Key Points
Two-thirds of PCNSL have BCRs specific for SAMD14 and neurabin-I, 2 highly homologous CNS proteins.
Both antigens were posttranslationally modified and induced a strong BCR pathway activation and proliferation.
Abstract
To address the role of chronic antigenic stimulation in primary central nervous system lymphoma (PCNSL), we searched for autoantigens and identified sterile α-motif domain containing protein 14 (SAMD14) and neural tissue-specific F-actin binding protein I (neurabin-I) as autoantigenic targets of the B-cell receptors (BCRs) from 8/12 PCNSLs. In the respective cases, SAMD14 and neurabin-I were atypically hyper-N-glycosylated (SAMD14 at ASN339 and neurabin-I at ASN1277), explaining their autoimmunogenicity. SAMD14 and neurabin-I induced BCR pathway activation and proliferation of aggressive lymphoma cell lines transfected with SAMD14- and neurabin-I-reactive BCRs. Moreover, the BCR binding epitope of neurabin-I conjugated to truncated Pseudomonas exotoxin-killed lymphoma cells expressing the respective BCRs. These results support the role of chronic antigenic stimulation by posttranslationally modified central nervous system (CNS) driver autoantigens in the pathogenesis of PCNSL, serve as an explanation for their CNS tropism, and provide the basis for a novel specific treatment approach.
Introduction
Primary central nervous system lymphoma (PCNSL) is a rare aggressive B-cell non-Hodgkin lymphoma affecting the brain, the leptomeninges, the spinal cord, or the eyes. Recently, a high frequency of activating CD79b and MYD88 mutations linking the BCR and TLR9 pathways and high expression of PD-1 ligands were reported.1,2 The reason for the selective tropism in the central nervous system (CNS), which is traditionally regarded as an immune privileged site, is still unclear. The tetrad of overrepresentation of autoreactivity-linked VH4-34, persistent functional variable region genes despite ongoing somatic hypermutation, the negativity for Epstein-Barr virus (EBV), and the transcriptional upregulation of the NF-kB pathway suggest chronic B-cell receptor (BCR) activation and prompted us to search for antigens of the BCRs from PCNSL.3-6 The hypothesis of chronic BCR stimulation contributing to the malignant transformation of B cells is old,7 and several specific target antigens of BCR from various B-NHLs have been identified.8-11 A recently published study proposed auto- and polyreactive BCRs in PCNSL.12
Methods
Patients
The study had been approved by the local ethics committee (Ärztekammer des Saarlandes). Snap-frozen PCNSL specimens from 21 HIV-negative patients were obtained from the institutes of pathology and neuropathology of the universities of Saarland, Kiel, Würzburg, and Erlangen. Pairs of serum and CSF samples from a second series of 22 patients with PCNSL were obtained from the Department of Neurology of Tübingen University. Additional sera and whole blood samples were obtained from the departments of hematology & oncology of Freiburg and Saarland universities. Sera from patients with secondary CNS manifestations or with testicular manifestations of diffuse large B-cell lymphoma (DLBCL) treated within the RICOVER-6013 trial of the German High-Grade Non-Hodgkin Lymphoma Study Group served as a control. Blood samples of multiple sclerosis were obtained from the Department of Neurology of Saarland Medical School. Cryospecimens of primary testicular lymphoma were obtained from the institutes of pathology of the universities of Würzburg and Ulm. Recombinant Fabs of other B cell-neoplasia as controls for PCNSL were constructed in cooperation with the Senckenberg Institute of pathology of the Goethe University in Frankfurt am Main.
Ig variable region gene PCR
Fragments of PCNSL cryosections were digested with 2 µL proteinase K (Roche PCR grade) for 4 hours at 55°C, followed by heat inactivation at 95°C for 10 minutes. Seminested polymerase chain reactions (PCRs) for VH, Vκ, and Vλ genes were performed (30 and 44 cycles, respectively), using the Expand High Fidelity PCR kit (Roche), as described by Küppers et al.14,15 The resulting variable region genes were sequenced and analyzed with IMGT-V-Quest for functionality, V(D)J segment usage, and mutations. Whenever both a functional immunoglobulin (Ig) heavy and light chain variable region gene was obtained, these genes were cloned into a modified pCES vector for the expression of recombinant BCRs as Fab fragments.16 Fabs were expressed and purified as described previously.17 Variable gene fragments were re-extended at the 5′ and 3′ ends, according to the proper Ig germline genes. Complete V genes were inserted via ApaLI and XhoI for Vκ or Vλ in front of a κ- or λ-constant region gene, respectively, and via NcoI and BstEII for VH in front of a γ-1 constant region gene into a modified pCES-1 vector for expression of the Fab fragment.16 Fabs were expressed in Escherichia coli TG1 strain and purified as described previously.16,18,19
Screening for antigenic BCR targets
ELISA for BCR and serum antibody reactivity against SAMD14 and neurabin-I
Sterile α-motif domain containing protein 14 (SAMD14) and neural tissue-specific F-actin binding protein I (neurabin-I) were recombinantly expressed with a C-terminal FLAG tag in HEK293 under the control of a CMV promoter (pSFI). Total cell extracts were bound at a concentration of 10 µg/mL to Nunc Maxisorb plates precoated overnight at 4°C with murine anti-FLAG antibody at a dilution of 1:2500 (vol/vol; Sigma, Munich). Blocking was performed with 1.5% (wt/vol) gelatin in TBS, and washing steps were performed with TBS-Tx (TBS, 0.1% [vol/vol] Tx100). Individual recombinant Fabs (10 µg/mL), sera (1:100), and CSF (1:50) were used. Enzyme-linked immunosorbent assays (ELISAs) were conducted according to standard protocols with biotinylated goat anti-human IgG (heavy and light chain), goat anti-human IgA, and anti-human IgM (all Dianova), all at dilutions of 1:2500, or subclass-specific sheep anti-human IgG1, IgG2, IgG3, and IgG4 (Binding Site, Birmingham, United Kingdom) at dilutions of 1:5000. After this, correspondent biotinylated secondary antibodies were used for the immunoassays for IgG subclasses. For detection, peroxidase-labeled streptavidin (Roche) was used at a dilution of 1:50 000.
Identification of the BCR-binding epitope of SAMD14 and neurabin-I
For the determination of the binding region of SAMD14 and neurabin-I, recombinant fragments of different lengths from both antigens were constructed with C-terminal FLAG tags and expressed as described earlier.
Western blots of SAMD14 and neurabin-I
Lysates of PCNSL tissue specimens, whole peripheral blood cells, or lymphoblastoid cell lines (LCLs) were loaded and separated in a 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membrane, using a transblot semidry transfer cell (Bio Rad). After blocking overnight at 4°C in phosphate-buffered saline/10% nonfat dry milk, transferred proteins were incubated with recombinant and PCNSL-patient-derived SAMD14/neurabin-I reactive BCRs at a concentration of 2 µg/mL for 1 hour at room temperature, or with rabbit anti N-terminus neurabin-I antibody (ABIN 1108406) at 1:500 for 1 hour or with murine anti-FLAG-antibody at 1:2000 for 1 hour, followed by 1 hour incubation at room temperature with murine anti-His antibody at 1:2000 (Qiagen), with HRP-labeled anti-mouse IgG antibody (BioRad) for His6-tagged PCNSL-derived recombinant BCRs, with goat HRP-labeled anti-rabbit antibody (Bio Rad) at 1:3000 for the anti-neurabin-I antibody, and by HRP-labeled anti-mouse-IgG antibody at 1:3000 for western blots with point-mutated FLAG-tagged full-length fragments of SAMD14 and neurabin-I and the chemiluminescence reagent was used for immunoblot detection.
Moreover, SAMD14 and neurabin-I from more than 80 established cell lines were screened by western blot for N-hyperglycosylation.
Deglycosylation of SAMD14 and neurabin-I
Equal amounts of denatured lysates from PCNSL specimens were treated with specific exo- or endoglycosidases (ie, PNGase-F, o-glycosidase, α-2[3,6,8,9]-neuraminidase, position-specific β-1-4-galactosidase, or β-N-acetylglucosaminidase; EDEGLY-Kit, Sigma).
Site-directed mutagenesis
Using QuickChange II Site-Directed Mutagenesis Kit (Stratagene) and SAMD14 and neurabin-I full-length FLAG-tagged DNA fragments, several candidate N-glycosylation sites within or adjacent to the BCR binding epitopes were mutagenized after prediction of the most likely glycosylation sites (http://www.cbs.dtu.dk/services/NetNGlyc/). In each case, the amino acid asparagine (N) was replaced by glutamine (Q), disabling N-glycosylation. This exchange was performed for SAMD14 with N339Q and N372Q and for neurabin-I with N1208Q, N1258Q, N1277Q, N1289Q, and N1301Q. These FLAG-tagged mutants were cloned into pRTS and expressed in LCLs derived from patients with N-hyperglycosylated SAMD14 and neurabin-I (patient 20) and from patients with normally glycosylated SAMD14 and neurabin-I (patient 21).
Lymphoblastoid cell lines
LCLs were established by infection of PBMCs with EBV, as described earlier.21
Expression of SAMD14/neurabin-I, MAZ antigens, and respective immunotoxins
BCR-binding epitopes of neurabin-I, SAMD14, and MAZ as a control were recombinantly expressed with C-terminal FLAG tags in HEK293 cells.11 Immunotoxins consisting of the BCR-binding epitope of neurabin-I (amino acids 1226-1251) conjugated to Pseudomonas exotoxin A were recombinantly expressed in E coli BL21, as described previously by others.22
Expression of SAMD14/neurabin-I reactive BCR in OCI-Ly3, TMD-8, and U2932
As no EBV-negative PCNSL cell line exists, to the best of our knowledge, the DLBCL ABC type cell lines OCI-Ly3, TMD-8, and U2932 were chosen as model cell lines. OCI-Ly3 harbors mutated MYD88 and CARD11, TMD-8 mutated MYD88, and mutated CD79B, but wild-type CARD11; U2932 mutated TAK1, but wild-type CARD11 and MYD88.23-26 Each cell line was transfected with a modified pRTS-1 expression vector with a variable region heavy chain and constant regions Cγ1-Cγ4 with membrane coding exons TM1 and TM2 for the transmembrane region and the cytoplasmic tail followed by a 2A sequence and the light chain variable region and light chain constant region gene inserted via SfiI sites.27 Both VH and Vk were derived from the SAMD14/neurabin-I reactive PCNSL-BCR from patient 18 or as a control from a patient with chronic lymphocytic leukemia and a BCR directed against MAZ.11 Transfection of OCI-Ly3, TMD8, and U2932 cells was performed on ice after 3 washing steps with RPMI at a cell density of 2 × 107/mL in RPMI without fetal calf serum. 2 × 106 cells were transfected with 5 µg plasmid DNA by electroporation, using Gen Pulser (BioRad) with a 0.2 cm cuvette at 140V with 30-millisecond pulses. Subsequently, cells were immediately put on ice again and cultured in RPMI 1640 medium supplemented with 20% FCS. Cell lines stably expressing surface BCR with reactivity against SAMD14/neurabin-I were selected with hygromycin 250 µg/mL.28 Successful transfection was verified by variable region gene PCRs, by western blot and flow cytometry of the FLAG-tagged recombinant BCRs.
Proliferation and BCR pathway activation assays
For western blot analysis of the BCR pathway activation of transfected OCI-Ly3, cells either expressing a BCR with reactivity against SAMD14/neurabin-I or a different antigen, respectively, 1 × 106 cells were incubated with no antigen, neurabin-I/SAMD14 at 5 µg/mL, MAZ at 5 µg/mL, or anti-IgM at 1 µg/mL. For western blot analysis of the BCR pathway, activation of transfected TMD8 and U2932 cells either expressing recombinant BCR with reactivity against SAMD14/neurabin-I by doxycycline induction or not expressing recombinant BCR by lack of doxycycline induction, 1 × 106 cells were incubated with no antigen, neurabin-I/SAMD14 at 5 µg/mL, LRPAP1 at 5 µg/mL, or anti-IgM at 1 µg/mL. As primary antibodies, rabbit antibodies against pTyr525/526 SYK diluted 1:2000, pTyr759 PLCγ2 diluted 1:1000, pTyr223 BTK diluted 1:1000, and pTyr96 BLNK diluted 1:1000 (B-cell signaling sampler kit, CST), rabbit antibody against actin diluted 1:2000 (Sigma), and murine antibody against MYC at a concentration of 1 µg/mL (Santa Cruz) were used, followed by washing steps and incubation with POX-conjugated anti-rabbit or anti-mouse antibodies diluted at 1:3000.
For the analysis of cytoplasmatic calcium changes by flow cytometry, a FACS Canto analyzer was used, as well as Fluo-4/AM dye. Transfected OCI-Ly3 cells either expressing BCRs with reactivity against SAMD14/neurabin-I or against MAZ11 were resuspended in calcium- and magnesium-free phosphate-buffered saline and loaded with Fluo-4/AM dye (final concentration, 2 μM; Invitrogen) for 30 minutes at room temperature, as described previously.11 Antigen was added, followed by flow cytometry of the cells. Ionomycin (10 ng/μL, Sigma-Aldrich) was used as a positive control for the release of calcium from internal stores. Calcium levels were repeatedly analyzed immediately after adding the antigen to the dye-loaded cells and mixing.
Cell proliferation was determined by a nonradioactive assay (EZ4U, Biomedica), according to the manufacturer’s instructions In short, 4 × 104/mL OCI-Ly3 cells expressing BCRs with reactivity against SAMD14/neurabin-I or MAZ were seeded in 200 µL cell cultures. To detect possible quantitative differences between the recombinantly expressed epitope regions of neurabin-I or MAZ, both were used at a concentration of 1 µg/mL. After 24-hour incubation at 37°C, 20 µL chromophore substrate was added to each well.
Cytotoxicity, apoptosis, and dye exclusion assays
For the analysis of the direct cytotoxic effects of immunotoxins, an LDH release assay was used. 5 × 103/well OCI-LY3, TMD8, or U2932 cells stably transfected with doxycycline-inducible expression of a SAMD14/neurabin-I-reactive BCR were incubated with neurabin-I-ETA′ (5 µg/mL), MAZ-ETA′ (5 µg/mL), or no immunotoxin. Percentage specific lysis was determined as (experimental lysis − spontaneous lysis)/(maximum lysis − spontaneous lysis) × 100. Maximum lysis was determined by adding 10% Triton X-100. Lactate dehydrogenase (LDH) was measured according to the protocol of the LDH assay kit (Roche, Mannheim, Germany). ELISA read-out was performed using a Victor II (PerkinElmer, Rodgau, Germany).
For the analysis of apoptosis, 5 × 105 cells/mL suspension of OCI-LY3 cells stably transfected to express a BCR with reactivity either against the SAMD14/neurabin-I or against MAZ11 were treated with neurabin-I/ETA′, MAZ/ETA′ (both at 0.5 µg/mL), or staurosporine (1 µg/mL) for 24 hours at 37°C, 5% CO2. After the incubation, the cells were washed twice with phosphate-buffered saline and resuspended in 500 µL binding buffer. Five microliters AnnexinV-FITC and 10 µL propidium iodide were added to each cell suspension and incubated for 10 minutes at room temperature, followed by analysis by FACS Canto. In addition, the effects of the immunotoxins were measured by trypan blue assays at 0, 24, and 48 hours.
Statistical analysis
Statistical significance of N-glycosylation and antibody status was analyzed with Fisher’s exact test. Statistical significance of proliferation and cytotoxicity assays was analyzed by multiple t test, using Prism 7.
Results
SAMD14 and neurabin-I as antigenic targets of BCRs from PCNSL
The median age of the 21 patients from whom PCNSL specimens were obtained was 64 (range, 36-77) years. Functional Ig heavy and light chain genes were successfully amplified from 12 patients with PCNSL, including 1 case with 2 functional heavy chain genes (case 3), and 2 cases with 2 functional light chain genes (case 15 and 17). Four (33%) of the 12 patients had VH gene segments of VH4-34. All cases with amplified V region genes were somatically mutated: 1 case carried an unmutated VH gene, but a mutated Vk segment; 2 other cases carried unmutated Vk genes, but mutated VH genes (supplemental Table 1, available on the Blood Web site).
The screening of the protein macroarrays representing 7390 distinct human proteins for reactivity with pooled recombinant BCRs, derived of 12 PCNSL cases, identified 1 candidate autoantigen with a strong reactivity on the Unipex1 membrane: an expression clone of SAMD14 variant 2 spanning from amino acids 192 to 417 (supplemental Figure 1). No reactivities were detected on the Unipex2 membrane.
An ELISA with recombinant SAMD14 variant 2 expressed with a C-terminal FLAG-tag in HEK293 confirmed SAMD14 as the only target antigen of 8/12 (67%) PCNSL-BCRs (Figure 1A).
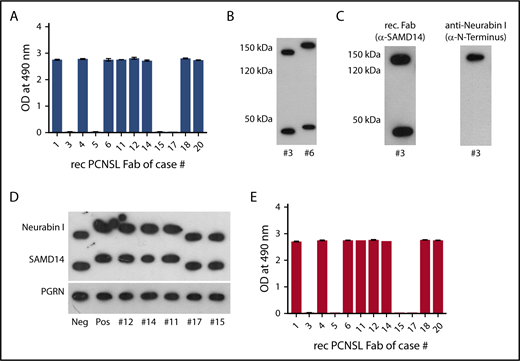
SAMD14 and neurabin-I as target antigen of recombinant PCNSL-derived BCRs. (A) ELISA of SAMD14 reactivity of PCNSL-derived recombinant BCRs. The columns represent adsorbence at OD 490 nm. (B) Western blots of PCNSL tissue lysates using SAMD14-specific recombinant BCR. Lane 1: PCNSL tissue from patient #3 with a BCR not reacting with SAMD14. Lane 2: PCNSL tissue from patient #6 with SAMD14 reactive BCR. Two proteins, one at 150 kDa (above) and at 45 kDa (bottom) of PCNSL, are targeted by the recombinant PCNSL-derived BCR, with both antigens from patient #6 having a higher molecular weight compared to the antigens from patient #3. (C) Identification of neurabin-I as the second antigen. Western blot of PNCSL tissue from patient #3. Lane 1: the SAMD14-specific recombinant PCNSL-derived BCR targets proteins at 145 kDa and 45 kDa; lane 2: stripped western blot for reprobing with a commercial antibody directed against the N terminus of neurabin-I, identified the 145 kDa band as neurabin-I. (D) Second isoform of SAMD14/neurabin-I in patients with SAMD14/neurabin-I-reactive lymphoma BCR. Western blots of PNCSL tissues from patients with SAMD-14/neurabin-I-specific lymphoma cell BCRs (lanes 2, 3, 4, and 5) showed a higher molecular weight than from patients with BCR specificities other than SAMD-14/neurabin-I (lanes 1, 6, and 7), whereas bands for progranulin were not different. (E) ELISA for neurabin-I reactivity of individual PCNSL-derived recombinant BCRs. The columns represent adsorbence at OD 490 nm, consistent with reactivity of the respective recombinant BCRs against neurabin-I.
SAMD14 and neurabin-I as target antigen of recombinant PCNSL-derived BCRs. (A) ELISA of SAMD14 reactivity of PCNSL-derived recombinant BCRs. The columns represent adsorbence at OD 490 nm. (B) Western blots of PCNSL tissue lysates using SAMD14-specific recombinant BCR. Lane 1: PCNSL tissue from patient #3 with a BCR not reacting with SAMD14. Lane 2: PCNSL tissue from patient #6 with SAMD14 reactive BCR. Two proteins, one at 150 kDa (above) and at 45 kDa (bottom) of PCNSL, are targeted by the recombinant PCNSL-derived BCR, with both antigens from patient #6 having a higher molecular weight compared to the antigens from patient #3. (C) Identification of neurabin-I as the second antigen. Western blot of PNCSL tissue from patient #3. Lane 1: the SAMD14-specific recombinant PCNSL-derived BCR targets proteins at 145 kDa and 45 kDa; lane 2: stripped western blot for reprobing with a commercial antibody directed against the N terminus of neurabin-I, identified the 145 kDa band as neurabin-I. (D) Second isoform of SAMD14/neurabin-I in patients with SAMD14/neurabin-I-reactive lymphoma BCR. Western blots of PNCSL tissues from patients with SAMD-14/neurabin-I-specific lymphoma cell BCRs (lanes 2, 3, 4, and 5) showed a higher molecular weight than from patients with BCR specificities other than SAMD-14/neurabin-I (lanes 1, 6, and 7), whereas bands for progranulin were not different. (E) ELISA for neurabin-I reactivity of individual PCNSL-derived recombinant BCRs. The columns represent adsorbence at OD 490 nm, consistent with reactivity of the respective recombinant BCRs against neurabin-I.
None of the recombinant control BCRs derived from 12 peripheral DLBCL, 19 MCL, and 31 FL cases reacted against SAMD14 (supplemental Figures 2 and 3). Western blot of denatured PCNSL lysates with the recombinant BCRs as primary antibodies confirmed reactivity against SAMD14, a protein of 45 kDa. An additional band of approximately 140 kDa was observed (Figures 1B-D). To investigate whether this was a hypothetical polymer of SAMD14 or the highly homologous SAM domain of neurabin-I, the western blot was stripped and repeated with an anti-neurabin-I antibody reactive against the nonhomologous N-terminus of neurabin-I and not the homologous SAM domain. This identified neurabin-I as a second target containing a highly homologous SAM domain (Figure 1C). ELISAs with fragments of different lengths of SAMD14 variant 2 and of neurabin-I identified an epitope spanning from amino acid 274 to 417 of SAMD14 variant 2 and an epitope spanning from amino acid 995 to 1345 of neurabin-I (supplemental Figures 4 and 5) as the BCR-binding region. In further analysis for SAMD14, amino acids 306 to 324 and for neurabin-I amino acids 1226 to 1251 were identified as the regions with the highest affinity. No reactivities of the PCNSL-BCRs were detected on an infectious disease epitope microarray.
Hyper-N-glycosylation of SAMD14 and neurabin-I
Both SAMD14 variant 2 and neurabin-I had a higher molecular weight in patients with PCNSL with reactive lymphoma BCRs compared with patients without reactive lymphoma BCRs and healthy control patients (Figure 1C,E). Deglycosylation with PNGase F/β-n-acetylglucosaminidase led to the disappearance of differences in molecular weights of both SAMD14 and neurabin-I between patients with and without SAMD14/neurabin-I-reactive lymphomas. Neither deglycosylation with O-glycosidase, α-2(3,6,8,9)-neuraminidase nor β-1-4-galactosidase (Figure 2A-B) induced this disappearance of differences in molecular weights. In 7/15 patients with PCNSL with sufficient material for western blot analysis, hyperglycosylated SAMD14 and neurabin-I were detected in cryosections and (only weakly) in the peripheral blood from those patients of whom the latter was available. The hyperglycosylated SAMD14 and neurabin-I isoforms were also expressed in monocytes, lymphocytes, and granulocytes from patients with PCNSL with SAMD14/neurabin-I-reactive BCRs (supplemental Figure 6). Hyperglycosylated SAMD14/neurabin-I was not observed in the peripheral blood cell lysates from 400 healthy control patients, 50 residents of nursery homes, or 20 patients with multiple sclerosis, nor in cryospecimen of 4 cases of testicular lymphoma or any of more than 80 established cell lines derived from human malignancies (data not shown). This resulted in a significant association of the hyperglycosylated SAMD14/neurabin-I isoforms with PCSNL (P < .0001). Subsequently, using patient-derived LCLs and point-mutated FLAG-tagged constructs of SAMD14 and neurabin-I, Asn 339 was identified to bear the hyperglycosylated Asn residue of SAMD14, and Asn 1277 of neurabin-I (Figure 2C). These glycosylation sites consisted of atypical N-L-E-Q.
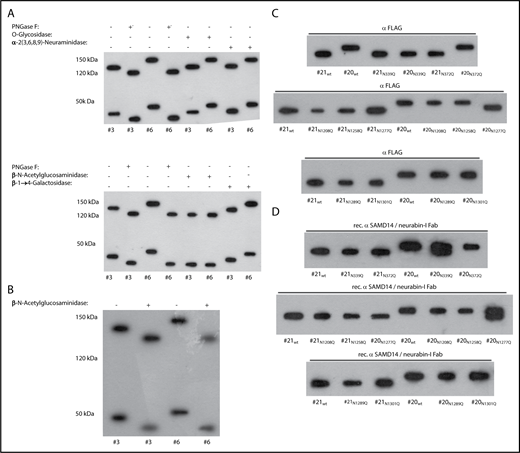
N-Hyperglycosyation of SAMD14 and neurabin-I in PCNSL. (A) N-hyperglycosylation of SAMD14 and neurabin-I in patients with SAMD14/neurabin-I-reactive lymphoma BCRs. Deglycosylation of SAMD14 and neurabin-I from PCNSL tissue with a panel of endo- or exoglycosidases (PNGase-F, O-glycosidase, neuraminidase, β-n-acetylglycosidase and galactosidase). (Top) Lane 1: untreated PCNSL tissue from patient #3 (without SAMD14 reactive lymphoma BCR). Lane 2: PNGase-F treated PCNSL tissue from patient #3. Lane 3: untreated PCNSL tissue from patient #6 (with SAMD14-reactive lymphoma BCR). Lane 4: PNGase-F-treated PCNSL tissue from patient #6. Lane 5: O-glycosidase-treated PCNSL tissue from patient #3. Lane 6: O-glycosidase-treated PCNSL tissue from patient #6. Lane 7: α-2(3,6,8,9)-neuraminidase-treated PCNSL tissue from patient #3. Lane 8: α-2(3,6,8,9)-neuraminidase treated PCNSL tissue from patient #6. (Bottom) Lane 1: untreated PCNSL tissue from patient #3. Lane 2: PNGase-F-treated PCNSL tissue from patient #3. Lane 3: untreated PCNSL tissue from patient #6. Lane 4: PNGase-F-treated PCNSL tissue from patient #6. Lane 5: β-N-acetylglucosaminidase-treated PCNSL tissue from patient #3. Lane 6: β-N-acetylglucosaminidase-treated PCNSL tissue from patient #6. Lane 7: β-1→4-galactosidase-treated PCNSL tissue from patient #3. Lane 8: β-1→4-galactosidase-treated PCNSL tissue from patient #6. The treatment with PNGase-F and β-N-acetylglucosaminidase resulted in the disappearance of the differences in molecular weights of both antigens from patients #3 and #6. (B) Deglycosylation of SAMD14 and neurabin-1 from PCNSL tissue only with β-N-acetylglucosaminidase. The treatment with β-N-acetylglucosaminidase resulted in the disappearance of the differences in molecular weights of both SAMD14 and neurabin-I from patients #3 and #6. (C) Identification of the hyperglycosylated Asn residues. Western blots of several constructs of SAMD14 and neurabin-I with different mutants of N-glycosylation sites expressed in LCLs derived from patients with and without hyperglycosylated SAMD14 and neurabin-I. Anti-FLAG antibodies were used as primary antibodies. These western blots showed the disappearance of the gain in molecular weight for the point-mutated, full-length proteins N339Q SAMD14 and N1277Q neurabin-I expressed in LCLs of patients with PCNSL with SAMD14/neurabin-I antibodies. (D) Identification of the hyperglycosylated Asn residues. Western blots of several constructs of SAMD14 and neurabin-I with different mutants of N-glycosylation sites expressed in LCLs derived from patients with and without hyperglycosylated SAMD14 and neurabin-I. Instead, Anti-FLAG primary antibody recombinant SAMD14/neurabin-I-specific Fab was used as primary antibody. These western blots show double bands as they unspecifically stain for both wild-type SAMD14 (hyperglycosylated) and nonhyperglycosylated N339Q SAMD14, and for both wild-type neurabin-I (hyperglycosylated) and N1277Q neurabin-I.
N-Hyperglycosyation of SAMD14 and neurabin-I in PCNSL. (A) N-hyperglycosylation of SAMD14 and neurabin-I in patients with SAMD14/neurabin-I-reactive lymphoma BCRs. Deglycosylation of SAMD14 and neurabin-I from PCNSL tissue with a panel of endo- or exoglycosidases (PNGase-F, O-glycosidase, neuraminidase, β-n-acetylglycosidase and galactosidase). (Top) Lane 1: untreated PCNSL tissue from patient #3 (without SAMD14 reactive lymphoma BCR). Lane 2: PNGase-F treated PCNSL tissue from patient #3. Lane 3: untreated PCNSL tissue from patient #6 (with SAMD14-reactive lymphoma BCR). Lane 4: PNGase-F-treated PCNSL tissue from patient #6. Lane 5: O-glycosidase-treated PCNSL tissue from patient #3. Lane 6: O-glycosidase-treated PCNSL tissue from patient #6. Lane 7: α-2(3,6,8,9)-neuraminidase-treated PCNSL tissue from patient #3. Lane 8: α-2(3,6,8,9)-neuraminidase treated PCNSL tissue from patient #6. (Bottom) Lane 1: untreated PCNSL tissue from patient #3. Lane 2: PNGase-F-treated PCNSL tissue from patient #3. Lane 3: untreated PCNSL tissue from patient #6. Lane 4: PNGase-F-treated PCNSL tissue from patient #6. Lane 5: β-N-acetylglucosaminidase-treated PCNSL tissue from patient #3. Lane 6: β-N-acetylglucosaminidase-treated PCNSL tissue from patient #6. Lane 7: β-1→4-galactosidase-treated PCNSL tissue from patient #3. Lane 8: β-1→4-galactosidase-treated PCNSL tissue from patient #6. The treatment with PNGase-F and β-N-acetylglucosaminidase resulted in the disappearance of the differences in molecular weights of both antigens from patients #3 and #6. (B) Deglycosylation of SAMD14 and neurabin-1 from PCNSL tissue only with β-N-acetylglucosaminidase. The treatment with β-N-acetylglucosaminidase resulted in the disappearance of the differences in molecular weights of both SAMD14 and neurabin-I from patients #3 and #6. (C) Identification of the hyperglycosylated Asn residues. Western blots of several constructs of SAMD14 and neurabin-I with different mutants of N-glycosylation sites expressed in LCLs derived from patients with and without hyperglycosylated SAMD14 and neurabin-I. Anti-FLAG antibodies were used as primary antibodies. These western blots showed the disappearance of the gain in molecular weight for the point-mutated, full-length proteins N339Q SAMD14 and N1277Q neurabin-I expressed in LCLs of patients with PCNSL with SAMD14/neurabin-I antibodies. (D) Identification of the hyperglycosylated Asn residues. Western blots of several constructs of SAMD14 and neurabin-I with different mutants of N-glycosylation sites expressed in LCLs derived from patients with and without hyperglycosylated SAMD14 and neurabin-I. Instead, Anti-FLAG primary antibody recombinant SAMD14/neurabin-I-specific Fab was used as primary antibody. These western blots show double bands as they unspecifically stain for both wild-type SAMD14 (hyperglycosylated) and nonhyperglycosylated N339Q SAMD14, and for both wild-type neurabin-I (hyperglycosylated) and N1277Q neurabin-I.
Antibody reactivity against SAMD14 and neurabin-I
SAMD14/neurabin-I autoantibodies were frequently detected by ELISA in the sera and CSF from patients with PCNSL. The sera and CSF from 8/22 (36.4%) patients from a cohort different from the 1 from whom the PCNSL specimens were obtained contained anti-SAMD14/neurabin-I antibodies (supplemental Figure 7), as was the case with 10 sera from a third series of 39 patients with PCNSL, resulting in a total of 18/61 (29.5%) serum antibody-positive patients. Antibody serum titers ranged from 1:800 up to 1:3200, and CSF titers ranged from 1:800 up to 1:1600 (Table 1). The 8 SAMD14/neurabin-I antibody-positive serum/CSF pairs were tested for their Ig classes and IgG subclasses. All of them were IgG, with 7/8 (87.5%) belonging to the IgG1 subclass (supplemental Figures 8 and 9). Moreover, there were no SAMD14/neurabin-I antibodies in the sera from 18 patients with secondary CNS manifestations of peripheral DLBCL, sera of 24 patients with DLBCL with testicular manifestation, 92 healthy control patients, and sera and CSF of 21 patients with multiple sclerosis (supplemental Figures 10-13 and 15), resulting in a significant association of SAMD14/neurabin-I antibodies with PCNSL (P < .0001).
Titers of SAMD14/neurabin-I antibodies in serum/CSF pairs
| Case Serum/CSF . | Anti-SAMD14/neurabin-I-Serum titers . | Anti-SAMD14/neurabin-I- CSF titers . |
|---|---|---|
| 1 | 1:1600-1:3200 | 1:1600 |
| 2 | 1:3200 | 1:1600 |
| 5 | 1:1600 | 1:1600 |
| 10 | 1:800−1:1600 | 1:800 |
| 14 | 1:1600 | 1:1600 |
| 15 | 1:1600 | 1:800 |
| 19 | 1:1600 | 1:800 |
| 21 | 1:1600 | 1:800 |
| Case Serum/CSF . | Anti-SAMD14/neurabin-I-Serum titers . | Anti-SAMD14/neurabin-I- CSF titers . |
|---|---|---|
| 1 | 1:1600-1:3200 | 1:1600 |
| 2 | 1:3200 | 1:1600 |
| 5 | 1:1600 | 1:1600 |
| 10 | 1:800−1:1600 | 1:800 |
| 14 | 1:1600 | 1:1600 |
| 15 | 1:1600 | 1:800 |
| 19 | 1:1600 | 1:800 |
| 21 | 1:1600 | 1:800 |
BCR pathway activation and induction of proliferation by SAMD14 and neurabin-I
Western blot analysis of the BCR signaling pathway showed a strong activation after the addition of the cognate antigen neurabin-I to transfected TMD8, U2932, and OCI-Ly3 cells expressing a SAMD14/neurabin-I-reactive BCR, as demonstrated by a strong upregulation of pTyr525/526 SYK, pTyr96 BLNK, pTyr759 PLCγ2, and pTyr223 BTK and a significant increase in MYC expression (Figure 3A; supplemental Figure 18). Furthermore, flow cytometric analysis of cytoplasmic calcium levels of the OCI-Ly3 cells transfected to express a SAMD14/neurabin-I-reactive BCR indicated a strong increase of cytoplasmatic calcium levels after incubation with SAMD14/neurabin-I, but not the control antigen MAZ (supplemental Figure 18), which had been shown to be the antigenic target derived from the malignant cells of a patient with chronic lymphocytic leukemia.11 In correspondence to this, incubation with the BCR-binding neurabin-I epitope resulted in an increased proliferation of the transfected U2932, TMD8, and OCI-LY3 cell lines expressing SAMD14/neurabin-I-reactive BCR (Figure 3B; supplemental Figure 18) in a tetrazolium formazan (EZ4U) assay.
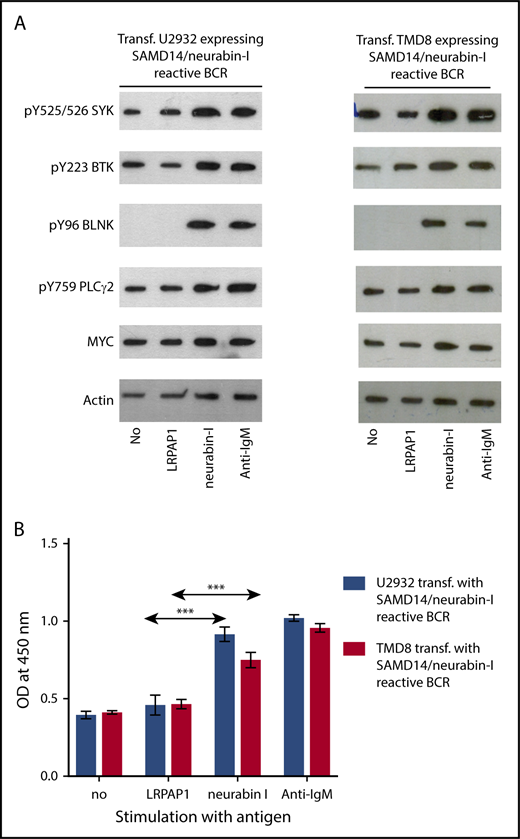
BCR pathway activation and proliferation induced by BCR-specific antigens. (A) Activation of the BCR signaling pathway. The western blot analysis of the BCR signaling pathway shows a strong activation by the addition of SAMD14 in transfected TMD8 and U2932 cells expressing a SAMD14/neurabin-I-reactive BCR or by the addition of anti-IgM. The addition of the cognate antigens or anti-IgM antibody results in the upregulation of pTyr525/526 SYK, pTyr96 BLNK, pTyr759 PLCγ2, and pTyr223 BTK and led to an overexpression of c-Myc. (B) Proliferation induction of aggressive lymphoma cell lines by BCR-specific antigens. The columns (formazan at an OD of 450 nm) represent the proliferation of cells measured by reduction of tetrazolium salt to colored formazan by viable cells (EZ4U assay). Addition of epitope containing region of neurabin-I resulted in a significant increase in proliferation of TMD8 and U2932 cells expressing SAMD14/neurabin-I-reactive BCRs. Experiments were performed in triplicates for each condition and repeated for 3 times (***P ≤ .001).
BCR pathway activation and proliferation induced by BCR-specific antigens. (A) Activation of the BCR signaling pathway. The western blot analysis of the BCR signaling pathway shows a strong activation by the addition of SAMD14 in transfected TMD8 and U2932 cells expressing a SAMD14/neurabin-I-reactive BCR or by the addition of anti-IgM. The addition of the cognate antigens or anti-IgM antibody results in the upregulation of pTyr525/526 SYK, pTyr96 BLNK, pTyr759 PLCγ2, and pTyr223 BTK and led to an overexpression of c-Myc. (B) Proliferation induction of aggressive lymphoma cell lines by BCR-specific antigens. The columns (formazan at an OD of 450 nm) represent the proliferation of cells measured by reduction of tetrazolium salt to colored formazan by viable cells (EZ4U assay). Addition of epitope containing region of neurabin-I resulted in a significant increase in proliferation of TMD8 and U2932 cells expressing SAMD14/neurabin-I-reactive BCRs. Experiments were performed in triplicates for each condition and repeated for 3 times (***P ≤ .001).
BCR-mediated targeting of PCNSL by BCR antigen/drug conjugates
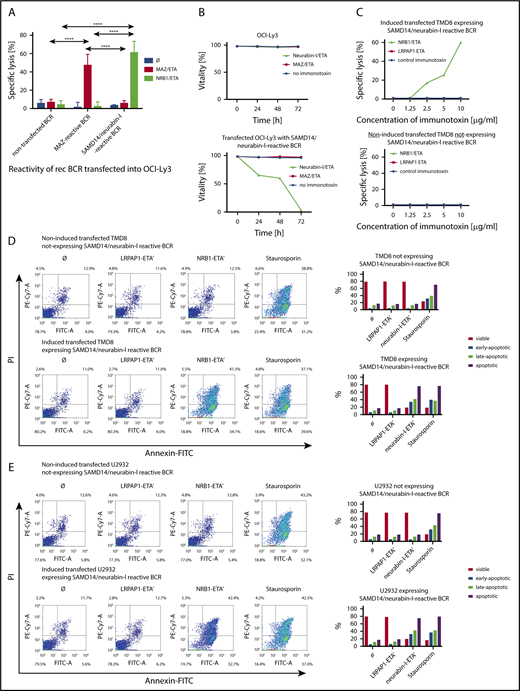
The transfected cell lines OCI-Ly3, U2932, and TMD8 expressing a patient-derived BCR with specificity for SAMD14/neurabin-I were specifically killed by incubation with a neurabin-I-ETA′ drug conjugate while remaining unaffected when incubated with the same toxin conjugated to irrelevant control antigens (MAZ or LRPAP1). The specific killing was demonstrated in an LDH release assay (Figure 4A). As shown by trypan exclusion, 100% of the lymphoma cells expressing a BCR with specificity for SAMD14/neurabin-I were dead after 72 hours of incubation with the BCR antigen for reverse targeting-toxin conjugate (Figure 4B). The toxic effect of the neurabin-I/ETA′ was dose-dependent (Figure 4C). In accordance to this, an increase of apoptotic cells was detected after incubation with neurabin-I/ETA′ in U2932, TMD8, and OCI-Ly3 cells expressing SAMD14/neurabin-I-reactive BCRs (Figure 4D-E; supplemental Figure 19), as shown by an Annexin-V/PI assay.
Specific killing of aggressive lymphoma cells using BCR-antigen/toxin constructs. (A) LDH-release assay. Percentage specific lysis of nontransfected OCI-Ly3 cells or OCI-LY3 cells transfected to express either MAZ-specific or SAM-specific BCRs after incubation with 5 μg/mL neurabin-I/ETA or MAZ/ETA, respectively, for 24 hours. Experiments were performed in triplicates and repeated 3 times (****P ≤ .0001). (B) Trypan blue exclusion assay. Cell viability of transfected OCI-LY3 cells expressing SAMD14/neurabin-I-specific BCRs or nontransfected OCI-Ly3 cells after incubation with 2 μg/mL neurabin-I/ETA or MAZ/ETA was determined by trypan blue staining after 24 and 48 hours, showing the time-course effect. (C) Dose dependency of cytotoxicity determined by LDH release assay. Percentage specific lysis of transfected TMD8 cells either doxycycline-induced or noninduced after incubation with different concentrations (1.25-10 μg/mL neurabin-I/ETA′ or LRPAP1/ETA′ immunotoxins for 24 hours), showing a dose-dependent effect. (D-E) Annexin-V assay. Characterization of transfected TMD8 and U2932 cells with either doxycycline-induced expression of SAMD14/neurabin-I reactive BCR or noninduced, nonexpressing recombinant BCRs by annexin-V and PI staining after 24-hour cultivation in the presence of SAM/ETA, LRPAP1/ETA, or Staurosporine. In the bottom line, bar graphs represent the proportion of vital, early, late, and total apoptotic cells.
Specific killing of aggressive lymphoma cells using BCR-antigen/toxin constructs. (A) LDH-release assay. Percentage specific lysis of nontransfected OCI-Ly3 cells or OCI-LY3 cells transfected to express either MAZ-specific or SAM-specific BCRs after incubation with 5 μg/mL neurabin-I/ETA or MAZ/ETA, respectively, for 24 hours. Experiments were performed in triplicates and repeated 3 times (****P ≤ .0001). (B) Trypan blue exclusion assay. Cell viability of transfected OCI-LY3 cells expressing SAMD14/neurabin-I-specific BCRs or nontransfected OCI-Ly3 cells after incubation with 2 μg/mL neurabin-I/ETA or MAZ/ETA was determined by trypan blue staining after 24 and 48 hours, showing the time-course effect. (C) Dose dependency of cytotoxicity determined by LDH release assay. Percentage specific lysis of transfected TMD8 cells either doxycycline-induced or noninduced after incubation with different concentrations (1.25-10 μg/mL neurabin-I/ETA′ or LRPAP1/ETA′ immunotoxins for 24 hours), showing a dose-dependent effect. (D-E) Annexin-V assay. Characterization of transfected TMD8 and U2932 cells with either doxycycline-induced expression of SAMD14/neurabin-I reactive BCR or noninduced, nonexpressing recombinant BCRs by annexin-V and PI staining after 24-hour cultivation in the presence of SAM/ETA, LRPAP1/ETA, or Staurosporine. In the bottom line, bar graphs represent the proportion of vital, early, late, and total apoptotic cells.
Discussion
We identified SAMD14 and the highly homologous SAM domain of neurabin-I29 as the predominant antigenic targets of the BCR from PCNSLs. The specificity of the PCNSL BCRs was not only demonstrated by the negative reaction of the PCNSL-BCRs with the remaining more than 7000 other proteins represented in the macroarray used for screening, but also by the absence of any reactivity against more than 200 pathogens including various bacterial, fungal, parasitic, and viral pathogens. The recombinant BCRs in the present study were not reactive against galectin-3 (supplemental Figure 17).12 Moreover, SAMD14/neurabin-I are specific antigens of PCNSL-derived BCRs, because none of the BCRs derived from 12 DLBCL, 19 MCL, and 31 follicular lymphoma cases reacted with these proteins, which are preferentially expressed in the CNS.
SAMD14 and neurabin-I share a highly homologous SAM domain, neurabin-I has a further SAM-like neurabin domain, and both SAMD14 and neurabin-I are primarily expressed in the CNS (www.proteinatlas.org and www.genevisible.com). The SAMD14 gene, located on chromosome 17q21.33, consists of 14 exons and a coding sequence of 417 amino acids. Little is known about the function of SAMD14. The gene coding for neurabin-I, also called protein phosphatase 1 regulatory subunit 9A, is located on chromosome 7q21.3 and has 29 exons with 5 different splicing forms with coding sequences from amino acids 1095 to 1374. Among other functions, neurabin-I is a regulator of protein phosphatase 1 and binds specifically to F-actin from neural tissues. Overexpression was observed in the dorsolateral prefrontal cortex of bipolar disorder and is believed to interact with N-methyl-D-aspartate receptor signaling pathways that regulate the actin cytoskeleton and spines of dendritic cells.30 Moreover, neurabin-I was found to be specifically overexpressed in hepatosplenic T-cell lymphoma.31
SAMD14 and neurabin-I were N-hyperglycosylated in all patients with PCNSL-derived BCRs with reactivity for these antigens, but not in any other patient or control. Posttranslationally modified antigens are a well-known phenomenon in autoimmunity.17,32-35 The associated autoreactive BCRs and antibodies are specifically reactive against the posttranslationally modified autoantigenic isoforms,17,33 whereas for other antigens, the BCRs or paraproteins did not differentiate between the posttranslationally modified and the wild-type isoform.10,34,36,37 The latter is also the case for the PCNSL-derived BCRs in this study, which reacted with both the normally glycosylated and the hyper N-glycosylated SAMD14 and neurabin-I isoforms. Peptides of hyper-N-glycosylated SAMD14/neurabin-I are probably specifically recognized by CD4+ T cells, which stimulate non-isoform-specific SAMD14/neurabin-I reactive B cells similar to hyperphosphorylated SLP2 in plasma cell disorders.38 SAMD14/neurabin-I autoantibodies showed no light chain restriction, but were observed in high titers in serum and CSF, indicating 2 things. First, it suggested a partly intrathecal production, and second, they probably do not originate directly from the lymphoma cells, but from related polyclonal B cells with the same specificity that have undergone plasmablastic differentiation, in contrast to the IgM+ and IgD+ PCNSL cells, which characteristically stop differentiation before class switch.39
For SAMD14, the residue of ASN 339, and for neurabin-I, the residue of ASN 1277, were identified as the sites of N-hyperglycosylation. Interestingly, these 2 N-glycosylation sites are made of atypical N-L-E-Q patterns, which might further trigger autoimmunity. Functional effects of the N-hyperglycosylation of both proteins are not clear and remain to be analyzed in future studies.
In the era of stereotactic biopsies, expression cloning of recombinant BCRs from PCNSL tissue represents a particular challenge because of the rare availability of sufficient fresh tissue. Despite the cooperation of the major PCNSL centers in the German-speaking countries, many PCNSL specimens included in this study were rather old (the oldest more than 25 years), and it was impossible to obtain sera or CSF from all patients of whom cryospecimens were analyzed. Therefore, for the analysis of serum and CSF samples, we had to turn to a second cohort of patients. The detection of polyclonal antibodies against SAMD14/neurabin-I, which were found in the sera and CSF of nearly one-third of patients in a second (independent) PCNSL validation cohort, supports the hypothesis that the malignant transformation evolves from a polyclonal B-cell response against the N-hyperglycosylated, and primarily in the CNS-expressed, SAMD14 and neurabin-I autoantigens as a nonrandom early step in PCNSL, followed by transformation of a subset of SAMD14/neurabin-I-reactive B-cells into a malignant clone.
To investigate whether the reactivity of the lymphoma BCRs against SAMD14 and neurabin-I continues to play a sustaining role for established aggressive lymphoma, 3 representative ABC-type DLBCL cell lines with different genetic backgrounds were used, as these are biologically closely related to PCNSL.23-26 These lines were transfected to express a SAMD14/neurabin-I-reactive BCR, as no established EBV-negative PCNSL cell line exists so far. Addition of the antigen SAMD14/neurabin-I to this transfected cell lines resulted in a strong BCR pathway activation accompanied by elevation of cytoplasmatic calcium levels, a stronger expression of MYC, and a significantly increased proliferation.
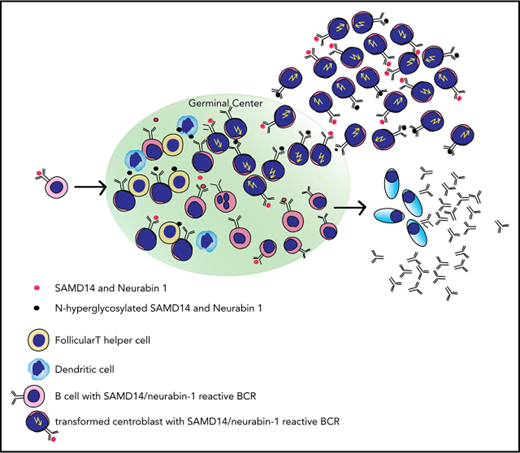
The results of this study support the hypothesis that activation of the lymphoma BCRs by their cognate CNS antigen produces a strong growth advantage even for the transformed aggressive lymphoma cells. This is also supported and might explain the persistence of functional BCRs in PCNSL, despite ongoing somatic hypermutation. The proliferative effects of the chronic BCR activation by SAMD14 and neurabin-I, together with the fact that both SAMD14 and neurabin-I are predominantly expressed in the CNS,29 serve also as an explanation for the CNS tropism of the respective lymphomas (supplemental Figure 20; scheme of suspected role of SAMD14/neurabin-I).
Finally, the specific reactivity of lymphoma BCRs from the majority of patients with PCNSL against SAMD14/neurabin-I might provide the basis for a specific therapeutic approach that uses BCR antigens for reverse targeting. Because SAMD14/neurabin-I are cytoplasmic proteins, treatment strategies using SAMD14/neurabin-I as a targeting moiety for directing a toxic payload to tumor cells should not bind to cells expressing membrane receptors other than a BCR with specificity for SAMD14/neurabin-I. Because it is the major physiologic task of the BCR to bind and internalize its specific antigen, SAMD14/neurabin-I BCR antigens for reverse targeting were readily internalized (data not shown) and, when conjugated to a toxin, killed the lymphoma cells after release of the toxin (ETA), with 100% of the cells being dead after 72 hours, as demonstrated by cytotoxicity and apoptosis assays (Figure 4B) and dye exclusion. In contrast to approaches that target the idiotypic region of the BCR and that must be designed for each individual patient, because of the predominance of BCRs with specificity for SAMD14/neurabin-I in PCNSL, SAMD14/neurabin-I can be used as bait to target a majority of patients with PCNSL.40,41 In addition to drug conjugates, the epitope regions of SAMD14 and neurabin-I could as well be conjugated to or used as the targeting moiety of CAR-T cells, thus binding to and activating T cells against the BCR-expressing cells. Because approaches using the BCR antigen for targeting the malignant B cells have a higher specificity, they can be expected to be less toxic than the currently available bispecific T-cell engaging antibodies or CAR-T cells with specificity for CD19.41
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors appreciate the help of Nadine Schneider and Ralf Küppers for the seminested PCRs on variable region genes. The authors are grateful to the entire team of the José-Carreras-Center for Immuno- and Gene Therapy and the Department of Internal Medicine I of Saarland University Medical School. Furthermore, the authors are grateful to Ralf Ketter from the Department of Neurosurgery of Saarland University Medical School, and Hans-Otto Reiber and Peter Lange from the Neurochemisches Labor of the Department of Neurology of Göttingen University Medical Centre. The authors thank Bernhard Thurner for proofreading the manuscript. Cryopreserved PCNSL samples from Würzburg were provided with the help of the Interdisciplinary Bank of Biomaterials and Data Würzburg (http://www.ibdw.ukw.de).
This work was supported by a grant from Sander-Stiftung, a charity foundation in Munich, Germany. L.T. received a grant for a clinical research rotation from the University of Saarland.
Authorship
Contribution: L.T., K.-D.P., and M.P. designed the study; S.A., M.B., C.S., V.P., M.Z., S.W., P.R., M.W., M.S., W.K., C.M., A.R., P.M., S.H., M.L.-H., A.M., H.S, E.S., G.I., R.B., R.M.B., S.S., and Y.-J.K. collected the cryopreserved PCNSL tissues, serum, and CSF samples from patients with primary central nervous system lymphoma, primary testicular lymphoma, diffuse large B-cell lymphoma and multiple sclerosis; L.T., M.K., E.R., and N.F. performed the cloning and recombinant expression of the Igs; N.F. performed the protein array experiments and deglycosylation experiments; E.R. performed the site-directed point mutagenesis; M.K. and E.R. performed sequencing; L.T. and M.P. are responsible for data analysis, interpretation of results, and writing of the manuscript; and all authors read and approved the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Lorenz Thurner, Department of Internal Medicine I, Saarland University Medical School, D-66421 Homburg/Saar, Germany; e-mail: lorenz.thurner@uks.eu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal