained from Medicare claims, which capture all inpatient and outpatient red cell– or platelet-transfusion events. We defined TD as ≥2 transfusion events, occurring ≥5 days apart, within 30 days before death or hospice enrollment. This definition mirrors prior studies using Medicare data, with the exception that some analyses defined the anchoring window as 30 days before death only.18-21 We found that such an anchoring produces incorrect results due to guarantee-time bias in TD ascertainment (supplemental Figure 1, available on the Blood Web site). To assess the sensitivity of our results and illustrate bias in prior studies, we repeated all analyses using alternative criteria: ≥3 transfusions within 60 days or ≥4 transfusions within 90 days. As supplementary sensitivity analyses, we studied various levels of TD severity (based on number of transfusion events) and TD based on the use of red cell– or platelet-transfusion only.
The primary end points included use of hospice services at the time of death and duration of hospice length of stay. Medicare records dates of enrollment and potential disenrollment from hospice, so for patients with multiple episodes of enrollment/disenrollment (eg, because of transfusion needs), we used only the last episode to ascertain the terminal hospice stay. Secondary end points were based on the National Quality Forum Performance Measures for Palliative and End-of-Life Care: death in the inpatient setting, admission to an intensive care unit (ICU) within 30 days of death, receipt of chemotherapy within 14 days of death, hospice enrollment <3 days before death, and outpatient referral to hospice (defined as hospice admission >2 days after discharge from any preceding hospitalization).6,22 We also evaluated Medicare spending within 30 days before death, summing up payments for outpatient and inpatient services, inflation adjusted to 2013 dollars using the US Personal Consumption Expenditures health-by-function price index.23,24 We identified chemotherapy administration recorded in Medicare claims, as previously described, with the caveat that the use of oral agents (including palliative hydroxyurea, oral chlorambucil, or tyrosine kinase inhibitors) was not consistently observed.21,24,25
Explanatory variables included patient’s age, sex, race/ethnicity (according to Medicare files), marital status, receipt of Medicaid coinsurance (indicator of disability or poverty), county-level indicators of population size and poverty prevalence, the National Cancer Institute–modified Charlson comorbidity index (a weighted score of comorbidities associated with mortality, based on Medicare claims from 12 months preceding death or hospice enrollment), preexisting dementia, indicator of poor performance status (validated as a measure of self-reported functional status), histological type of leukemia, time from diagnosis to death, and year of death.24,26,27
Statistical analysis
Distribution of variables was tabulated without any univariate statistical testing, because even minute differences would result in high statistical significance in this large dataset. We studied binary end points in robust Poisson models, which directly estimate relative risk (RR).28 Relative duration of hospice enrollment was studied in a 2-part model with the first (logistic) equation for the binary risk of hospice nonenrollment and the second (robust Poisson) equation for the hospice length of stay. All models adjusted for the same set of clinically relevant explanatory variables described above, regardless of statistical significance, and estimates are given with 95% confidence intervals (CIs). Linearized temporal trends in outcomes were evaluated in univariate robust Poisson models using calendar year as independent variable and reporting 2-sided P for the Wald test of model coefficient as Ptrend. P values <.05 were considered statistically significant. All analyses were conducted using SAS 9.4 (SAS Institute, Cary, NC) and Stata 15.1/MP (StataCorp, College Station, TX).
Results
Among 21 033 patients whose median age at diagnosis was 79 years (interquartile range [IQR], 73 to 84), 4141 (20%) were transfusion dependent (Table 1). Broken down by specific histology, the prevalence of TD was higher in AML (32%), ALL (22%), and CMML (22%) than in CML (15%) or CLL (7%) and increased over time (Figure 2A). Transfusion-dependent patients were, on average, younger (median age 77 vs 79 years) and more often male, married, and less likely to have poor baseline performance status or dementia. Overall survival from diagnosis was 11.4 months (95% CI, 11.0-11.7), ranging from 3.8 months (95% CI, 3.7-4.0) in AML to 34.6 months (95% CI, 33.5-35.8) in CLL (supplemental Table 1). Use of any chemotherapy since the leukemia diagnosis (observable in Medicare claims) was recorded in 39% of patients who enrolled in hospice before death and in 45% of those who did not. Furthermore, use of chemotherapy was 39% in patients without TD and 56% in those with TD, and it varied by histology.
Characteristics and outcomes of Medicare beneficiaries with leukemia, stratified by TD status before death or hospice enrollment
| . | All patients (n = 21 033) . | Not transfusion dependent (n = 16 892) . | Transfusion dependent (n = 4141) . |
|---|---|---|---|
| Leukemia histology | |||
| AML | 8 918 (43) | 6 082 (36) | 2836 (68) |
| ALL | 652 (3) | 511 (3) | 141 (3) |
| CLL | 8 420 (40) | 7 821 (46) | 599 (15) |
| CML | 1 536 (7) | 1 305 (8) | 231 (6) |
| CMML | 1 507 (7) | 1 173 (7) | 334 (8) |
| Age group, y | |||
| 65 to <70 | 2 528 (12) | 1 914 (11) | 614 (15) |
| 70 to <75 | 4 193 (20) | 3 203 (19) | 990 (24) |
| 75 to <80 | 5 133 (24) | 4 055 (24) | 1078 (26) |
| 80 to <85 | 4 804 (23) | 3 915 (23) | 889 (21) |
| ≥85 | 4 375 (21) | 3 805 (23) | 570 (14) |
| Sex | |||
| Female | 9 172 (44) | 7 491 (44) | 1681 (41) |
| Male | 11 861 (56) | 9 401 (56) | 2460 (59) |
| Race/ethnicity | |||
| White non-Hispanic | 18 342 (87) | 14 784 (88) | 3558 (86) |
| White Hispanic | 753 (4) | 582 (3) | 171 (4) |
| Black | 1 267 (6) | 1 049 (6) | 218 (5) |
| Asian or other | 671 (3) | 477 (3) | 194 (5) |
| Marital status | |||
| Married | 10 891 (52) | 8 438 (50) | 2453 (59) |
| Other | 10 142 (48) | 8 454 (50) | 1688 (41) |
| Medicaid coinsurance | |||
| No | 18 159 (86) | 14 535 (86) | 3624 (88) |
| Yes | 2 874 (14) | 2 357 (14) | 517 (13) |
| Comorbidity index* | |||
| 0 | 4 211 (20) | 3 339 (20) | 872 (21) |
| 1 | 4 582 (22) | 3 604 (21) | 978 (24) |
| 2 | 4 126 (20) | 3 281 (19) | 845 (20) |
| 3 | 3 046 (15) | 2 487 (15) | 559 (14) |
| 4 | 2 151 (10) | 1 729 (10) | 422 (10) |
| ≥5 | 2 917 (14) | 2 452 (15) | 465 (11) |
| Performance status* | |||
| Not poor | 13 732 (65) | 10 703 (63) | 3029 (73) |
| Poor | 7 301 (35) | 6 189 (37) | 1112 (27) |
| Dementia* | |||
| Absent | 19 073 (91) | 15 054 (89) | 4019 (97) |
| Present | 1 960 (9) | 1 838 (11) | 122 (3) |
| Poverty prevalence (%)† | |||
| 0 to <5 | 5 900 (28) | 4 614 (27) | 1286 (31) |
| 5 to <10 | 5 865 (28) | 4 700 (28) | 1165 (28) |
| 10 to <20 | 5 391 (26) | 4 371 (26) | 1020 (25) |
| ≥20 | 3 158 (15) | 2 611 (16) | 547 (13) |
| Unrecorded | 719 (3) | 596 (4) | 123 (3) |
| Population size† | |||
| ≥1 million | 11 458 (54) | 9 099 (54) | 2359 (57) |
| 250 000-1 million | 5 879 (28) | 4 804 (28) | 1075 (26) |
| <250 000 | 3 696 (18) | 2 989 (18) | 707 (17) |
| Hospice enrollment at death | |||
| No | 11 803 (56) | 9 631 (57) | 2172 (52) |
| Yes | 9 230 (44) | 7 261 (43) | 1969 (48) |
| Chemotherapy within 14 d of death | |||
| No | 18 667 (89) | 15 107 (89) | 3560 (86) |
| Yes | 2 366 (11) | 1 785 (11) | 581 (14) |
| Inpatient death | |||
| No | 11 908 (57) | 9 645 (57) | 2263 (55) |
| Yes | 9 125 (43) | 7 247 (43) | 1878 (45) |
| ICU within 30 d of death | |||
| No | 13 636 (65) | 11 054 (65) | 2582 (62) |
| Yes | 7 397 (35) | 5 838 (35) | 1559 (38) |
| Less than 3 d on hospice (n = 9230)‡ | |||
| No | 7 360 (80) | 5 915 (82) | 1445 (73) |
| Yes | 1 870 (20) | 1 346 (19) | 524 (27) |
| Outpatient hospice referral (n = 9230)‡ | |||
| No | 4 468 (48) | 3 380 (47) | 1088 (55) |
| Yes | 4 762 (52) | 3 881 (53) | 881 (45) |
| . | All patients (n = 21 033) . | Not transfusion dependent (n = 16 892) . | Transfusion dependent (n = 4141) . |
|---|---|---|---|
| Leukemia histology | |||
| AML | 8 918 (43) | 6 082 (36) | 2836 (68) |
| ALL | 652 (3) | 511 (3) | 141 (3) |
| CLL | 8 420 (40) | 7 821 (46) | 599 (15) |
| CML | 1 536 (7) | 1 305 (8) | 231 (6) |
| CMML | 1 507 (7) | 1 173 (7) | 334 (8) |
| Age group, y | |||
| 65 to <70 | 2 528 (12) | 1 914 (11) | 614 (15) |
| 70 to <75 | 4 193 (20) | 3 203 (19) | 990 (24) |
| 75 to <80 | 5 133 (24) | 4 055 (24) | 1078 (26) |
| 80 to <85 | 4 804 (23) | 3 915 (23) | 889 (21) |
| ≥85 | 4 375 (21) | 3 805 (23) | 570 (14) |
| Sex | |||
| Female | 9 172 (44) | 7 491 (44) | 1681 (41) |
| Male | 11 861 (56) | 9 401 (56) | 2460 (59) |
| Race/ethnicity | |||
| White non-Hispanic | 18 342 (87) | 14 784 (88) | 3558 (86) |
| White Hispanic | 753 (4) | 582 (3) | 171 (4) |
| Black | 1 267 (6) | 1 049 (6) | 218 (5) |
| Asian or other | 671 (3) | 477 (3) | 194 (5) |
| Marital status | |||
| Married | 10 891 (52) | 8 438 (50) | 2453 (59) |
| Other | 10 142 (48) | 8 454 (50) | 1688 (41) |
| Medicaid coinsurance | |||
| No | 18 159 (86) | 14 535 (86) | 3624 (88) |
| Yes | 2 874 (14) | 2 357 (14) | 517 (13) |
| Comorbidity index* | |||
| 0 | 4 211 (20) | 3 339 (20) | 872 (21) |
| 1 | 4 582 (22) | 3 604 (21) | 978 (24) |
| 2 | 4 126 (20) | 3 281 (19) | 845 (20) |
| 3 | 3 046 (15) | 2 487 (15) | 559 (14) |
| 4 | 2 151 (10) | 1 729 (10) | 422 (10) |
| ≥5 | 2 917 (14) | 2 452 (15) | 465 (11) |
| Performance status* | |||
| Not poor | 13 732 (65) | 10 703 (63) | 3029 (73) |
| Poor | 7 301 (35) | 6 189 (37) | 1112 (27) |
| Dementia* | |||
| Absent | 19 073 (91) | 15 054 (89) | 4019 (97) |
| Present | 1 960 (9) | 1 838 (11) | 122 (3) |
| Poverty prevalence (%)† | |||
| 0 to <5 | 5 900 (28) | 4 614 (27) | 1286 (31) |
| 5 to <10 | 5 865 (28) | 4 700 (28) | 1165 (28) |
| 10 to <20 | 5 391 (26) | 4 371 (26) | 1020 (25) |
| ≥20 | 3 158 (15) | 2 611 (16) | 547 (13) |
| Unrecorded | 719 (3) | 596 (4) | 123 (3) |
| Population size† | |||
| ≥1 million | 11 458 (54) | 9 099 (54) | 2359 (57) |
| 250 000-1 million | 5 879 (28) | 4 804 (28) | 1075 (26) |
| <250 000 | 3 696 (18) | 2 989 (18) | 707 (17) |
| Hospice enrollment at death | |||
| No | 11 803 (56) | 9 631 (57) | 2172 (52) |
| Yes | 9 230 (44) | 7 261 (43) | 1969 (48) |
| Chemotherapy within 14 d of death | |||
| No | 18 667 (89) | 15 107 (89) | 3560 (86) |
| Yes | 2 366 (11) | 1 785 (11) | 581 (14) |
| Inpatient death | |||
| No | 11 908 (57) | 9 645 (57) | 2263 (55) |
| Yes | 9 125 (43) | 7 247 (43) | 1878 (45) |
| ICU within 30 d of death | |||
| No | 13 636 (65) | 11 054 (65) | 2582 (62) |
| Yes | 7 397 (35) | 5 838 (35) | 1559 (38) |
| Less than 3 d on hospice (n = 9230)‡ | |||
| No | 7 360 (80) | 5 915 (82) | 1445 (73) |
| Yes | 1 870 (20) | 1 346 (19) | 524 (27) |
| Outpatient hospice referral (n = 9230)‡ | |||
| No | 4 468 (48) | 3 380 (47) | 1088 (55) |
| Yes | 4 762 (52) | 3 881 (53) | 881 (45) |
All data are n (%).
Based on Medicare claims within 12 months prior to death or hospice enrollment.
In patient’s county of residence, according to the US Department of Agriculture.
Only patients enrolled in hospice at death.
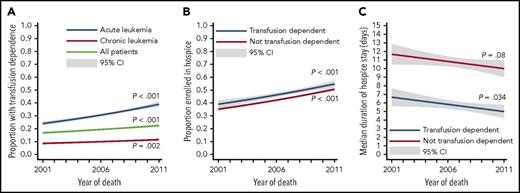
Trends in transfusion dependence and hospice use. The panels illustrate proportions of patients who were transfusion dependent at the time of death (A), who were enrolled in hospice at the time of death (B), and in median duration of hospice stay (C). The P values and CIs were calculated from generalized linear regression (A-B) or quantile regression (C).
Trends in transfusion dependence and hospice use. The panels illustrate proportions of patients who were transfusion dependent at the time of death (A), who were enrolled in hospice at the time of death (B), and in median duration of hospice stay (C). The P values and CIs were calculated from generalized linear regression (A-B) or quantile regression (C).
Overall, 44% of patients were enrolled in hospice at EOL, more for acute (47%) than for chronic (41%) leukemias. This proportion increased significantly between 2001 and 2011 (overall from 35% to 49%, for groups with and without TD, Figure 2B). There was a reciprocal decrease in inpatient deaths and chemotherapy use at EOL (Ptrend < .001) and no significant change in the proportion of hospice stays < 3 days (Ptrend = .11), whereas the rate of terminal ICU admissions increased (Ptrend < .001; supplemental Figure 2). Median duration of hospice enrollment was 9 days (IQR, 3-28) for acute and chronic leukemias, but it was shorter for transfusion-dependent patients (6 vs 11 days; P < .001). This median duration decreased slightly over time (Ptrend = .015; Figure 2C). Multiple episodes of hospice enrollment and disenrollment were noted in 5% of patients, and the data were not significantly different between those with and without TD (P = .14).
Adjusting for baseline characteristics, TD was associated with a slightly higher likelihood of hospice enrollment at EOL (RR, 1.08; 95% CI, 1.04-1.12; Table 2). Use of hospice also increased significantly with age and was substantially more common among white non-Hispanic patients, as well as those with acute leukemia or dementia. There were also weak associations with sex, comorbidities, and length of survival from diagnosis. Regardless of TD status, patients who used hospice at EOL had markedly lower rates of inpatient death, ICU admissions, and chemotherapy use at EOL, as well as lower median Medicare spending in the last 30 days of life (Figure 3).
Multivariable models for primary end points: hospice enrollment at EOL and length of terminal hospice stay (N = 21 033)
| . | Hospice enrollment . | Hospice length of stay* . | |||||
|---|---|---|---|---|---|---|---|
| RR . | 95% CI . | P . | RR . | 95% CI . | P . | ||
| TD | 1.08 | 1.04-1.12 | <.001 | 0.49 | 0.44-0.54 | <.001 | |
| Leukemia histology | |||||||
| Acute | Reference | <.001 | Reference | .62 | |||
| Chronic | 0.82 | 0.79-0.85 | 0.97 | 0.87-1.09 | |||
| Age at diagnosis, y | |||||||
| 65 to <70 | Reference | <.001 | Reference | <.001 | |||
| 70 to <75 | 1.08 | 1.01-1.15 | 1.23 | 1.02-1.49 | |||
| 75 to <80 | 1.24 | 1.16-1.32 | 1.28 | 1.06-1.53 | |||
| 80 to <85 | 1.35 | 1.27-1.44 | 1.38 | 1.15-1.65 | |||
| ≥85 y | 1.46 | 1.37-1.56 | 1.54 | 1.28-1.85 | |||
| Sex | |||||||
| Female | Reference | <.001 | Reference | <.034 | |||
| Male | 0.90 | 0.87-0.93 | 0.90 | 0.81-0.99 | |||
| Race/ethnicity | |||||||
| White non-Hispanic | Reference | <.001 | Reference | .47 | |||
| White Hispanic | 0.90 | 0.82-0.99 | 0.90 | 0.68-1.19 | |||
| Black | 0.84 | 0.78-0.91 | 0.89 | 0.71-1.11 | |||
| Asian or other | 0.65 | 0.57-0.73 | 0.85 | 0.65-1.10 | |||
| Marital status | |||||||
| Not married | Reference | .11 | Reference | .21 | |||
| Married | 1.03 | 0.99-1.06 | 0.92 | 0.83-1.02 | |||
| Baseline health indicators | |||||||
| Medicaid coinsurance | 0.91 | 0.86-0.96 | <.001 | 1.17 | 1.00-1.36 | .046 | |
| Comorbidity index† | 0.95 | 0.94-0.96 | <.001 | 0.92 | 0.89-0.94 | <.001 | |
| Poor performance status† | 1.05 | 1.01-1.09 | .006 | 1.03 | 0.93-1.14 | <.61 | |
| Dementia† | 1.14 | 1.08-1.20 | <.001 | 1.54 | 1.33-1.79 | <.001 | |
| Poverty prevalence, %‡ | |||||||
| 0 to <5 | Reference | .028 | Reference | .27 | |||
| 5 to <10 | 0.98 | 0.94-1.02 | 1.11 | 0.98-1.25 | |||
| 10 to <20 | 1.01 | 0.96-1.05 | 1.10 | 0.97-1.24 | |||
| ≥20 | 0.93 | 0.88-0.99 | 1.13 | 0.97-1.32 | |||
| Unrecorded | 1.05 | 0.97-1.14 | 1.24 | 0.96-1.60 | |||
| Population size‡ | |||||||
| ≥1 million | Reference | <.001 | Reference | .004 | |||
| 250 000-1 million | 1.10 | 1.06-1.14 | 1.12 | 1.01-1.24 | |||
| <250 000 | 0.99 | 0.95-1.04 | 1.22 | 1.08-1.38 | |||
| Survival from diagnosis, mo | |||||||
| <6 | Reference | .040 | Reference | <.001 | |||
| 6 to <12 | 1.03 | 0.98-1.08 | 1.86 | 1.67-2.07 | |||
| 12 to <24 | 1.05 | 1.00-1.10 | 2.09 | 1.84-2.37 | |||
| ≥24 mo | 1.08 | 1.03-1.13 | 2.27 | 2.02-2.56 | |||
| Year of death§ | 1.04 | 1.04-1.05 | <.001 | 1.02 | 1.00-1.03 | .011 | |
| . | Hospice enrollment . | Hospice length of stay* . | |||||
|---|---|---|---|---|---|---|---|
| RR . | 95% CI . | P . | RR . | 95% CI . | P . | ||
| TD | 1.08 | 1.04-1.12 | <.001 | 0.49 | 0.44-0.54 | <.001 | |
| Leukemia histology | |||||||
| Acute | Reference | <.001 | Reference | .62 | |||
| Chronic | 0.82 | 0.79-0.85 | 0.97 | 0.87-1.09 | |||
| Age at diagnosis, y | |||||||
| 65 to <70 | Reference | <.001 | Reference | <.001 | |||
| 70 to <75 | 1.08 | 1.01-1.15 | 1.23 | 1.02-1.49 | |||
| 75 to <80 | 1.24 | 1.16-1.32 | 1.28 | 1.06-1.53 | |||
| 80 to <85 | 1.35 | 1.27-1.44 | 1.38 | 1.15-1.65 | |||
| ≥85 y | 1.46 | 1.37-1.56 | 1.54 | 1.28-1.85 | |||
| Sex | |||||||
| Female | Reference | <.001 | Reference | <.034 | |||
| Male | 0.90 | 0.87-0.93 | 0.90 | 0.81-0.99 | |||
| Race/ethnicity | |||||||
| White non-Hispanic | Reference | <.001 | Reference | .47 | |||
| White Hispanic | 0.90 | 0.82-0.99 | 0.90 | 0.68-1.19 | |||
| Black | 0.84 | 0.78-0.91 | 0.89 | 0.71-1.11 | |||
| Asian or other | 0.65 | 0.57-0.73 | 0.85 | 0.65-1.10 | |||
| Marital status | |||||||
| Not married | Reference | .11 | Reference | .21 | |||
| Married | 1.03 | 0.99-1.06 | 0.92 | 0.83-1.02 | |||
| Baseline health indicators | |||||||
| Medicaid coinsurance | 0.91 | 0.86-0.96 | <.001 | 1.17 | 1.00-1.36 | .046 | |
| Comorbidity index† | 0.95 | 0.94-0.96 | <.001 | 0.92 | 0.89-0.94 | <.001 | |
| Poor performance status† | 1.05 | 1.01-1.09 | .006 | 1.03 | 0.93-1.14 | <.61 | |
| Dementia† | 1.14 | 1.08-1.20 | <.001 | 1.54 | 1.33-1.79 | <.001 | |
| Poverty prevalence, %‡ | |||||||
| 0 to <5 | Reference | .028 | Reference | .27 | |||
| 5 to <10 | 0.98 | 0.94-1.02 | 1.11 | 0.98-1.25 | |||
| 10 to <20 | 1.01 | 0.96-1.05 | 1.10 | 0.97-1.24 | |||
| ≥20 | 0.93 | 0.88-0.99 | 1.13 | 0.97-1.32 | |||
| Unrecorded | 1.05 | 0.97-1.14 | 1.24 | 0.96-1.60 | |||
| Population size‡ | |||||||
| ≥1 million | Reference | <.001 | Reference | .004 | |||
| 250 000-1 million | 1.10 | 1.06-1.14 | 1.12 | 1.01-1.24 | |||
| <250 000 | 0.99 | 0.95-1.04 | 1.22 | 1.08-1.38 | |||
| Survival from diagnosis, mo | |||||||
| <6 | Reference | .040 | Reference | <.001 | |||
| 6 to <12 | 1.03 | 0.98-1.08 | 1.86 | 1.67-2.07 | |||
| 12 to <24 | 1.05 | 1.00-1.10 | 2.09 | 1.84-2.37 | |||
| ≥24 mo | 1.08 | 1.03-1.13 | 2.27 | 2.02-2.56 | |||
| Year of death§ | 1.04 | 1.04-1.05 | <.001 | 1.02 | 1.00-1.03 | .011 | |
Only the second equation (for time on hospice) from the 2-part model is shown for clarity.
Based on Medicare claims within 12 months prior to death or hospice enrollment.
In patient’s county of residence, according to the US Department of Agriculture.
Linearized trend.
Indicators of EOL care quality among Medicare beneficiaries with leukemia, stratified by transfusion dependence and use of hospice at the end of life. (A) Proportions of patients dying in the inpatient setting, with an ICU admission in the last 30 days of life, or with chemotherapy administration in the last 14 days of life. (B) Median Medicare spending in the last 30 days of life (error bars indicate IQR).
Indicators of EOL care quality among Medicare beneficiaries with leukemia, stratified by transfusion dependence and use of hospice at the end of life. (A) Proportions of patients dying in the inpatient setting, with an ICU admission in the last 30 days of life, or with chemotherapy administration in the last 14 days of life. (B) Median Medicare spending in the last 30 days of life (error bars indicate IQR).
In the model evaluating the relative duration of hospice stay, TD was associated with 51% shorter time on hospice (relative duration, 0.49; 95% CI, 0.44-0.54). Advanced age, presence of dementia, residence in a less populated area, and longer survival from diagnosis were associated with longer time on hospice. In multivariable models for secondary end points, transfusion-dependent patients had a 37% higher risk of receiving hospice services for <3 days (RR, 1.37; 95% CI, 1.25-1.51), a slightly higher risk of dying in the inpatient setting (RR, 1.04; 95% CI, 1.00-1.08) or of an ICU admission before death (RR, 1.05; 95% CI, 1.00-1.10), as well as a lower likelihood of outpatient hospice referral (RR, 0.89; 95% CI, 0.84-0.94).
There was no significant heterogeneity between acute or chronic leukemias in the association between TD and hospice enrollment or duration of hospice stay (as evidenced by a nonsignificant Pinteraction = .06 and .20, respectively, supplemental Table 2). Similarly, the associations between TD and end points were not significantly different when all leukemia histologies were treated as separate categories in the analysis, with Pinteraction = .13 and .19, respectively. In contrast, TD was associated with less use of chemotherapy at EOL in acute leukemias (RR, 0.76; 95% CI, 0.69-0.84) but more use in chronic leukemias (RR, 1.79; 95% CI, 1.49-2.16, Pinteraction < .001). Among patients who enrolled in hospice, TD was associated with less frequent outpatient hospice referrals in chronic leukemias (RR, 0.74; 95% CI, 0.66-0.83) but not in acute leukemias (RR, 0.96; 95% CI, 0.90-1.02; Pinteraction < .001).
The results were consistent in sensitivity analyses using varying look-back windows for our definition of TD (60 or 90 days), restricted only to patients with leukemia recorded as the cause of death or to patients who died in 2006-2011 (supplemental Table 2). When TD was subcategorized by number of transfusion events (2, 3, or ≥4), patients with heavy transfusion use had a similar RR for hospice enrollment but shorter average hospice length of stay (supplemental Table 3). We also observed consistent associations between TD and main end points when evaluating TD based on red blood cell or platelet transfusion use only (supplemental Table 4). Based on death certificates, deaths were attributed to a cause other than leukemia in 21% of patients who had TD and in 46% of those who did not (supplemental Table 5). The percentages of deaths attributed directly to leukemia were 63% and 56% for hospice enrollees and nonenrollees, respectively.
Discussion
In this study of >21 000 Medicare beneficiaries with acute and chronic leukemias, we investigated the association among TD, hospice use, and EOL care quality measures. The analysis yielded 3 important findings that have significant implications for health care policy. First, hospice use is increasing overall for patients with leukemias, but the length of stay remains very short. Second, TD is associated with slightly higher use of hospice services, as well as a markedly (51%) shorter duration of hospice enrollment, indicating high need but less meaningful use of the service. Third, although the quality of EOL care remains poor for patients with leukemias, those who receive hospice care experience a lower risk of dying in the hospital or receiving futile chemotherapy and generate lower costs of care at EOL.
In contrast to reports from academic centers citing a 23% rate of hospice enrollment among older adults with AML and a 44% rate of chemotherapy administration at EOL, we found that hospice use has steadily increased up to 49% among Medicare beneficiaries with leukemia and that chemotherapy use at EOL was uncommon.29 Expanding upon studies in lymphomas and AML, this observation suggests that perspectives on EOL care obtained from academic institutions and from population at large may differ and that acceptance of hospice services across hematologic malignancies may be generally increasing in the community.19,25,30 Although experts have advocated for more hospice use in hematology in recent years,31,32 specialist hematologic oncologists have expressed a perception that hospice care may not be well suited to the needs of patients with leukemia.8,12 Because enrollment in hospice is endorsed by credentialing programs like the American Society of Clinical Oncology Quality Oncology Practice Initiative, administrative pressure to improve performance on this measure may result in increased terminal referrals.33 Additionally, rising rates of hospice use could reflect the grim reality that up to 50% of Medicare beneficiaries may not receive any leukemia-directed therapy, according to published studies.34,35 We note that detailed data on the use of all available therapies in various leukemias are lacking, because oral agents have not been identifiable in Medicare claims until recently. Emerging data suggest that >30% of older patients may not receive even relatively nontoxic tyrosine kinase inhibitors for CML.36 Interestingly, we observed only a minor difference (39% vs 45%) in any recorded chemotherapy between hospice users and nonusers, suggesting that the use of chemotherapy itself may not be a significant barrier to hospice in leukemias. Despite marked differences in the clinical course and management of diseases like AML and CLL (and resulting variation in transfusion dependence), the trends in hospice use or in the indicators of EOL care quality were remarkably similar for acute and chronic leukemias. To elucidate these trends, enrollment patterns should be studied in other hematologic malignancies and in diverse care settings.
Unfortunately, it is clear that TD is associated with suboptimal use of hospice care services. We found, and confirmed in multiple sensitivity analyses, a slightly higher rate of hospice use among transfusion-dependent patients. This was initially surprising, because we hypothesized a lower rate based on a study in myelodysplastic syndrome.18 Upon further investigation, this prior result may be affected by guarantee-time bias caused by anchoring of the look-back window for transfusion claims to the date of death rather than the date of hospice enrollment. We replicated this biased association and provide a detailed explanation in supplemental Figure 1. Because essentially all patients with TD had to discontinue transfusions upon hospice enrollment, counting the relevant claims in a 30-day period that includes the hospice stay leads to misclassification of those patients as not transfusion dependent. This bias was mitigated by extending the look-back window (to 60 or 90 days) or by anchoring it correctly to the date of hospice entry. Furthermore, several explanations conceptually support our result, suggesting that an even higher RR for hospice use in transfusion-dependent patients might have been observed had there been no barrier to enrollment. First, TD correlates with leukemia severity and resulting marrow failure, which may indicate to patients a terminal phase of their disease. It may also cause exhaustion from frequent visits and related symptoms and make patients more inclined to use hospice. In this context, one would expect a high need for hospice services in the transfusion-dependent population; thus, the observed borderline RR of 1.08 may still indicate a significant barrier to enrollment. Second, patients dying before reaching the transfusion-dependent stage, as a result of acute events or treatment-related toxicities, would be less likely to use hospice. Third, patients with TD had a higher proportion of “leukemia” recorded as the cause of death (79% vs 54%). Although death certificates frequently misclassify true causal attributions, patients who really die of sudden noncancer-related events like accidents or myocardial infarction might be less likely to receive hospice care. Nevertheless, the association between TD and hospice use persisted in a sensitivity analysis limited to patients with leukemia recorded as the cause of death. It was also similar for chronic and acute leukemias, despite a higher prevalence of cardiovascular causes of death in chronic leukemias (21% vs 4%).
Most importantly, we found that TD was associated with a markedly reduced hospice length of stay that was already shorter in leukemia (9 days) than the national average (19 days) among cancer patients.5,37,38 Accordingly, transfusion-dependent patients had a 37% increased risk of using hospice for <3 days. Consequently, these patients and their families barely gleaned many of the palliative and psychosocial services available under hospice benefit. Novel strategies are needed to engage leukemia patients and help them to access hospice earlier than in the last week of life, a point underscored by the fact that their average hospice length of stay has not improved. The association of TD with short hospice stays cannot be explained by a rapid clinical deterioration in AML, because it was the same in chronic leukemias. Rather, it may relate to the difficulties that hematologists report in predicting the terminal phase of blood cancers or reflect patients’ preference to hold off on enrollment until the last days of life because of refusal to discontinue transfusion support.7 Short survival of transfusion-dependent patients on hospice may reflect, in part, their more advanced terminal status, but we argue that the difference would not be as pronounced if they were allowed to enroll early, without having to forsake the transfusion support. We observed a low rate of multiple hospice enrollment episodes (5%), regardless of the TD status, suggesting that once a decision to pursue hospice care is made, it is rarely reversed to obtain a transfusion. Nevertheless, 1 recent study found that >60% of AML patients who disenrolled from hospice received subsequent transfusions.19
The need to improve meaningful use of hospice through adjustment of policy and clinical practice relates to our third key finding: the strong correlation between hospice care and improved measures of EOL care quality. Hospice enrollees had a lower likelihood of dying in the hospital (overall 3% vs 75%), less chemotherapy use at EOL, and lower average Medicare spending in the last 30 days of life. By virtue of their median age, most leukemia patients in the United States have Medicare coverage, and Medicare policy surrounding the hospice benefit is a major factor shaping the use of hospice services.39,40 Recognizing when hospice care becomes the optimal strategy for further management of a leukemia could have significant societal benefits, because pervasive futile interventions during the terminal phase of the disease are expensive and may be detrimental to the overall quality of life.41,42 More important than cost considerations are the issues of goal-concordant care and preferred place of death, especially that standard indicators of EOL care quality endorsed by the National Quality Forum are not universally accepted as optimal by hematologists. Most cancer patients prefer to die at home, yet 43% of patients included in our analysis died in the hospital, 35% spent time in the ICU during the last month of life, and 20% of those who enrolled in hospice did so for <3 days.43 Although we observed a decreasing use of chemotherapy at EOL, we notably excluded deaths within 30 days of diagnosis, often as a result of aggressive chemotherapy. In contrast, a recent study that examined all deaths among Medicare beneficiaries with AML showed a slight increase in chemotherapy use at the EOL.19 Different approaches to systemic therapy may explain the heterogeneous association between TD and use of chemotherapy at EOL in acute and chronic leukemias, because palliative oral therapy with tyrosine kinase inhibitors (in CML) or chlorambucil (in CLL) may continue longer than intensive regimens for AML/ALL. This issue may become increasingly important as patients with leukemia on palliative oral agents, like the B-cell receptor signaling inhibitors in CLL, begin to face the need to discontinue these agents when considering hospice enrollment. Aggressive EOL care remains quite prevalent in leukemia and should be a focus of further research, interventions, and policy initiatives. This may include “open hospice” models that allow transfusions and other interventions that could potentially prolong life. Such interventions can still represent high-quality goal-concordant care that helps patients and families to achieve important objectives even when cure is no longer possible. Transfusions can address palliative needs related to breathlessness, bothersome bleeding, and profound fatigue. Arguably, relieving these symptoms should be a goal similar to treatment of pain, constipation, or obstructive symptoms typical for patients with solid tumors.44,45
One promising strategy to improve the EOL care in oncology has been to incorporate specialist palliative care earlier in the course of illness, alongside active cancer therapy. The focus of such efforts is distinct from the EOL care provided by hospice, yet early palliative care enhances prognostic understanding, reduces inpatient deaths and ICU admissions, and increases hospice use and length of stay.46,47 The approach has shown success among recipients of stem cell transplantation and is being tested in AML patients undergoing induction chemotherapy (NCT02975869).48
There are several limitations inherent in our approach. First, observed associations may not be causal (eg, the association between overall hospice use and TD is likely confounded by underlying disease severity). Because we included multiple histologies and categorized them into 2 broad groups, we could not use some of the claims-based constructs that approximate disease severity in specific hematologic malignancies.18,34 Second, the population represented in our dataset is limited to fee-for-service Medicare, and findings may not translate to managed-care plan enrollees, younger patients, or other diseases. The definition of TD is not uniform, even in clinical research, and we cannot exclude some ascertainment bias regarding transfusions during prolonged hospital admissions (recorded as single events in Medicare files). We guarded against this bias by examining alternative TD definitions in sensitivity analyses. Finally, some weak associations from our models (RR, 0.90-1.10) are “statistically significant” but may not be clinically revealing.
In conclusion, the association between TD and suboptimal use of hospice services indicates an important opportunity to improve the EOL care for older Americans with leukemias. Our population-derived data add richer context to what surveys had told us about hematologists’ views on hospice care, as well as highlight the need to further investigate patients’ and caregivers’ perspectives, conspicuously missing from the literature.49 From the perspective of health care policy, our analysis supports testing “open hospice access” initiatives (ie, Medicare Care Choices Model) that could allow leukemia patients to overcome enrollment barriers related to transfusion needs. Recognizing the advantage associated with hospice care in terms of quality care, decreased resource use, and Medicare spending, explicit policy adjustments addressing enrollment barriers may be warranted.
Presented in part at the 59th annual meeting of the American Society of Hematology, Atlanta, GA, 9 December 2017.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors acknowledge the efforts of the National Cancer Institute, the Office of Research, Development and Information, Centers for Medicare & Medicaid Services, Information Management Services, Inc., and the SEER Program tumor registries in the creation of the SEER-Medicare database.
The collection of cancer incidence data used in this study was supported by the California Department of Public Health, as part of the statewide cancer-reporting program mandated by California Health and Safety Code section 103885; the National Institutes of Health National Cancer Institute’s SEER program under contract HHSN261201000140C awarded to the Cancer Prevention Institute of California, contract HHSN261201000035C awarded to the University of Southern California, and contract HHSN261201000034C awarded to the Public Health Institute; and the Centers for Disease Control and Prevention’s National Program of Cancer Registries, under agreement U58DP003862-01, awarded to the California Department of Public Health. T.W.L. is supported by a Sojourns Scholars Award from the Cambia Health Foundation and a Mentored Research Scholar Grant from the American Cancer Society (MRSG-15-185-01-PCSM). A.J.O. is supported by a Research Scholar Grant from the American Cancer Society (128608-RSGI-15-211-01-CPHPS) and an Institutional Development Award Program Infrastructure for Clinical and Translational Research (IDeA-CTR) grant from the National Institutes of Health, National Institute of General Medical Sciences (U54GM115677).
The ideas and opinions expressed in this article are those of the authors, and endorsement by the State of California Department of Public Health, the National Cancer Institute, and the Centers for Disease Control and Prevention or their contractors and subcontractors is not intended and should not be inferred.
Authorship
Contribution: T.W.L. designed research, interpreted data, and wrote the manuscript; P.C.E. interpreted data and wrote the manuscript; and A.J.O. curated the data, conducted all statistical analyses, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Thomas W. LeBlanc, Duke Cancer Institute, 2424 Erwin Rd, Suite 602, Durham, NC 27705; e-mail: thomas.leblanc@duke.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal