An integrated genomic analysis presented by Walker et al in this issue of Blood confirms that the primary genetic event partially determines the pattern of secondary genetic events in untreated multiple myeloma (MM).1
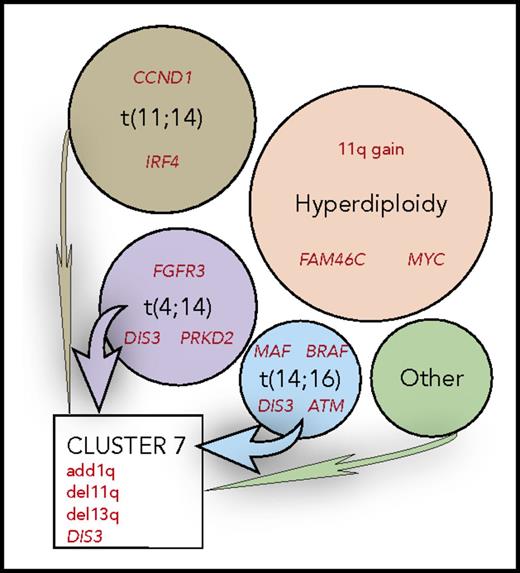
MM can be divided into nonoverlapping molecular subtypes, shown in circles, based on the primary genetic event, identified by black text. These appear to partially determine the profile of secondary genetics events, summarized in red text. Clustering of the copy number abnormalities identifies novel clusters with convergent evolution, such as cluster 7, identifying patients with a poor prognosis.
MM can be divided into nonoverlapping molecular subtypes, shown in circles, based on the primary genetic event, identified by black text. These appear to partially determine the profile of secondary genetics events, summarized in red text. Clustering of the copy number abnormalities identifies novel clusters with convergent evolution, such as cluster 7, identifying patients with a poor prognosis.
Purified tumor samples from 1273 newly diagnosed MM patients, with associated clinical and outcome data, were analyzed by RNA sequencing, whole exome sequencing, and in a majority of cases whole genome sequencing. The sequence data were analyzed to identify the expression of RNA transcripts, genomic structural variants (translocations, deletions, insertions, inversions), single nucleotide variants, indels, loss of heterozygosity, and copy number abnormalities affecting whole chromosomes, segments of chromosomes, and individual genes.
It seems well established that the initiating oncogenic events common to most premalignant monoclonal gammopathy of undetermined significance and MM tumors include at a minimum 1 of 7 primary immunoglobulin heavy chain gene translocations or hyperdiploidy, which is manifested by trisomies of several odd-numbered chromosomes (3, 5, 7, 9, 11, 15, 19, and 21).2-4 Secondary, progressive genetic events appear to be shared among the different kinds of tumors even though the prevalence of a specific progression event can differ for different subtypes of MM tumors.5,6 The authors of this study provide some novel information regarding secondary events and their relation to primary events.
First, using several methods involving either a frequency-based approach or a functionally based approach, they have identified a total of 63 apparent driver genes, with 17 potentially actionable. This includes several novel putative oncogenes (PTPN11, PRKD2, SF3B1, IDH1, and IDH2) and several putative tumor suppressor genes (UBR5, HUWE1), all of which are mutated only infrequently in MM tumors. They also identify important pathways based on the 63 genes: MEK/ERK (50%), NF-κB signaling (14%), G1/S cell cycle transition (5%), epigenetic regulators (24%), and RNA processing (18%).
Second, they provide multiple examples of presumptive secondary genetic events that are nonrandomly associated with tumors that have different primary events (see figure). In one case, it appears that both synonymous and nonsynonymous mutations in CCND1 are predetermined by the t(11;14) translocation, which dysregulates CCND1. Also, mostly nonsynonymous mutations in MAF are present uniquely in tumors with the t(14;16) translocation. Similarly, FGFR3 has activating mutations only in t(4;14) tumors, in which FGFR3 is upregulated by the translocation. This also may be true for unique mutations at aa594 in BRAF in t(14;16) tumors because of an APOBEC mutational signature that is associated with t(14;16) tumors. In other tumors, it is unclear why certain genetic events are more prevalent, for example, 11q+, FAM46C mutations, MYC rearrangements in hyperdiploid tumors; or PRDK2 and DIS3 mutations predominantly in t(4;14) tumors; or BRAF, DIS3, and ATM mutations in t(14;20) tumors. Their data also confirm one of their earlier observations that NRAS mutations are found predominantly in t(11;14) and hyperdiploid tumors, whereas KRAS mutations are more randomly distributed among tumors with different primary events.7 Curiously, there is a predominance of mutations at codon 61 of NRAS vs more evenly distributed mutations at codons 12, 13, and 61 of KRAS.7 In addition to the nonrandom association of secondary events in different types of MM tumors, the figure also shows an example of the convergent evolution of secondary events (1q+, 11q−, 13q−, DIS3 mutation) in nonhyperdiploid MM tumors with different primary events.
Third, based on variant allele frequencies, they show that oncogenic mutations are more likely to be clonal than tumor suppressor gene mutations, with the notable exception of TP53. They suggest that the higher variant allele frequency suggests an earlier time of the mutation or a greater selection pressure for oncogenic mutations.
Fourth, they show that an increasing number of driver abnormalities are associated with a poor prognosis. Driver abnormalities include not only mutated driver genes, but also translocations, chromosomal and segmental chromosome gains and losses, loss of heterozygosity, and APOBEC mutational signature. Given the low prevalence of mutated driver genes in tumors, it seems likely that these other events are largely responsible for differing clinical outcomes. The recent study by Bolli et al5 clearly shows that it is mostly these other abnormalities and not mutated driver genes that effect clinical prognosis. In fact, both papers find that TP53 is the only mutated driver gene that is a very strong predictor of clinical outcomes.
In conclusion, this is an impressive collection of clinical and genomic data on 1273 newly diagnosed MM tumors that will be available for others to use for additional insights.
Conflict-of-interest disclosure: The authors declare no competing financial interests.