Abstract
The introduction of JAK inhibitors, leading to regulatory approval of ruxolitinib, represents a major therapeutic advance in myelofibrosis (MF). Most patients experience reduction in splenomegaly and improved quality of life from symptom improvement. It is a paradox, however, that, despite inhibition of signaling downstream of disease-related driver mutations, JAK inhibitor treatment is not associated with consistent molecular or pathologic responses in MF. Furthermore, there are important limitations to JAK inhibitor therapy including development of dose-limiting cytopenias and/or nonhematological toxicities such as neuropathy or opportunistic infections. Over half of the patients discontinue treatment within 3 years of starting treatment. Although data are sparse, clinical outcome after JAK inhibitor “failure” is likely poor; consequently, it is important to understand patterns of failure to select appropriate salvage treatment(s). An algorithmic approach, particularly one that incorporates cytogenetics/molecular data, is most helpful in selecting stem cell transplant candidates. Treatment of transplant-ineligible patients relies on a problem-based approach that includes use of investigational drugs, or consideration of splenectomy or radiotherapy. Data from early phase ruxolitinib combination studies, despite promising preclinical data, have not shown clear benefit over monotherapy thus far. Development of effective treatment strategies for MF patients failing JAK inhibitors remains a major unmet need.
Introduction
Currently, no treatment other than allogeneic stem cell transplantation (SCT) produces consistent molecular and/or pathologic responses in primary myelofibrosis (PMF) or post–essential thrombocythemia (ET)/post–polycythemia vera (PV) myelofibrosis (MF). The only JAK inhibitor currently licensed by the US Food and Drug Administration for treatment of intermediate- or high-risk MF is ruxolitinib. Consequently, the most mature safety and efficacy data for JAK inhibitors in MF therapy relate to ruxolitinib. The development of other JAK inhibitors for MF therapy has been slow either due to initial toxicity concerns (eg, fedratinib, pacritinib),1,2 or apparent lack of advantage over ruxolitinib in terms of key study end points (eg, momelotinib),3,4 or for proprietary reasons (eg, BMS-911543) (Table 1).
Summary phase 3 clinical trial data for JAK inhibitors used for MF therapy
| Trial . | n* . | Treatment arm(s) . | Control arm . | Prior JAK inhibitor allowed? . | Median follow-up (median drug exposure) . | Study drug discontinuation rate . | Dose modifications† . | Spleen volume response‡ . | Symptom response . |
|---|---|---|---|---|---|---|---|---|---|
| COMFORT-I | 155 | Ruxolitinib | Placebo | No | 268 wk (149 wk) | 72% | 57% | 42%; P < .001 | 46%; P < .001 |
| COMFORT-II | 146 | Ruxolitinib | BAT | No | 4.7 y (2.6 y) | 73% | n.a. | 28%; P < .001 | Not applicable |
| JAKARTA | 193 | Fedratinib,400 mg QD,500 mg QD | Placebo | No | n.a. (30 wk, 400 mg) (28 wk, 500 mg) | 25%, 400 mg | 24%, 400 mg | 36%, 400 mg; P < .001 | 36%, 400 mg; P < .001 |
| 33%, 500 mg | 37%, 500 mg | 40%, 500 mg; P < .001 | 34%, 500 mg; P < .001 | ||||||
| PERSIST-I | 220 | Pacritinib,400 mg QD | BAT | No | 22.9 mo (15.6 mo) | 59%§ | 47% | 19%; P = .0003 | 19%, 0.24 |
| PERSIST-II | 211 | Pacritinib,400 mg QD,200 mg BID | BAT, including ruxolitinib, 45% | Yes | n.a. (24 wk) | 40%, QD§ | 20%, QD | 15% QD; P = .02 | 17% QD; P = .7 |
| 29%, BID§ | 12%, BID | 22% BID; P = .001 | 32% BID; P = .01 | ||||||
| SIMPLIFY-I | 215 | Momelotinib,200 mg QD | Ruxolitinib,20 mg BID | No | 24 wk (21 wk) | 19% | 26% | 27%, noninferiority | 28%, noninferiority |
| Met; P = .01 | Not met; P = .98 | ||||||||
| SIMPLIFY-II | 104 | Momelotinib,200 mg QD | BAT, including ruxolitinib, 88% | Yes | 168 d (19.5 wk) | 34% | 16% | 7%; P = .9 | 26%; P = .0006 |
| Trial . | n* . | Treatment arm(s) . | Control arm . | Prior JAK inhibitor allowed? . | Median follow-up (median drug exposure) . | Study drug discontinuation rate . | Dose modifications† . | Spleen volume response‡ . | Symptom response . |
|---|---|---|---|---|---|---|---|---|---|
| COMFORT-I | 155 | Ruxolitinib | Placebo | No | 268 wk (149 wk) | 72% | 57% | 42%; P < .001 | 46%; P < .001 |
| COMFORT-II | 146 | Ruxolitinib | BAT | No | 4.7 y (2.6 y) | 73% | n.a. | 28%; P < .001 | Not applicable |
| JAKARTA | 193 | Fedratinib,400 mg QD,500 mg QD | Placebo | No | n.a. (30 wk, 400 mg) (28 wk, 500 mg) | 25%, 400 mg | 24%, 400 mg | 36%, 400 mg; P < .001 | 36%, 400 mg; P < .001 |
| 33%, 500 mg | 37%, 500 mg | 40%, 500 mg; P < .001 | 34%, 500 mg; P < .001 | ||||||
| PERSIST-I | 220 | Pacritinib,400 mg QD | BAT | No | 22.9 mo (15.6 mo) | 59%§ | 47% | 19%; P = .0003 | 19%, 0.24 |
| PERSIST-II | 211 | Pacritinib,400 mg QD,200 mg BID | BAT, including ruxolitinib, 45% | Yes | n.a. (24 wk) | 40%, QD§ | 20%, QD | 15% QD; P = .02 | 17% QD; P = .7 |
| 29%, BID§ | 12%, BID | 22% BID; P = .001 | 32% BID; P = .01 | ||||||
| SIMPLIFY-I | 215 | Momelotinib,200 mg QD | Ruxolitinib,20 mg BID | No | 24 wk (21 wk) | 19% | 26% | 27%, noninferiority | 28%, noninferiority |
| Met; P = .01 | Not met; P = .98 | ||||||||
| SIMPLIFY-II | 104 | Momelotinib,200 mg QD | BAT, including ruxolitinib, 88% | Yes | 168 d (19.5 wk) | 34% | 16% | 7%; P = .9 | 26%; P = .0006 |
BAT, best available therapy; BID, twice daily; n.a., not available; QD, once daily.
Number of patients in study drug arm(s).
May or may not include dose interruptions.
Primary end point assessment.
Excluding discontinuations due to study hold.
Why is JAK inhibitor therapy not disease modifying in MF?
The 3 canonical gene mutations largely restricted to Philadelphia-negative myeloproliferative neoplasms (MPNs), namely JAK2V617F, MPL exon 10, and CALR exon 9 mutations, represent true drivers of disease phenotype in various disease models, acting via constitutive activation of the cytokine receptor-JAK-STAT signaling pathways.5 Consequently, it is puzzling that despite potent in vitro inhibitory activity, none of the JAK inhibitors tested in the clinic has shown consistent molecular and/or pathologic responses thus far. Several lines of speculative reasoning can be put forth in this regard. First, clinical responses may be correlated with JAK2V617F variant allele frequency, which is highly variable in MF (1%-100%), generally with higher levels seen in post-ET/PV MF than in PMF.6,7 In 1 study, spleen responses in patients with progressive or massive splenomegaly who were treated with ruxolitinib were significantly higher in those with JAK2V617F allele burden ≥50%.8 Second, the frequency of mutations in noncanonical MPN-relevant genes (eg, ASXL1, SRSF2) may influence response to JAK inhibitors. In 1 study, spleen responses to momelotinib therapy were significantly higher in patients with CALR-mutated/ASXL1-unmutated status (73%) as compared with those with the reverse profile (25%).9 Similarly, in a ruxolitinib treated cohort, those with mutations in ≤2 noncanonical genes had ninefold higher odds of spleen response as compared with those with mutations in ≥3 such genes.10 Third, although there are data from healthy volunteers,11,12 few JAK inhibitor studies have provided high-quality pharmacodynamics data correlating target inhibition with clinical response in MF patients. Consequently, suboptimal treatment responses may be partly related to inadequate drug exposure in some patients. In the phase 1/2 ruxolitinib study, a dose- and time-dependent decrease in phosphorylated STAT-3 (pSTAT-3) levels was demonstrated.13 In contrast, mean pSTAT3 reduction from baseline was similar across dose levels with fedratinib treatment, although patients with higher levels of pSTAT3 inhibition were more likely to achieve a spleen response.14 More optimal inhibition of downstream signaling may not be feasible however due to competing toxicity concerns, such as myelosuppression. Fourth, some have proposed heterodimeric JAK-STAT activation as a mechanism of persistence of MPN cells despite sustained JAK2 inhibition.15 Finally, some have argued that JAK inhibitors would be more efficacious if used earlier in the disease course (proliferative/cellular phase), where, similar to data with interferon-α, there may be a greater likelihood of improving clinical end points with accompanying favorable changes in BM architecture.16-19
Incidence and patterns of JAK inhibitor treatment failure in MF
A rough proxy for JAK inhibitor failure in MF is the treatment discontinuation rate in clinical trials. The most mature follow-up data are available for ruxolitinib; the discontinuation rate in COMFORT-I and COMFORT-II trials was ∼50% at 3 years and 75% at 5 years.20-23 In the COMFORT trials, 25% to 35% of patients discontinued ruxolitinib due to treatment-related adverse events.22,23 The incidence of new or worsening grade 3/4 anemia (38%) or thrombocytopenia (12%) is generally highest in the first 6 months of ruxolitinib treatment and decreases therafter.22 The most common nonhematological adverse events are diarrhea (36%) and peripheral edema (33%).23 Infections of special interest among ruxolitinib-treated patients have included urinary tract infection (25%), pneumonia (13%), herpes zoster infection (12%), sepsis (8%), and tuberculosis (1%).23
The discontinuation rate may be higher with other JAK inhibitors, although direct comparisons are rare and treatment follow up is shorter (Table 1); furthermore, in the setting of second-line JAK inhibitor treatment, the disease biology may have evolved.1-4,24,25 Specific nonhematological toxicities of concern with other JAK inhibitors included initial concerns about Wernicke encephalopathy with fedratinib,1,26 although more recent data have questioned this association,27 and regulatory applications for this drug are planned in 2018. Peripheral neuropathy has been reported with momelotinib.4,28,29 Pacritinb was placed on hold in February 2016 after initial reports of patient deaths related to cardiac failure and intracranial hemorrhage emerged from the PERSIST trials; the clinical hold was lifted in January 2017 after review of more mature data from these studies and a new dose finding study in MF patients has been initiated.2,24,30
Occurrence of adverse events frequently leads to dose reduction/interruption, which may lead to loss of response. This translates into unsatisfactory therapeutic benefit for some patients. For example, in the COMFORT-I study, 57% of patients in the ruxolitinib randomized arm required dose reductions due to adverse events.22 Given that achieving/maintaining spleen response is more dose sensitive as compared with symptom response,31 loss of spleen response in the setting of dose reduction/interruption is likely an important contributor to treatment discontinuation. Correspondingly, in COMFORT-I, there was a progressive decrease in the proportion of patients with spleen response, from 42% at week 24 to 19% at week 264.22
Rates of leukemic transformation in studies have been relatively low possibly reflecting the short follow-up, reported as 3.2% and 5.5% in the COMFORT-I and COMFORT-II trials, respectively.22,23 Finally, treatment discontinuation due to comorbid illness is not uncommon given the age distribution of MF patients.
Prognosis of MF patients after discontinuation of JAK inhibitor therapy
Relatively little data exist regarding clinical outcome of patients following discontinuation of JAK inhibitor therapy. When available, these data largely pertain to patients with intermediate- and high-risk MF, reflecting the patient profile in clinical trials. Data from a subset of patients who received ruxolitinib in the phase 1/2 study were recently published.34 Of the 56 patients who discontinued treatment, median survival time was only 14 months. Survival was not different based on reason for discontinuation (66% had relapsed disease, 11% leukemic transformation). Patients with platelet count <100 × 109/L at the time of discontinuation had a significantly worse overall survival (OS). Of 42 informative patients, 14 (33%) had acquired a new deleterious mutation (60% ASXL1). The patients with molecular clonal evolution had significantly worse survival than those without (6 vs 16 months).
Selection of salvage therapy after JAK inhibitor failure in MF
Selection of a treatment approach is challenging and has to consider clinical (eg, anemia/thrombocytopenia grade, spleen size), biological (eg, poor-risk molecular or cytogenetic abnormalities), and patient-specific (eg, age, performance status) factors, and the reason(s) for JAK inhibitor failure (eg, inadequate dosing due to drug-related toxicity). Application of a traditional clinical prognostic model,35 supplemented by molecular/cytogenetic data,36 is likely most useful in identifying those patients who may benefit from SCT.37 For SCT-ineligible patients, in the absence of an identifiable treatment target,38 there is no algorithmic treatment approach and a more traditional symptom/problem-based approach is frequently necessary. Clearly, one has to consider patient eligibility for available clinical trials; if the reason for JAK inhibitor failure relates to a drug-class effect (eg, dose-limiting thrombocytopenia), then an agent with a novel mode of action is preferred. In other select instances, participation in a clinical trial with an alternative JAK inhibitor may be appropriate. Some patients may be stabilized or clinical response restored with judicious addition of drugs with known activity in MPNs such as interferon-α or hydroxyurea to the JAK inhibitor, although robust safety data for such combinations is lacking.39-41
Stylized cases to illustrate specific treatment challenges
Case 1
A 72-year-old woman developed splenomegaly with a 30-pound weight loss, drenching night sweats, and bone pain. The hemoglobin was 9.6 g/dL, white blood cell count (WBC) was 16.1 × 109/L (1% blasts), and platelet count was 104 × 109/L. The lower spleen edge extended to the pelvic brim. A BM biopsy confirmed JAK2V617F-positive PMF; the karyotype was 46,XX,del(13)(q12q22)[17]/46,XX,[3]. The patient had a Dynamic International Prognostic Scoring System (DIPSS) score of 3 (intermediate-2 risk) and was started on ruxolitinib treatment (15 mg twice daily). By week 4, she had near resolution of constitutional symptoms with significant decrease in spleen size, and by 3 months, the CBC parameters had normalized. After 18 months, progressive spleen growth, that was unresponsive to ruxolitinib 20 mg twice daily, was observed. The hemoglobin was 9.4 g/dL; WBC, 107.6 (1% blasts) × 109/L; and platelets, 23 × 109/L. A repeat BM biopsy was unchanged from baseline; the karyotype was 46,XX,del(13)(q12q22)[12]/46,idem,der(6)t(1;6)(q21;p21.3)[4]/46,idem,t(1;8)(q21;p23),der(8)t(1;8)(q21;p23)[4].
Summary
This patient had disease relapse by revised IWG-MRT criteria based on loss of anemia and spleen responses.32 There are limited treatment options for transplant-ineligible patients with severe thrombocytopenia in the setting of JAK inhibitor treatment. Treatment-related thrombocytopenia is commonly seen in the first 8 to 12 weeks of JAK inhibitor treatment and is generally grade 1/2 in severity. Such patients may require a period of dose reduction or, less commonly, treatment interruption. When seen independent of treatment effect, development of ≥grade 3 thrombocytopenia in MF indicates advanced disease, and is associated with a median OS of 12 to 18 months,42 and significantly worse leukemia-free survival.43 In this patient, the thrombocytopenia likely reflects disease evolution given its late occurrence, association with loss of spleen response and acquisition of new cytogenetic abnormalities. Clonal cytogenetic evolution, which can be seen in 25% of patients during JAK inhibitor treatment, has been associated with inferior OS and leukemia-free survival.44
Although second-line JAK inhibitor treatment may produce clinically relevant spleen responses in a subset of ruxolitinib-refractory or intolerant MF patients,25 there is limited data regarding the safety of JAK inhibitors in patients with severe thrombocytopenia (platelet count <50 × 109/L).45,46 Clinical trials with pacritinib have focused on this niche; in the PERSIST-1 study, which randomized JAK inhibitor-naive MF patients to either pacritinib 400 mg per day or best available therapy, 16% and 17% of patients on the pacritinib arm had pretreatment platelet counts of <50 and 50 × 109/L to 99 × 109/L, respectively.2 While the pacritinib arm met its primary end point of spleen volume response, of the patients with baseline grade 2 and 3 thrombocytopenia, 63% and 57%, respectively, had a ≥1 grade worsening of thrombocytopenia. Further, adverse events and resulting dose modifications were higher in patients with baseline thrombocytopenia. In the PERSIST-2 study that randomized MF patients with baseline platelet count <100 × 109/L between pacritinib 400 mg once daily or 200 mg twice daily, or best available therapy, 51% and 42% of patients on the pacritinib arms had platelet count <50 × 109/L, respectively.24,30 A third of pacritinib treated patients had ≥1 grade worsening of thrombocytopenia, however the incidence of hematological adverse events was similar for patients with baseline platelet count <50 × 109/L vs ≥50 × 109/L. While the pacritinib twice daily arm met both co-primary end points of spleen and symptom response, this analysis was compromised by study truncation due to clinical hold. Consequently, additional data are awaited from the pacritinib dose-finding study before its safety in MF patients with severe thrombocytopenia is confirmed.
The indications for splenectomy have evolved in the JAK inhibitor era, with the main indication now being alleviation of severe treatment-refractory cytopenias, rather than relief from splenomegaly-associated mechanical symptoms. It should be noted however that robust data supporting consistent improvement of cytopenias following splenectomy in this setting are lacking. In 1 small study, patients were able to safely discontinue ruxolitinib prior to splenectomy; the immediate postoperative mortality was consistent with estimates preceding widespread use of JAK inhibitors and survival outcome after splenectomy was significantly worse in patients with severe thrombocytopenia that was unrelated to ruxolitinib use.47 In another study of 120 advanced MF patients (96% intermediate-2/high-risk), the median OS after splenectomy was 1.5 years; although the study period was between 2001 to 2016, the outcome of patients who had previously received JAK inhibitors was not reported.48 Splenic irradiation can effectively relieve splenic pain, however its utility in the setting of severe thrombocytopenia is limited by the risk of protracted cytopenias.49
There are several case reports of successful use of thalidomide or pomalidomide to augment the platelet count in MF patients with severe thrombocytopenia being treated with ruxolitinib.46,50 However, the use of such treatment combinations may not be possible outside of a clinical trial setting. Preliminary data suggest that anti-fibrotic agents such as PRM-151 can improve platelet counts in thrombocytopenic MF patients.51 This patient underwent splenectomy with improvement of platelet count to the 50-70 × 109/L range that was durable for several months postoperatively.
Case 2
A 55-year-old man was noted to have abnormal CBC results during routine preoperative assessment, with hemoglobin 13.8 g/dL, WBC 84.1 (3% blasts) × 109/L, and platelets 136 × 109/L. He had minimal symptoms aside from daily night sweats; the spleen was palpable at 24cm below the costal margin. BM findings were consistent with JAK2V617F-positive PMF with normal karyotype; risk stratification showed DIPSS score of 3 (intermediate-2 risk). He was started on fedratinib treatment (300 mg once daily), with prompt resolution of night sweats. After 6 months, the spleen size was significantly smaller. After 14 months, progressive leukocytosis (88.2 × 109/L; 18% monocytes, 10% blasts) and thrombocytopenia (46 × 109/L) were noted. A repeat BM biopsy showed 12% blasts and normal karyotype. Next generation sequencing revealed JAK2V617F, ASXL1Q858* and U2AF1Q157P mutations; the latter 2 mutations were absent at diagnosis.
Summary
Although this patient did not meet revised IWG-MRT criteria for ‘progressive disease’ or ‘leukemic transformation,’32 the patient likely has ‘accelerated-phase’ MF. An operational definition identifies characteristics that predict for median survival of ≤12 months. In 1 study, blood or BM blasts ≥10%, platelet count <50 × 109/L and/or chromosome 17 abnormalities defined accelerated phase disease.42 Interestingly, the majority of patients who had blast transformation transitioned through an interim accelerated phase, rather than progressing directly from chronic phase MF. In another study of PMF patients, risk factors associated with >80% 2-year mortality included monosomal karyotype, inv(3)/i(17q) abnormalities, or any 2 of the following: circulating blasts >9%, leukocytes ≥40 × 109/L, or other unfavorable karyotype.52 A simplified definition of accelerated phase is presence of 10% to 19% blasts in the blood or bone marrow.53 Development of monocytosis has also been associated with rapid disease progression and short survival; in some cases morphological and/or molecular (eg, ASXL1, TET2, SRSF2 mutations) characteristics intermediate between PMF and chronic myelomonocytic leukemia are observed.54-57
This patient was appropriately referred for allogeneic SCT as the only potentially curative option given the poor outcome predicted with conventional therapies.58 A recently developed prognostic tool (MIPSS70/MIPSS70-plus) was shown to be superior to IPSS or DIPSS-plus in risk-stratifying transplant-age PMF patients.36 Although data are limited, prior exposure to JAK inhibitors does not appear to adversely affect posttransplantation outcomes; conversely, in 1 study, superior survival (91% OS at 2 years) and lower nonrelapse mortality were observed for patients achieving clinical improvement response with JAK inhibitor treatment prior to allogeneic SCT.59 The occurrence of serious adverse events related to JAK inhibitor discontinuation is likely related to the timing of such discontinuation relative to start of conditioning therapy.37,59,60
There are limited treatment options for patients who are ineligible for allogeneic SCT. Use of higher than conventional doses of ruxolitinib (up to 200 mg twice daily) as monotherapy has shown limited anti-leukemic activity (CR/CRi rates 0% to 17%) in blast-phase MPN.61,62 Similarly, use of hypomethylating agents (decitabine or 5-azacitidine) alone has shown CR/CRi rates of 14% to 24% in accelerated or blast-phase MPN or myelodysplastic/myeloproliferative neoplasms (MDS/MPN).63,64 In a dose-finding study of combined ruxolitinib and decitabine in accelerated or blast phase MPN, CR/CRi responses were seen in a third of patients.65 Similarly, addition of 5-azacitidine to ruxolitinib has been studied in patients with MDS/MPN, with preliminary data showing 50% overall response rates66 Small case series have indicated the feasibility of combining ruxolitinib with low-intensity chemotherapy (eg, low-dose cytosine arabinoside) for treating accelerated or blast-phase MPN, generally with short-term disease control.67-69 The feasibility of continuous ruxolitinib treatment in combination with induction-type intensive chemotherapy has also been demonstrated in a small number of blast-phase MPN patients; primary refractoriness was not observed and half of the patients were successfully bridged to allogeneic SCT.70
The feasibility of an induction-maintenance strategy of low-dose abdominal radiation therapy has also been demonstrated in a small series of accelerated phase MF patients.71 In addition to the expected response in decreasing hepatosplenomegaly, there was marked reduction in leukocytosis and circulating blasts for several months. This patient underwent allogeneic SCT and was alive at last follow up.
Case 3
A 53-year-old woman presented with 15-pound weight loss and mild pancytopenia, evolving to RBC-transfusion requiring anemia over 6 months. The hemoglobin was 8.2 g/dL, WBC 3.4 × 109/L (0% blasts) and platelets 143 × 109/L. The spleen edge was palpable at the level of the umbilicus. BM findings were consistent with JAK2V617F-positive PMF with normal karyotype; risk stratification showed DIPSS score of 3 (intermediate-2 risk). Momelotinib treatment was started at 300 mg once daily. The spleen became nonpalpable by week 12; RBC transfusions were no longer needed, with peak hemoglobin (11.8 g/dL) reached 18 months after starting treatment. After 4 years of relatively stable counts, the hemoglobin steadily declined without identifiable cause. A CBC showed hemoglobin 6.7 g/dL, WBC 5.3 × 109/L (1% blasts), and platelets 101 × 109/L. A repeat BM biopsy was unremarkable with no evidence of excess blasts or clonal evolution.
Summary
This patient had disease relapse by revised IWG-MRT criteria based on loss of anemia response.32 For anemia that develops or worsens on JAK inhibitor treatment, a distinction as to whether this reflects worsening MF pathophysiology or drug-related myelosuppression is clinically relevant. In this patient, an exhaustive evaluation failed to reveal a cause for loss of anemia response such as adverse drug interaction, chronic blood loss, hemolysis, or inflammatory condition. Further, the late onset of anemia following an initial response, in the absence of dose-changes, suggested worsening MF pathophysiology rather than momelotinib-related myelosuppression.
Reversal of anemia that develops during JAK inhibitor treatment is challenging, particularly if there is no improvement with dose reduction. While erythropoiesis stimulating agents (ESA), androgens (eg, danazol) and immunomodulatory drugs (eg, pomalidomide, lenalidomide) have been combined with ruxolitinib, the results have been suboptimal. In a phase 2 study of danazol plus ruxolitinib in 14 MF patients with anemia, there were no anemia responses per IWG-MRT criteria; the trial was halted due to suboptimal efficacy.72 In a retrospective observational study of 43 MF patients, the objective response rate (40%) to ESA treatment was reportedly unaffected by concurrent ruxolitinib use.73 While not specified, reversal of ruxolitinib-induced anemia was implied since 24 patients started ESA treatment after ruxolitinib in this study. Similar responses have been reported for 13 patients on the COMFORT-II study who received ESA concurrent with ruxolitinib.74 In contrast, in a phase 1b/2 trial of pomalidomide (0.5mg/day) plus ruxolitinib in 38 MF patients with anemia, 58% of patients discontinued treatment after median 12 cycles. Worsening of anemia was the most frequent adverse event (34%). A ≥2g/dL hemoglobin increase or RBC transfusion independence was seen in 4 (11%) of patients.75 A phase 2 study of lenalidomide plus ruxolitinib in MF patients was terminated early due to excessive toxicity.76
Modest anemia and/or platelet responses can be seen with prednisone (starting dose 0.5-1mg/day) monotherapy in MF.77 Similarly, treatment with INCB039110, a selective JAK1 inhibitor that downregulates pro-inflammatory cytokines, produced a ≥ 50% decrease in the number of RBC units transfused in 21/39 (54%) transfusion-dependent MF patients in a phase 2 study, although 45% of patients developed infections.78 Six of fifteen (40%) patients treated with PRM-151 had protocol-defined anemia responses, and no drug-related myelosuppression was observed.51 The role of activin receptor type IIA transforming growth factor-β ligand-traps in alleviating MF-related anemia is currently under study. Updated results from an ongoing study showed anemia responses in 6/17 (35%) anemic or transfusion-dependent MF patients receiving sotatercept monotherapy; while the drug was well tolerated, 19/24 (79%) discontinued treatment after a median 5 cycles.79 A phase 2 study with the related drug, luspatercept, is currently recruiting patients. This patient was tapered off momelotinib treatment. At last follow up, her hemoglobin had stabilized in the 7-8 g/dL range. She elected for observation off therapy with a plan for clinical trial enrollment or referral for transplantation if her anemia worsened.
Case 4
A 67-year-old woman developed profound fatigue, high-grade fevers, 20-pound weight loss, abdominal distention, and RBC-transfusion dependence over 6 months. The hemoglobin was 5.1 g/dL, WBC 106.4 × 109/L (3% blasts) and platelets 236 × 109/L. Physical examination revealed massive hepatosplenomegaly and BM biopsy was consistent with JAK2V617F-positive PMF with normal karyotype; the DIPSS score was 6 (high risk disease). She was treated with an investigational JAK inhibitor. Within 2-4 weeks, her fevers resolved and by the end of 3 months, her spleen was approximately 50% smaller by palpation and RBC transfusion frequency decreased by 50%. She did relatively well for a year, when bilateral cervical lymphadenopathy was noted. CT scan confirmed bilateral, partially conglomerate, centrally necrotic lymph nodes. Ultrasound-guided core biopsy showed necrotizing granulomatous inflammation with acid fast bacilli; culture and polymerase chain reaction results were consistent with Mycobacterium tuberculosis.
Summary
Infections are a major cause of morbidity and mortality in MF; in 1 study, the incidence rate was 3.9%/year, with 78% bacterial, 11% viral and 2% fungal, and 9% of infection cases had a fatal outcome.80 In a nationwide survey of 780 PMF patients in Japan, infection and leukemic transformation were the leading causes of death.81 The risk of infections is likely amplified by JAK inhibitors, which have significant anti-inflammatory and immunosuppressive properties. The pathophysiology relates to disruption of key cytokine networks and impairment of dendritic cell, natural killer cell and T-cell function.13,82,83 In 1 study, the incidence of infections in ruxolitinib-treated vs control patients was 44% and 22%, respectively (P < .001).80 In a recent meta-analysis, ruxolitinib treatment was associated with a significantly higher risk of herpes zoster infections in 3 randomized control trials (odds ratio = 7.39) and in 2 extended phase 3 trials (odds ratio = 5.2).84 In 6 postmarketing studies, the most frequent infections were herpes zoster (8%), bronchitis (6.1%) and urinary tract infections (6%). This meta-analysis acknowledged potential underestimation of risk since infections may not have been systematically reported in trial-related publications. They also cited 28 published case reports describing 31 patients with serious opportunistic infections, most frequently tuberculosis,85 hepatitis B reactivation86 and Pneumocystis jirovecii infection.87 Other infections included cryptococcal meningoencephalitis,88 progressive multifocal leukoencephalopathy,89 toxoplasmosis retinitis,90 cytomegalovirus retinitis91 herpes simplex reactivation92 and Epstein-Barr virus-driven lymphoproliferative disorder.93 Based on these risks, some have advocated screening for specific infections prior to starting JAK inhibitor treatment.94 During JAK inhibitor treatment, latent/occult infections should be treated if adequate secondary prophylaxis is available, and surveillance for monitoring for reactivation of such infections should be considered. Examples of the former include use of antiviral therapies in patients with occult hepatitis B infection, similar to those undergoing B-lymphocyte depleting therapies, or isoniazid therapy in patients with latent tuberculosis.95,96 As to whether JAK inhibitor treatment can be continued or reintroduced in patients with treatable opportunistic infections has to be decided on a case-by-case basis.97,98 Of particular relevance to treatment of mycobacterial infections is the potential for adverse drug interactions with ruxolitinib. In 1 report, ruxolitinib treatment was not interrupted during anti-mycobacterial therapy that included rifampin.98 Despite the predicted significant decrease in plasma ruxolitinib levels due to strong induction of CYP3A4 by rifampin, the treatment response was maintained. This discrepancy likely results from the relative abundance of clinically active ruxolitinib metabolites.12 This patient had interruption of JAK inhibitor treatment in conjunction with initiation of antimycobacterial therapy. Unfortunately, there was a progressive decline in her condition and she elected for hospice care.
Conclusions
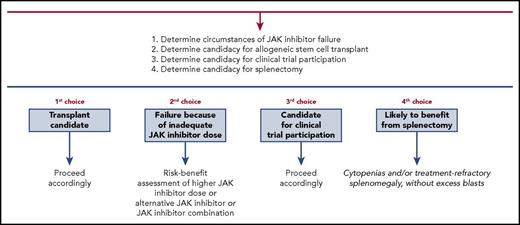
Development of effective treatment strategies for MF patients failing JAK inhibitors remains a major unmet need. Treatment decisions are individualized and selection of treatment strategies, particularly for transplant-ineligible patients, can be challenging. We present an algorithm (Figure 1) that highlights 1 possible treatment approach for this patient population.
Treatment algorithm for myelofibrosis patients failing JAK inhibitor therapy.
Authorship
Contribution: A.P. was primary author; and A.P. and A.T. reviewed and approved the final manuscript draft.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Animesh Pardanani, Division of Hematology, Mayo Clinic, 200 First St SW, Rochester, MN 55905; e-mail: pardanani.animesh@mayo.edu.