TO THE EDITOR:
Hypoxia is associated with an increased risk of thrombosis.1,2 Hypoxia is usually caused by high altitude,3 but it can be elicited by chronic alcoholism,4,5 chronic smoking,6 and clinical conditions such as lung failure,7,8 sickle cell anemia,9 and nonalcoholic fatty liver disease.10 Notably, deficiencies of protein S (PS), a natural anticoagulant that inhibits coagulation factor IXa, also occur in sickle cell anemia and at high altitude.11-16 This later circumstance purports a possibility that hypoxia causes a PS deficiency, in turn, elevating thrombotic risk. The cellular response to hypoxia is mediated by the dimeric transcription factor hypoxia inducible factor 1 (HIF1). The HIF1α subunit of HIF1 is expressed constitutively in many tissues, and an O2-dependent signaling! system continuously degrades HIF1α.17 Conversely, O2 deficiency prevents HIF1α degradation and stabilizes HIF1α.18 In this study, we demonstrate that HIF1 downregulates PS expression, a finding that suggests a molecular link between hypoxia and thrombosis.
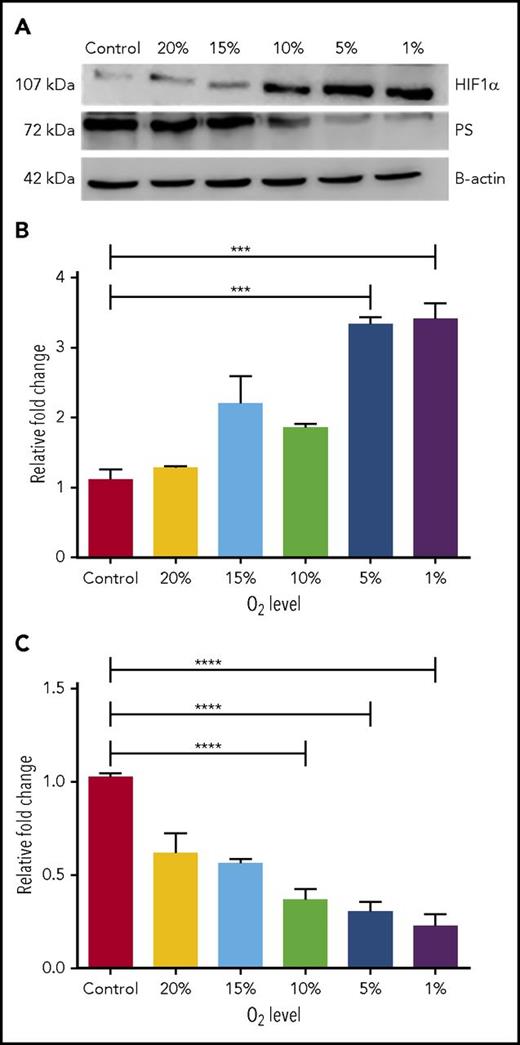
Because PS is produced primarily in liver, we used human hepatocarcinoma (HEPG2) cells, the standard cell system for studying PS expression.19 We cultured HEPG2 cells at normoxia (22% O2) and under hypoxic conditions ranging from 20% to 1% O2. PS levels were assessed by immunoblotting. Increasing hypoxia reduced PS protein level to 20% and concurrently increased HIF1α stability (Figure 1A). We also measured by quantitative polymerase chain reaction (qPCR) the effect of hypoxia on transcription of HIF1α (Figure 1B) and PS (Figure 1C). This analysis revealed that decreased O2 levels enhanced HIF1α transcription (from 100% to 340%). Conversely, a stepwise decrease in O2 (from 20% to 1%) progressively downregulated PS transcription (from 100% to 20%). Notably, hypoxia had no measureable effect on transcription or expression of another natural anticoagulant, TFPI (supplemental Figure 1A-B, available on the Blood Web site).
HIF1α downregulates PS expression in hypoxic HEPG2 cells. (A) Representative immunoblots showing relative PS and HIF1α protein levels in HEPG2 cells grown at different O2 concentrations. (B) Relative HIF1α mRNA levels in HEPG2 cells grown at different concentrations of O2. (C) Relative PS mRNA levels in HEPG2 cells grown at different concentrations of O2. ****P < .0001.
HIF1α downregulates PS expression in hypoxic HEPG2 cells. (A) Representative immunoblots showing relative PS and HIF1α protein levels in HEPG2 cells grown at different O2 concentrations. (B) Relative HIF1α mRNA levels in HEPG2 cells grown at different concentrations of O2. (C) Relative PS mRNA levels in HEPG2 cells grown at different concentrations of O2. ****P < .0001.
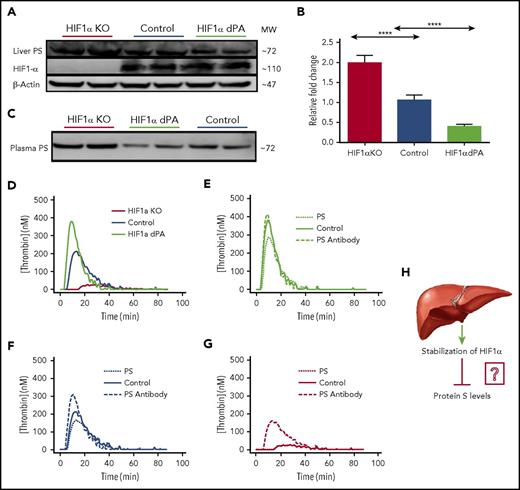
The inverse relationship between HIF1α and PS levels as a function of O2 concentration suggested that HIF1 protein might regulate PS expression. We confirmed this conjecture by modulating HIF1α expression in mouse liver. The HIF1α P564A mutant (HIF1α dPA) is resistant to degradation, resulting in sustained, elevated HIF1 protein abundance, even under normal O2 concentrations. We collected liver samples from HIF1α liver-specific knockout mice, knockout mice expressing HIF1α dPA in the liver (gift from William Kim, University of North Carolina-Chapel Hill),20,21 and control (nonknockout) mice and analyzed the samples by immunoblotting (in duplicate) and qPCR. Setting the control mice HIF1 expression levels at 100%, we found that the HIF1α dPA mice had an expectedly high (∼325%) HIF1 expression. Compared with control mice (100%), PS protein level in the liver from HIF1α liver-specific knockout mice was elevated (∼220%) and diminished in liver from HIF1α dPA mice (∼50%) (Figure 2A; supplemental Table 1). Likewise, we observed corresponding alterations in PS transcription. Compared with control mice, PS messenger RNA (mRNA) increased by twofold (±0.25) in HIF1α knockout mice, and PS mRNA was reduced to 0.3-fold (±0.15) in HIF1α mice that expressed HIF1α dPA (Figure 2B).
HIF1α regulates PS expression in the mouse liver. (A) Representative immunoblots showing relative PS and HIF1α protein levels in livers from HIF1α liver-specific knockout mice, control mice, and liver-specific HIF1α dPA mice. Each blot contained samples from 2 mice belonging to each category. The reported data are representative of 3 separate immunoblots, and the blot densities are quantified in supplemental Table 1. (B) Relative PS mRNA levels in the livers of control mice, HIF1α liver-specific knockout (KO) mice, and liver-specific HIF1α dPA mice (n = 5 per group). (C) Representative immunoblots assessing relative amounts of PS in plasmas from HIF1α liver-specific knockout mice, liver-specific HIF1α dPA mice, and control mice. (D) The graph shows relative thrombin generation by the plasmas from HIF1α liver-specific knockout mice, control mice, and liver-specific HIF1α dPA mice. (E) The graph shows thrombin generation by plasma from liver-specific HIFα dPA mice that was inhibited by addition of exogenous PS. (F) The graph shows relative thrombin generation from control mouse plasma following addition of anti-PS antibody or exogenous PS. (G) Thrombin generation by plasma from HIF1α liver-specific knockout mice in the presence of PS or anti-PS antibody. (H) Model depicting stabilization of HIF1α in mouse liver suppresses PS expression. ****P < .0001.
HIF1α regulates PS expression in the mouse liver. (A) Representative immunoblots showing relative PS and HIF1α protein levels in livers from HIF1α liver-specific knockout mice, control mice, and liver-specific HIF1α dPA mice. Each blot contained samples from 2 mice belonging to each category. The reported data are representative of 3 separate immunoblots, and the blot densities are quantified in supplemental Table 1. (B) Relative PS mRNA levels in the livers of control mice, HIF1α liver-specific knockout (KO) mice, and liver-specific HIF1α dPA mice (n = 5 per group). (C) Representative immunoblots assessing relative amounts of PS in plasmas from HIF1α liver-specific knockout mice, liver-specific HIF1α dPA mice, and control mice. (D) The graph shows relative thrombin generation by the plasmas from HIF1α liver-specific knockout mice, control mice, and liver-specific HIF1α dPA mice. (E) The graph shows thrombin generation by plasma from liver-specific HIFα dPA mice that was inhibited by addition of exogenous PS. (F) The graph shows relative thrombin generation from control mouse plasma following addition of anti-PS antibody or exogenous PS. (G) Thrombin generation by plasma from HIF1α liver-specific knockout mice in the presence of PS or anti-PS antibody. (H) Model depicting stabilization of HIF1α in mouse liver suppresses PS expression. ****P < .0001.
Liver-specific HIF1α alterations showed immediate effects on plasma PS levels. PS levels increased by 2 (±0.4)–fold in plasma from HIF1α knockout mice, whereas PS was reduced by 2 (±0.12) –fold in plasma from HIF1α dPA mice (Figure 2C). Changes in plasma PS were also reflected in the extent of thrombin generation. Plasma from HIF1α knockout mice produced fivefold less thrombin than control mouse plasma, and plasma from HIF1α dPA mice produced 1.5-fold more thrombin than control mice (Figure 2D). Data were analyzed by Graph-Pad Prism analysis software. Results were expressed as mean ± SD, and P values are presented in the figures as *P < .01; **P < .001, ****P < .0001.
To confirm that the variations in thrombin generation were due directly to changes in PS levels, we measured the effects of adding exogenous PS and a PS-specific antibody to plasmas from the aforementioned mice. Thrombin generation was not affected by supplementation of 450 nM anti-PS antibody into plasma from HIF1α dPA mice, whereas 150 nM PS supplementation reduced thrombin generation by 25% (Figure 2E). Supplementation of control mouse plasma with exogenous PS decreased thrombin generation, and addition of anti-PS antibody increased thrombin generation (Figure 2F). PS supplementation of plasma from HIF1α knockout mice increased thrombin generation, whereas anti-PS antibody had no significant effect (Figure 2G). These results confirmed that the changes in plasma PS levels were directly responsible for the variations in thrombin generation. We note that pathological stabilization of HIF1α often occurs in cancer and metabolic disorders that are also associated with higher risks of thrombosis and increased procoagulant activity. The foregoing results demonstrating suppression of PS activity in mice with hyperstable HIF1α dPA suggest a molecular explanation for increased thrombosis and procoagulant activity in cancer and other disorders.
Conclusion
Our results show that stabilization of HIF1 in the liver, a normal response to hypoxia, is associated with reduced PS expression that results in a lower plasma PS level (Figure 2H) and, in turn, increased likelihood of thrombosis. Although HIF1 is a well-documented, general transcriptional activator, it has also been identified as a transcriptional repressor. For example, HIF1 interacts with genes such as AIF,22 cyclin D1,23 and Bid24 and reduces their transcription under hypoxia. In addition, HIF1α regulates transcription of a variety of microRNAs that, in turn, regulate expression of various target mRNAs25 ; in these cases, HIF1 can be said to be an indirect transcriptional regulator of these mRNAs. The ease of culture of HEPG2 cells compared with normal hepatocytes offers a useful in vitro model for future studies of HIF1 in PS gene regulation. We have demonstrated a reciprocal relationship between the expression of HIF1 and PS. We will next determine whether HIF1-mediated PS downregulation occurs by a direct or indirect HIF1 transcriptional repressor function. This study will open a new direction for targeting hypoxia-mediated thrombotic disorders.
The aforementioned study is approved by Institutional Biosafety Committee (16400) and Institutional Animal Care and Use Committee (3504) of LSU Health Science Center.
The online version of this article contains a data supplement.
Acknowledgments
The authors thank Fokhrul Hossain and Samarpan Majumder (Department of Genetics, LSU Health Science Center) for help with qPCR figures.
This work was funded by the LSU Health Science Center Special Appropriation Award (0101500039) (R.M.) and National Institutes of Health, National Heart, Lung, and Blood Institute grant 1R01 HL 118557-01A1-07.
Authorship
Contribution: V.S.P. and R.M. contributed to study design; V.S.P., S.A., and A.D. collected and analyzed experimental data; D.C. and G.S. maintained the mice and collected liver and plasma samples; V.S.P. wrote the initial version of the manuscript; A.D. assisted in revision of the manuscript; and R.M. conceived the study, interpreted the results, and wrote the final version of the manuscript.
Conflict of interest disclosure: The authors declare no competing financial interests.
Correspondence: Rinku Majumder, Room 7114, Medical Education Building, 1901 Perdido St, New Orleans, LA 70112; e-mail: rmajum@lsuhsc.edu.
References
Author notes
A.D. and S.A. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal