Abstract
Mature T- and natural killer (NK)–cell neoplasms comprise a group of morphologically, immunophenotypically, molecularly, and clinically heterogeneous disorders with generally unfavorable outcome. Results of first-line chemotherapy are unsatisfactory for the most common T-cell lymphomas (peripheral T-cell lymphoma, not otherwise specified; angioimmunoblastic T-cell lymphoma; anaplastic large cell lymphomas; anaplastic lymphoma tyrosine kinase–negative) as well as for many other entities. High-dose therapy followed by autologous hematopoietic stem cell transplantation (HSCT) is widely recommended for consolidation after a complete or partial remission is achieved. However, about one-third of patients never reach transplantation because of early relapse or refractoriness. Targeted therapies have recently been developed; combinations with chemotherapy may improve outcomes, but long-term results from prospective studies are largely missing. In this situation, allogeneic HSCT remains a valuable treatment option inducing long-lived remissions in about 30% to 50% of patients with relapsed and refractory T-cell lymphoma able to proceed to transplantation. Results of allogeneic transplantation for consolidation in first remission are less defined and its indications remain controversial. With growing evidence that haploidentical HSCT also works in lymphoma, more patients can be brought to transplantation. Decreasing the morbidity and mortality of allogeneic transplantation is a continuous challenge. Integrating new drugs into transplant concepts and setting up prospective studies involving allogeneic transplantation remain unmet needs that warrant urgent study in a group of disorders in which classical chemotherapy and new drugs have generated results, which are far from optimal until today.
Introduction
The mature T- and natural killer (NK)–cell neoplasms comprise a large spectrum of lymphoproliferative disorders with widely differing geographic distribution as well as distinct morphological, pathophysiological, molecular, and clinical characteristics.1,2 T-cell lymphomas are rare with an incidence of less than 1 case per 100 000 people in the United States.3 Altogether, they amount to 10% to 20% of all lymphomas, with the high percentages reported from Asia partly being the result of substantially higher number of patients with Epstein-Barr virus–driven NK/T-cell lymphoma in that part of the world.4 Likewise, adult T-cell leukemia/lymphoma (ATLL) is largely restricted to HTLV I-endemic regions in Southern Japan, the Caribbean, Central and South America, and intertropical Africa.5
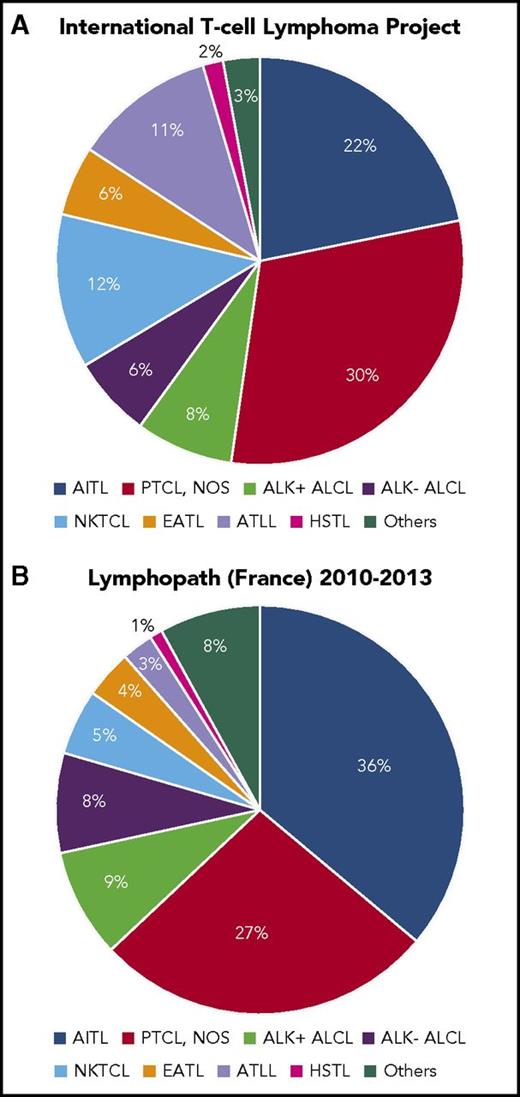
With increasing knowledge of their immunophenotypic and molecular characteristics the T- and NK-cell lymphomas have repeatedly been reclassified, mirroring important progress in basic science, detection of new risk factors, the description of new potentially drugable targets, and their therapeutic exploitation.6 In 2017, the World Health Organization classification of lymphoid neoplasms1 acknowledges >20 different T-cell lymphoma entities that broadly segregate into lymphomas with predominant nodal involvement, extranodal involvement, leukemic, or cutaneous manifestations. Peripheral T-cell lymphoma, not otherwise specified (PTCL-NOS), angioimmunoblastic T-cell lymphoma (AITL), and the anaplastic large cell lymphomas (ALCL), anaplastic lymphoma tyrosine kinase (ALK)+ or ALK−, are the most frequent T-cell lymphoma subtypes, although relative frequencies of T-cell entities reported in different series vary substantially (Figure 1).2,7 Improved diagnostic methods7 and potentially real incidence changes over time contribute to an increase in cases with T-cell characteristics over time.8 NK/T-cell lymphoma, ATLL, primary intestinal T-cell lymphoma (enteropathy-associated T-cell lymphoma [EATL], and monomorphic epitheliotropic intestinal T-cell lymphoma), hepatosplenic lymphoma (HSTL), and the primarily cutaneous lymphomas mycosis fungoides (MF), Sezary syndrome (SS), and subcutaneous panniculitis-like T-cell lymphoma represent further important types of T- and NK-cell neoplasms with a characteristic clinical course.
Relative frequencies of major T-cell entities. Reported by the International T-cell Lymphoma Project2 (A) and Lymphopath7 (B). NKTCL, nasal NK/T-cell lymphoma.
This review on allogeneic hematopoietic stem cell transplantation (HSCT) in T-cell lymphomas will focus on 3 major entities (PTCL-NOS, AITL, ALCL ALK−). We will briefly report on other NK/ T-cell lymphomas for which sometimes only anecdotal transplant data do exist. Prospective studies are only available for patients undergoing transplantation as part of first-line therapy comparing allogeneic HSCT to high-dose therapy and autologous hematopoietic stem cell transplantation (HDT/ASCT). Data collected for patients with relapsed/ refractory T-cell lymphoma are retrospective and all methodological caveats of this approach do apply.
Prognostic factors
Most patients with T-cell lymphoma present in advanced stage; therefore, staging itself is not discriminatory enough. Several prognostic indices have been suggested.9-11 Although any scoring system has advantages and disadvantages most investigators stay will the International Prognostic Index originally developed for aggressive B-cell malignancies.12 The practical consequences of staging or prognostic factor analyses are more limited in T-cell than in the aggressive B-cell lymphomas because practical consequences are limited. Although most if not all T-cell lymphomas harbor typical cytogenetic and molecular abnormalities, none of these markers has been used alone or in combination with clinical risk factors to improve on the International Prognostic Index.13
First-line and conventional salvage therapy
Unfortunately, the rapidly increasing knowledge of genetic and epigenetic changes found in virtually all subtypes of T/NK-cell lymphomas13 has not been paralleled by the pace of therapeutic innovation achieved. The cyclophosphamide, doxorubicin hydrochloride, vincristine sulfate, and prednisone (CHOP) regimen first described in 197614 remains the cornerstone of first-line therapy for most T-cell lymphoma patients.15,16 The addition of etoposide17 or the administration of other cytotoxic agents alone or in combination18-20 have shown limited success. Also, combinations with the anti-CD52 antibody alemtuzumab or small molecules targeting surface antigens or molecular defects found in T-cell lymphoma have either failed21 or shown moderate improvement. For patients with relapsed or refractory disease, pralatrexate,20 romidepsin,22 belinostat,23 alisertib,24 lenalidomide,25 or the anti- CCR4 antibody mogamulizumab26 have been licensed in the United States or Japan but failed licensing in the European Union because of limited efficacy shown in phase 2 trials. The more successful molecules like the antibody-drug conjugate brentuximab vedotin (BV)27-29 or romidepsin, a selective inhibitor of histone deacetylases30 have been studied in combination with CHP31 or CHOP and investigation to improve first-line therapy continues. These agents may be successfully used for bridging patients to transplantation, also because of their mild to moderate toxicity.32 Their long-term efficacy especially when used as single agent seems more limited; none of the new drugs have become part of standard first-line therapy thus far.15,16
Patients with relapsed or refractory T-cell lymphoma have a particularly bleak prognosis33 ; they often fail to respond to further therapy. Chemotherapy regimens recommended to treat such patients have been identical to those for patients with relapsed aggressive B-cell lymphoma. Alternative cytotoxic drugs like bendamustine or pralatrexate may achieve mostly transient responses in one-third to one-half of patients treated.34,35 Salvage therapy is most valuable to bridge patients to transplantation. Excellent reviews on PTCL-NOS, AITL, and ATLL including the discussion of new treatment options have recently been published.36-38
Bringing patients to transplantation
Before assessing the role of transplantation in more detail, it is important to note that retrospective analyses of transplant outcomes are biased by the impossibility to report on patients not making it to transplantation because of relapse and refractory disease, age, frailty, toxicity of previous treatment, or lack of a suitable donor. Importantly, the few prospective studies on HDT/ASCT39-42 and allogeneic HSCT43,44 unanimously report that between 26% and 59% of patients could not undergo transplantation because of early progression or relapse (Table 1). Thus, one-fourth to more than one-third of patients will never proceed to transplantation. More chemotherapy given at shorter time intervals, as with any alternative strategy, failed to change this pattern of early failure and relapse.45
Prospective studies on autologous and allogeneic transplantation as part of first-line therapy in T-cell lymphoma
| Reference . | n . | Histology . | PFS/EFS . | OS . | n (%) of patients transplanted . | Cause of failure to proceed to transplantation (n) . |
|---|---|---|---|---|---|---|
| 39 | 160 | NOS, 62; AITL, 30; ALCL, ALK−, 3; EATL, 21; other, 16 | 44% at 5 y | 51% at 5 y | 115 (72) to autologous HSCT | PD (26), toxic death (7), protocol violation (2), mobilization fail (2), other (4) |
| 40 | 83 | NOS, 32; AITL, 27; ALCL, ALK−, 13; other, 11 | 36% at 3 y | 48% at 3 y | 55 (66) to autologous HSCT | PD (24), toxic death (2), patient decision (2) |
| 41 | 62 | NOS, 28; AITL, 10; ALCL, ALK+, 19; other, 5 | 30% at 12 y | 34% at 12 y | 46 (74) to autologous HSCT | PD (11), mobilization fail (2), patient decision (2) |
| 42 | 41 | NOS, 20; AITL, 12; other, 9 | 30% at 4 y | 39% at 4 y | 17 (41) to autologous SCT | PD (16), relapse (1), toxic death (1), mobilization fail (3), poor performance (2), patient decision (1) |
| 43 | 61 | NOS, 33; AITL, 14; ALCL, ALK−, 12; EATL, 2 | 26% at 4 y | 31% at 4 y | 38 (62) to allogeneic or autologous HSCT | PD (18), toxic death (5) |
| 44 | 58 | NOS, 25; AITL, 16; ALCL, ALK−, 7; other, 8 | 41% at 1 y | 69% at 1 y | 36 (62) to allogeneic or autologous HSCT | PD (17), toxic death (1), change of histology (2), no donor (5) |
| Reference . | n . | Histology . | PFS/EFS . | OS . | n (%) of patients transplanted . | Cause of failure to proceed to transplantation (n) . |
|---|---|---|---|---|---|---|
| 39 | 160 | NOS, 62; AITL, 30; ALCL, ALK−, 3; EATL, 21; other, 16 | 44% at 5 y | 51% at 5 y | 115 (72) to autologous HSCT | PD (26), toxic death (7), protocol violation (2), mobilization fail (2), other (4) |
| 40 | 83 | NOS, 32; AITL, 27; ALCL, ALK−, 13; other, 11 | 36% at 3 y | 48% at 3 y | 55 (66) to autologous HSCT | PD (24), toxic death (2), patient decision (2) |
| 41 | 62 | NOS, 28; AITL, 10; ALCL, ALK+, 19; other, 5 | 30% at 12 y | 34% at 12 y | 46 (74) to autologous HSCT | PD (11), mobilization fail (2), patient decision (2) |
| 42 | 41 | NOS, 20; AITL, 12; other, 9 | 30% at 4 y | 39% at 4 y | 17 (41) to autologous SCT | PD (16), relapse (1), toxic death (1), mobilization fail (3), poor performance (2), patient decision (1) |
| 43 | 61 | NOS, 33; AITL, 14; ALCL, ALK−, 12; EATL, 2 | 26% at 4 y | 31% at 4 y | 38 (62) to allogeneic or autologous HSCT | PD (18), toxic death (5) |
| 44 | 58 | NOS, 25; AITL, 16; ALCL, ALK−, 7; other, 8 | 41% at 1 y | 69% at 1 y | 36 (62) to allogeneic or autologous HSCT | PD (17), toxic death (1), change of histology (2), no donor (5) |
PD, progressive disease.
HDT and ASCT
First reports on HDT/ASCT in patients with relapsed or refractory T-cell lymphoma occurred many years ago, and the most important studies have systematically been reviewed recently.46 Following early negative experience, recent studies excluded patients with disease not responding to conventional therapy. Many studies involving HDT/ASCT are biased because intent-to-treat analyses including all patients being candidates for transplantation could not be performed and younger and healthier patients seem overrepresented. Moreover, the larger studies come from international registries and patients had differing first- (and second-) line therapy and were exposed to various high-dose regimens and variable supportive care. Three- to 5-year overall survival (OS) is between <40% and >60%, mirroring differences in individual patient and disease characteristics. The largest study published by Center for International Blood and Marrow Transplant Research (CIBMTR) in 2013 reported progression-free survival (PFS) and OS of 41% (95% confidence interval, 29-52) and 53% (95% confidence interval, 40-64), respectively, for 75 patients beyond a first but including patients in a second or further complete remission (CR).47
More precise data are available from prospectively evaluated patient cohorts transplanted for consolidation after a first complete or partial remission had been achieved. These studies have repeatedly been reviewed and a systematic review/meta-analysis has been published. The authors conclude that HDT/ASCT is a reasonable treatment option to offer to patients with PTCL as front-line consolidation with resulting OS rates of 54% to 68% and a relatively low nonrelapse-related mortality (NRM) ranging from 2% to 6%.46 Of note, prospective trials comparing consolidative HDT/ASCT with observation or alternative treatment do not exist. Recent retrospective analyses shed doubt if consolidative HDT/ASCT is indicated in all patients achieving remission with conventional chemotherapy.48
Allogeneic transplantation
The vast majority of allogeneic HSCT has been performed in patients with (multiply) relapsed or refractory T-cell lymphoma, often after failure of HDT/ASCT. All caveats with regard to selection bias also apply to retrospective series of allogeneic HSCT. Reports published 10 or more years ago do no longer reflect current practice because a strict upper age limit has been replaced by individual evaluation of physical and intellectual fitness. Progress in graft-versus-host disease (GVHD) prophylaxis and supportive care may have reduced transplant-related morbidity and mortality compared with NRM encountered decades ago. Search strategies and donor availability are massively changing after early studies gave convincing evidence that results of allogeneic HSCT using haploidentical family donors are comparable to those achieved with matched related or unrelated donors.49,50
Allogeneic HSCT for relapsed and refractory T-cell lymphoma
In 2004, Corradini et al published a report on 17 patients with PTCL-NOS, AITL, and ALCL who had been allografted from HLA-identical family donors (1 unrelated donor) after reduced-intensity conditioning (RIC) with thiotepa, cyclophosphamide, and fludarabine.51 Prophylaxis of GVHD consisted of cyclosporine A and short-course methotrexate. After a median follow-up of 28 months 14 of 17 patients were alive, with 12 in CR. This observation made the authors claim a graft-versus-lymphoma effect also in T-cell non-Hodgkin lymphoma. Evidence for a graft-versus-lymphoma-effect remained circumstantial, especially when taking into account the relative high relapse rates in larger series and some of the rare subtypes. The efficacy of donor lymphocyte infusions may be more convincing in that respect.
T-cell lymphoma subtypes, NRM rates, incidence of acute and chronic GVHD, relapse, PFS, and OS reported in publications including >20 adult patients with relapsed and refractory T-cell lymphoma are listed and referenced in Table 2.47,52-58 Table 2 contains only studies in which the majority of patients suffered from PTCL-NOS, AITL, and ALCL, ALK+, or ALK−, although most reports also include patients with other T-cell entities.54,57 Reports showing data on cutaneous T-cell lymphomas, HSTL, ATTL, NK/T-cell lymphoma, EATL, and pediatric ALK+ ALCL are discussed separately. The studies from CIBMTR, European Group for Blood and Marrow Transplantation (EBMT), France, and Germany comprising the bulk of patients reported in Table 2 face substantial heterogeneity with regard to the number of patients with distinct histologic subtypes, status of disease at transplantation, duration of disease before transplantation, donor selection, conditioning regimen used, GVHD prophylaxis, and other variables. Virtually all studies report on patients having matched related or unrelated donors; only the most recent studies include few patients with haploidentical donors. So far, significant differences in PFS or OS for patients with PTCL-NOS, AITL, and adult ALCL have not been reported. Unfortunately, the ALK status of ALCL patients is reported as unknown in many patients; too few patients with known ALK status remain to make comparisons with other entities. Early results of haploidentical transplantation in lymphoma are promising; significant differences in outcome compared with matched (un)related transplants have not yet been reported.49,50 The percentages of patients given RIC vs myeloablative conditioning varies from study to study. Although in the German report. all patients received myeloablative conditioning (MAC), patients described in the studies by Dodero et al,53 Delioukina et al,57 and Czajczynska et al58 received RIC. Patients from the other reports were exposed to MAC and RIC regimens at varying percentages. The only study specifically addressing the question of outcome differences in relation to the conditioning regimen did not find significant differences in OS and PFS with less NRM and higher relapse rates following RIC.47 Some studies also report on donor lymphocyte infusions. Taken together, ∼50% of patients responded, lending some support to the existence of a graft-versus-T-cell lymphoma effect. Approximately 50% of patients survive long-term after allogeneic HSCT; PFS averages ∼40% at 5 years. Larger study presented at the meetings of the American Society of Hematology 2017 and the European Society for Blood and Marrow Transplantation 2018 confirm these results.59,60
Results of allogeneic stem cell transplantation in T-cell lymphoma patients beyond first CR
| Reference . | n . | Histology; no. of patients* . | Median age, y . | NRM . | GVHD . | Relapse . | PFS . | OS . |
|---|---|---|---|---|---|---|---|---|
| 47 | 126 | NOS, 63 | 38 | MAC 34% | NR | MAC 37% | MAC 29% | MAC 31% |
| AITL, 12 | at 3 y | at 3 y | at 3 y | at 3 y | ||||
| ALCL, 51 | RIC 27% | RIC 42% | RIC 32% | RIC 50% | ||||
| at 3 y | at 3 y | at 3 y | at 3 y | |||||
| 52 | 77 | PTCL, 27 | 41 | 34% at 5 y | Acute 21% (grade 3-4) chronic NR | NR | 58% at 5 y | 63% at 5 y |
| AITL, 11 | 47 | 80% at 5 y | 80% at 5 y | |||||
| ALCL, 27 (8 ALK+) | 26 | 48% at 5 y | 55% at 5 y | |||||
| Other, 12 | 35 | NR | 33% at 5 y | |||||
| 93 | 66 | NOS, 23 | NR | 29% at day 100 | NR | NR | 46% at 1 y | 48% at 1 y |
| AITL, 12 | ||||||||
| ALCL, 11 | ||||||||
| T-LBL, 6 | ||||||||
| T-PLL, 7 | ||||||||
| Other, 7 | ||||||||
| 53 | 52 | NOS, 23 | 47 | 12% at 5 y | Acute, 22% (grade 2-4) chronic, 27% | 49% at 5 y | 39% at 5 y | 45% at 5 y |
| AITL, 9 | 44% at 5 y | 66% at 5 y | ||||||
| ALCL, 11 | 45% at 5 y | 54% at 5 y | ||||||
| No difference by histology | No difference by histology | |||||||
| 54 | 52 | NOS, 20 | 46 | 27% at 3 y | Acute, 21% | 43% at 3 y | 30% at 3 y | 41% at 3 y |
| AITL, 5 | (grade 2-4) | |||||||
| ALCL, 6 | Chronic, 27% | |||||||
| Other, 21 | (extensive) | |||||||
| 55 | 45 | AITL, 45 | 48 | 25% at 1 y | Acute, 29% (grade 2-4) Chronic, 54% | 20% at 3 y | 53% at 3 y | 64% at 3 y |
| 56 | 37 | NOS, 8 | 40 (50 for CTCL) | 29% at 5 y | Acute, 51% | 24% at 5 y | 47% at 5 y | 52% at 5 y |
| AILT, 4 | Chronic, 62% | |||||||
| ALCL, 6 (3 ALK+) | ||||||||
| Other, 6 | ||||||||
| CTCL, 13 | ||||||||
| 57 | 27 | NOS, 5 | 50 | 22% at 2 y | Acute, 33% (grade 2-3) Chronic, 85% | 30% at 2 y | 47% at 2 y | 55% at 2 y |
| AITL, 3 | No difference by histology | No difference by histology | ||||||
| ALCL, 2 (2 ALK+) | ||||||||
| CTCL, 11 | ||||||||
| 58 | 24 | NOS, 9 | 53 | 25% | Acute, 25% (grade 2-4) Chronic, 30% | 25% | NR | 42% at 3 y |
| AITL, 5 | ||||||||
| ALCL, 4 |
| Reference . | n . | Histology; no. of patients* . | Median age, y . | NRM . | GVHD . | Relapse . | PFS . | OS . |
|---|---|---|---|---|---|---|---|---|
| 47 | 126 | NOS, 63 | 38 | MAC 34% | NR | MAC 37% | MAC 29% | MAC 31% |
| AITL, 12 | at 3 y | at 3 y | at 3 y | at 3 y | ||||
| ALCL, 51 | RIC 27% | RIC 42% | RIC 32% | RIC 50% | ||||
| at 3 y | at 3 y | at 3 y | at 3 y | |||||
| 52 | 77 | PTCL, 27 | 41 | 34% at 5 y | Acute 21% (grade 3-4) chronic NR | NR | 58% at 5 y | 63% at 5 y |
| AITL, 11 | 47 | 80% at 5 y | 80% at 5 y | |||||
| ALCL, 27 (8 ALK+) | 26 | 48% at 5 y | 55% at 5 y | |||||
| Other, 12 | 35 | NR | 33% at 5 y | |||||
| 93 | 66 | NOS, 23 | NR | 29% at day 100 | NR | NR | 46% at 1 y | 48% at 1 y |
| AITL, 12 | ||||||||
| ALCL, 11 | ||||||||
| T-LBL, 6 | ||||||||
| T-PLL, 7 | ||||||||
| Other, 7 | ||||||||
| 53 | 52 | NOS, 23 | 47 | 12% at 5 y | Acute, 22% (grade 2-4) chronic, 27% | 49% at 5 y | 39% at 5 y | 45% at 5 y |
| AITL, 9 | 44% at 5 y | 66% at 5 y | ||||||
| ALCL, 11 | 45% at 5 y | 54% at 5 y | ||||||
| No difference by histology | No difference by histology | |||||||
| 54 | 52 | NOS, 20 | 46 | 27% at 3 y | Acute, 21% | 43% at 3 y | 30% at 3 y | 41% at 3 y |
| AITL, 5 | (grade 2-4) | |||||||
| ALCL, 6 | Chronic, 27% | |||||||
| Other, 21 | (extensive) | |||||||
| 55 | 45 | AITL, 45 | 48 | 25% at 1 y | Acute, 29% (grade 2-4) Chronic, 54% | 20% at 3 y | 53% at 3 y | 64% at 3 y |
| 56 | 37 | NOS, 8 | 40 (50 for CTCL) | 29% at 5 y | Acute, 51% | 24% at 5 y | 47% at 5 y | 52% at 5 y |
| AILT, 4 | Chronic, 62% | |||||||
| ALCL, 6 (3 ALK+) | ||||||||
| Other, 6 | ||||||||
| CTCL, 13 | ||||||||
| 57 | 27 | NOS, 5 | 50 | 22% at 2 y | Acute, 33% (grade 2-3) Chronic, 85% | 30% at 2 y | 47% at 2 y | 55% at 2 y |
| AITL, 3 | No difference by histology | No difference by histology | ||||||
| ALCL, 2 (2 ALK+) | ||||||||
| CTCL, 11 | ||||||||
| 58 | 24 | NOS, 9 | 53 | 25% | Acute, 25% (grade 2-4) Chronic, 30% | 25% | NR | 42% at 3 y |
| AITL, 5 | ||||||||
| ALCL, 4 |
MAC, myeloablative conditioning; NR, not reported; T-LBL, T-cell lymphoblastic lymphoma; T-PLL, T-cell prolymphocytic leukemia.
Only major subtypes are listed: numbers do not add up to the total number of study patients,
Allogeneic HSCT for consolidation of first remission
There have been 2 prospective studies addressing the question if allogeneic transplantation could improve survival of patients achieving remission after first-line therapy. Corradini et al51 reported a study including 61 newly diagnosed patients (median age, 48 years) with PTCL-NOS (33 patients), AITL (14 patients), ALK− ALCL (12 patients), and EATL (2 patients) who started treatment with 2 courses of CHOP plus alemtuzumab (33 mg before the first, and 30 mg before the second CHOP) followed by 2 courses of high-dose methotrexate (total dose, 1.6 g/m2), cyclophosphamide (1800 mg/m2), and high-dose cytarabine (12 g/m2). Patients finding a matched related or unrelated donor and achieving a complete or partial remission were to receive allogeneic HSCT. Finally, only 23 patients (38%) could undergo allogeneic transplantation, 14 patients (23%) without a suitable donor underwent autologous transplantation, and 24 patients (39%) received neither autologous nor allogeneic transplantation. Reasons for not reaching transplantation were early progression (18 patients), toxic death (5 patients), and physician’s decision in 1 patient. At a median follow-up of 44 months, 16 patients were still in CR, 4 patients had relapsed and died, and 3 patients died of pneumonia, encephalitis, and acute GVHD while in CR. Acute GVHD of grades 2-4 occurred in 40% and chronic GVHD was noted in 52% evaluable patients. Four-year OS and PFS for allografted patients was 69%, with no significant difference to patients receiving an autograft.
The second study was performed by the Lymphoma Study Association in France and the German High-Grade Lymphoma Study Group.44 Patients were randomized up front to ASCT or allogeneic HSCT. Both patient groups received 4 courses of CHOP plus etoposide followed by 1 course of rituximab, dexamethasone, cytarabine, and Platinol chemotherapy. Patients randomized to HDT/ASCT had their stem cells harvested after 2 or 3 courses of CHOP plus etoposide, and cells were reinfused after HDT using the carmustine, etoposide, cytarabine, and melphalan regimen. Patients randomized to allogeneic HSCT were to receive identical chemotherapy followed by myeloablative conditioning with fludarabine, busulfan, and cyclophosphamide if an HLA-identical family or unrelated donor was available. Patients with a 9/10 HLA-match were also accepted. The study was closed after 104 patients had been randomized because of statistical considerations; results of a planned interim analysis performed after 58 patients had been randomized are available. Very similar to the Italian experience, 38% of patients did not reach transplantation because of progressive disease in 17 patients, toxic death in 1, other reasons in 2, or lack of an HLA-identical donor. Follow-up was short with no significant survival difference between patients who had received an auto- or an allograft. The major problem of both studies was the high percentage of patients with early progression hindering a valid comparison of allogeneic HSCT and HDT/ASCT. More important to the patient, early progression compromises the results of both modalities. As of today, allogeneic HSCT in first CR cannot be recommended for the major T-cell subtypes.
Allogeneic HSCT for NK/T-cell lymphoma
NK/T-cell lymphoma is a rare type of T-cell lymphoma more frequent in Asia and South America than in other parts of the world. Disseminated NK/T-cell lymphoma is associated with a poor prognosis, although inclusion of methotrexate and l-asparaginase into first-line therapy improved prognosis significantly.61 Reports on allogeneic HSCT for NK/T-cell lymphoma are scarce; larger series have not been published. An earlier report from Japan summarized the outcome of 28 patients who received allogeneic HSCT after MAC (n = 23) and RIC (5). The median age of patients was 38 years who had received a median of 2 prior therapies. At a median follow-up of 34 months and the 2-year PFS and OS were 34% and 40%, respectively.62 A study from the Asia Lymphoma Study Group published in 2014 reports on 18 patients who had undergone modern first-line chemotherapy.63 With a median follow-up of 21 months, 5-year OS was 57%, event-free survival (EFS) was 51%. Although these results are promising, the authors acknowledge that many patients with NK/T-cell lymphoma following a very aggressive clinical course will never reach transplantation because even a short-lived remission cannot be achieved and time to set up transplantation is lacking. Furthermore, allogeneic HSCT must be compared with the results of modern radiochemotherapy. A report from 2011 describes a single case of successful donor lymphocyte infusion for NK/T-cell lymphoma relapsed after allogeneic HSCT indicating that a graft -versus-lymphoma effect may exist.64
Allogeneic HSCT for hepatosplenic T-cell lymphoma
Except for anecdotal cases65 and the few patients reported with the studies in relapsed/refractory T-cell lymphomas (Table 2), the only study specifically addressing the outcome of patients with HSTL undergoing transplantation was published by the EBMT.66 Eighteen patients receiving allogeneic transplantation had a median age of 39 years and had failed a median of 2 lines of therapy. With a median follow-up of 36 months for surviving patients, 9 of 18 patients (50%) were alive, 1 after relapse. The main cause of death after allogeneic HSCT was NRM, and only 1 patient had relapsed after transplantation. We concluded that allogeneic HSCT may be a valid treatment option for patients with relapsed/refractory HSTL. A recent consensus paper on indication and timing of transplantation in T-cell lymphoma recommends autologous or allogeneic transplantation as part of first-line therapy.67
Allogeneic HSCT in adult T-cell leukemia/lymphoma
Most reports on allogeneic HSCT for ATLL come from Asia reflecting the differing incidence of this disease in different parts of the world. By far the largest study was published by Hishizawa et al, who reported on 386 patients included in a nationwide survey from Japan.68 Three-year OS was 33%, relapse rate 21%, and NRM rate 37%. Older age, male sex, status other than CR, and the use of cord blood negatively influenced survival. These results were slightly worse than those reported from smaller studies published in 2005.69,70 The EBMT reported a smaller series of patients (n = 21) from European centers, Israel, and Lebanon.71 Three-year EFS and OS were 26% and 34%, respectively. These results were deemed comparable to those of the Japanese studies. In contrast, the most recent study from Japan reported dismal outcomes after allogeneic HSCT for 130 patients with relapsed ATLL: 3-year NRM was 37%, relapse rate was 51%, and OS was 13%.72 This study included patients with relapsed and refractory disease only.
Using a Markov decision analysis, Fuji et al73 demonstrate that chemotherapy followed by allogeneic HSCT is the optimal treatment strategy in patients with intermediate- or high-risk disease according to the modified ATL prognostic index.74 Thus, patients with acute type ATLL, poor performance status, high soluble interleukin-2 receptor level, high adjusted calcium level, and high C-reactive protein are candidates for allogeneic HSCT as part of first-line therapy. Waiting until relapse in such patients is not recommended especially after devastating results in relapsed/refractory patients were published. On the other hand, recommendations in favor of transplantation must be taken with caution, because conventional therapeutic options, at least for the less aggressive subtypes of ATLL, substantially improved in recent years.
Allogeneic HSCT for cutaneous T-cell lymphoma
Molina et al were among the first to demonstrate long-term survival after allogeneic HSCT in patients with advanced, multiply relapsed MF/SS.75 They reported 6 of 8 patients being alive without evidence of disease after a median follow-up of 56 months. The EBMT published a larger series of 60 patients with MF (n = 36) and SS (n = 24) who had undergone allogeneic HSCT from a matched related (n = 45) or unrelated (n = 15) donor. The patients’ median age was 47 years and the majority of patients suffered from advanced disease with a median of 4 prior treatments.76 The most recent update reported an OS of 48% and PFS of 33% at 5 years. NRM at 2 years had been 22% and the cumulative incidence of relapse was 47% at 3 years.77 Hosing et al from MD Anderson Cancer Center published a series of 47 patients with MF/SS at different stages.78 The median number of prior systemic therapies received was 6 with interferon, bexarotene, extracorporal photopheresis, and multiagent chemotherapy or targeted therapies administered to patients with lymph node or visceral involvement. OS at 4 years was 51%; PFS was 26% with significantly different results for patients with SS or MF (73% for SS, 12% for MF patients with large cells). The overall incidence of disease progression/ relapse was 50% and the cumulative NRM rate was 17% at 2 years.
Studies by CIBMTR on 129 patients and from France on 37 cases largely confirm these data.79,80 Taken together, results of allogeneic HSCT in patients with early disease CTCL look promising, patients with advanced disease after multiple therapies do significantly worse with relapse rates beyond 50% and NRM >20% impairing results.
Allogeneic HSCT for EATL
EATL is a rare type of peripheral T-cell lymphoma with a generally poor prognosis recently reclassified into EATL and monomorphic epitheliotropic intestinal T-cell lymphoma.1 Although some data on the results of HDT/ASCT exist,81,82 no information on allogeneic HSCT except for case reports is available.59,83 The high median age at diagnosis and the problem of bringing patients with severe gastrointestinal lesions in many instances refractory to conventional therapy to allogeneic HSCT seem important reasons why experience with allogeneic HSCT in EATL is very limited. Among the few patients reported, some patients died of early NRM, whereas other patients survived disease-free for years.
Allogeneic HSCT for pediatric ALK+ ALCL
First-line therapy of pediatric ALK+ ALCL consists of complex combination chemotherapy and is highly successful with 2-year-survival rates beyond 90%.84 For patients with relapsed or refractory disease, allogeneic transplantation has been proposed. The study by Woessmann et al reported on 20 children with a median age of 8.8 years at transplant, most of whom suffered from advanced disease.85 Six patients had progressed or relapsed during front-line therapy; 11 patients had relapsed within the first year after diagnosis. All but 3 patients were in CR (11 patients) or partial remission (3 patients) before transplantation. After variable, mostly myeloablative conditioning patients received transplants from a matched sibling (8 patients), a matched unrelated donor (8 patients), or a haploidentical family donor (4 patients). EFS at 3 years was 75% ± 10%. These data are confirmed by a Japanese study in which all 6 children receiving an allogeneic transplant for ALCL in second CR remained in continuous CR after a median follow-up of 42 months.86 Nowadays, children with relapsed or refractory ALK+ ALCL would also be candidates for treatment with BV or crizotinib. Both drugs have shown promising results in adults with ALK+ ALCL.27,87 Although their ultimate role in childhood ALCL awaits further study, relapses do occur during or after treatment with BV or crizotinib. Both drugs may have an important role for bridging patients to allogeneic transplantation.
Future directions
Allogeneic transplantation is a complex treatment modality with the depth of remission before transplantation, the conditioning regimen (myeloablative vs RIC), the matching of donor and recipient, and the prophylaxis of GVHD being the most important of the many factors ultimately deciding on the success of the procedure. New drugs such as lenalidomide, romidepsin, mogamulizumab, belinostat, or alisertib have also been investigated to improve the patient’s remission status before transplant.13 Unfortunately, except for BV in CD 30+ disease and crizotinib in ALK+ ALCL, survival rates have been somewhat disappointing and the search for better debulking agents before allogeneic HSCT continues. PD-1 or PD-1L antibodies show activity also in certain T-cell lymphoma entities.88 Administering such an antibody after allogeneic HSCT for relapsed Hodgkin lymphoma resulted in high response rates (77%), but also triggered severe GVHD leading to death in 26% of patients.89 Therefore, it is not yet clear which role PD-1(L) antibodies will play in T-cell lymphoma in general and after allogeneic HSCT.
In patients with relapsed and refractory B-cell lymphoma chimeric antigen receptor T cells directed against the CD 19 antigen have shown promising results.90,91 Targeting pan–T-cell antigens would not only kill malignant T cells but also would eliminate normal T cells, causing profound, long-lasting T-cell depletion and unacceptable immunosuppression. To circumvent such problems, antibodies with specificity for the T-cell receptor β-chain constant region (anti-TRBC) have recently been identified. Anti-TRBC1 chimeric antigen receptor T cells killed normal and malignant TRBC1+ cells in vitro and in a mouse model, leaving TRBC2+ T cells unaffected.92 If reproducible in the clinic, this or similar approaches will be extremely interesting to broaden the treatment armamentarium for a disease short of effective new treatment modalities.
Conclusions
Allogeneic transplantation of hematopoietic stem cells remains a valuable treatment option for patients with relapsed and refractory T-cell lymphoma. In some of the most aggressive entities (HSTL, ATLL), transplantation in first CR should be discussed. As with other hematological malignancies, advanced disease and multiple prior therapies predict for a higher risk of NRM and higher relapse rates. Thus, allogeneic HSCT should be considered early, in most instances after first relapse. If an individual patient will undergo allogeneic HSCT depends on the ability to induce another albeit transient remission or at least avoid overt progression, goals that unfortunately cannot be reached by one-third of transplant candidates. Donor availability, age and comorbidity thresholds, and alternative treatment options rapidly change and must be taken into account before a final decision is made. Guidelines must be read with great caution and cannot substitute for individualized decisions based on the totality of published and center experience. More structured approaches (prospective studies with comparison with alternative therapies) to evaluate the role of allogeneic HSCT are highly warranted.
Acknowledgments
The authors thank Laurence de Leval, Institute of Pathology, University Hospital Lausanne, Lausanne, Switzerland, for providing data on the epidemiology of T-cell lymphoma and reading the manuscript in consideration of the current classification of T- and NK-cell neoplasms.
This work was supported by the Deutsche Forchungsgemeinschaft EXC 1003 Cells in Motion–Cluster of Excellence, Münster, Germany.
Authorship
Contribution: N.S., G.L., and M.S. collected all information and prepared the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Norbert Schmitz, Department of Medicine A/Hematology, Oncology, Hemostaseology and Pulmonology, University of Münster, Albert Schweitzer Campus 1, D-48149 Münster, Germany; e-mail: norbert.schmitz@ukmuenster.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal