In this issue of Blood, Leins et al use hematopoietic stem cell (HSC) transplantation from young or old mice into young Rag1−/− hosts to demonstrate that T- and B-cell reconstitution and acquisition of immune function is dependent on blocking the age-associated higher expression of cell division control 42 (Cdc42) in HSCs from old animals transferred to a young host. They achieved this by treating old HSCs with the specific pharmacologic Cdc42 inhibitor CASIN [2-((6-phenyl-2,3,4,9-tetrahydro-1H-carbazol-1-yl)amino)ethanol], otherwise known as Pirl1-related compound 2.1
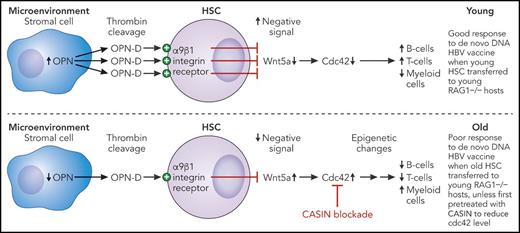
Shown are hypothetical dysregulated hematopoietic pathways in old bone marrow. In young mice, sufficient osteopontin fragment-D (OPN-D) is produced by thrombin cleavage of OPN from BM stromal cells to maintain tonic Wnt5a signaling such that Cdc42 levels are not raised. In old animals, however, lower availability of OPN-D allows higher expression of Wnt5a, which stimulates Cdc42 expression. This can be pharmacologically blocked by CASIN, resulting in normalization of epigenetic changes in HSCs otherwise associated with a skewing of immune cell output with fewer B-cell and T-cell progenitors, more myeloid cells, and poor vaccine responsiveness. The end result is that CASIN-treated old HSCs transferred to a young environment reconstitute an immune system with a distribution of B cells, T cells, and myeloid cells similar to that observed after transfer of young HSCs that is able to respond to de novo antigen challenge in the form of a DNA vaccine. Professional illustration by Patrick Lane, ScEYEnce Studios.
Shown are hypothetical dysregulated hematopoietic pathways in old bone marrow. In young mice, sufficient osteopontin fragment-D (OPN-D) is produced by thrombin cleavage of OPN from BM stromal cells to maintain tonic Wnt5a signaling such that Cdc42 levels are not raised. In old animals, however, lower availability of OPN-D allows higher expression of Wnt5a, which stimulates Cdc42 expression. This can be pharmacologically blocked by CASIN, resulting in normalization of epigenetic changes in HSCs otherwise associated with a skewing of immune cell output with fewer B-cell and T-cell progenitors, more myeloid cells, and poor vaccine responsiveness. The end result is that CASIN-treated old HSCs transferred to a young environment reconstitute an immune system with a distribution of B cells, T cells, and myeloid cells similar to that observed after transfer of young HSCs that is able to respond to de novo antigen challenge in the form of a DNA vaccine. Professional illustration by Patrick Lane, ScEYEnce Studios.
Leins et al compare the phenotypic and functional attributes of T and B cells in animals reconstituted with CASIN-treated old HSCs with those in unmanipulated young or old control mice and conclude that the observed age-associated attenuated immune responses are caused by intrinsic changes in the HSCs. Thus, reconstitution of the Rag1−/− hosts with CASIN-treated old HSCs results in a normalization of the proportions of T, B, and myeloid cells to the extent seen in hosts reconstituted with young HSCs, whereas reconstitution with untreated old HSCs results in a phenotype similar to that of unmanipulated old control mice. Crucially, the in vivo functionality of the reconstituted immune system is demonstrated by the finding that old animals respond less well to a DNA-based hepatitis B virus vaccine than young controls, which is also seen in young Rag1−/− hosts that receive HSCs from old animals. However, when HSCs from old animals are pretreated with CASIN before transplantation, the reconstituted animals respond to the vaccine almost as well as young control mice. These results unequivocally demonstrate that (in the presence of an intact thymus in a young host environment) HSCs transferred from old animals are functionally less competent because of the presence of elevated levels of Cdc42. Of course, in real-life aging, the HSCs, the thymus, and the systemic environment would be different in older individuals, so the relative contribution of these phenomena to physiological aging cannot be determined at present. However, it is interesting that the authors observe that the age-associated skewing of the distribution of immune cells, characterized as fewer B cells, more myeloid cells, fewer naïve T cells, more late-stage memory cells, and more regulatory T cells, is observed in these young animals reconstituted with (untreated) old HSCs, but is reversed by CASIN-treatment prior to transfer. These results suggest that the balance of immune cells in older individuals, which is commonly believed to be the result of lifelong exposures to pathogens or other extrinsic stimuli, is in fact at least partly due to intrinsic stem cell effects.
Many important questions are raised by the Leins et al study. First, what are the mechanisms responsible for the higher level of Cdc42 in old HSCs and why does this have negative effects? In earlier work, the authors showed that elevated Wnt5a expression in old HSCs had similar effects on cell polarization and myeloid skewing, and this was mediated by Cdc42.2 Downstream activities of Cdc42 include regulatory effects on tubulin and actin organization that could potentially be mediated through modulating histone H4 acetylation and affecting cell-cell contact and adhesion, the latter suggesting that interactions with the bone marrow (BM) niche may be crucial.3 Thus, ancient cell signaling pathways active in many cells and tissue types are involved in HSC aging. Although the authors refer to this as intrinsic, the next question is what causes the increased Wnt5a-Cdc42 activity in old HSCs. Here, the authors have made an important contribution by showing that levels of osteopontin (OPN) are lower in the BM of old mice, in the stromal cells, not the HSCs. They further determined that an OPN fragment (OPN-D) cleaved by thrombin binds to integrin α9β1 on the HSC surface, resulting in downregulation of Wnt5a.4 In the absence of sufficient OPN, this pathway is potentially compromised, resulting in greater Wnt5a expression (see figure). This raises the question of why there is less OPN expression in BM stroma of older individuals, a question that has not yet been answered.
What might be the translational relevance of these results? It is intriguing that in an early study by Kerber et al,5 which examined gene expression profiles for their association with aging and mortality in human families over 3 generations, the strongest association with negative outcomes was found for Cdc42. Such family studies are rare but powerful, putting Cdc42 firmly in the spotlight as a major regulator of human aging. Mechanisms accounting for these effects were not explored in the Kerber et al study, but Leins et al recently published data indicating that Cdc42 protein levels measured in the peripheral blood of 196 donors matched the chronological age of the participant (as established by methylation profiling) and were strongly positively associated with cardiovascular disease.6 Given the indications of the importance of Cdc42 in human aging and disease, it is a priori likely that the data from the mouse model presented by Leins et al and their earlier work will be of major translational relevance. Hence, it seems that Cdc42 levels may be valuable indicators of healthy human aging. Having thoroughly cased the joint, the time may be right to translate this knowledge into action by using pharmacologic approaches or by intervening in the OPN pathway.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal