In this issue of Blood, Thurner et al identified that two-thirds of patients with primary central nervous system lymphoma (PCNSL) have B-cell receptors (BCRs) specific for 2 autoantigens, SAMD14 and neurabin-1. These antigens are predominantly expressed in the central nervous system (CNS), induced strong BCR pathway activation, and autorecognition of them is therefore an attractive explanation for the CNS tropism of the disease.1
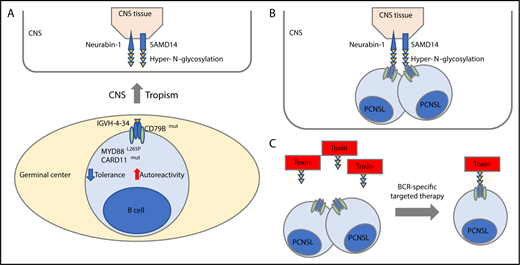
Hyper-N-glycosylated autoantigen-mediated CNS tropism. (A) PCNSL cells are derived from germinal center exit B cells that preferentially express BCR using the IGVH-4-34 genes that are known to produce antibodies with increased autoreactivity. Besides uniform activation of the BCR with CD79B mutations, PCNSL cells exhibit frequent cooccurring MYD88L265P and infrequent CARD11 mutations, both known genetic alterations associated with tolerance failure and survival of autoantigen recognizing B cells (lower). In this issue of Blood, Thurner et al identify that PCNSL patients exhibit a hyper-N-glycosylated form of SAMD14 and neurabin-1 with high expression in the CNS tissue (upper). (B) Thurner et al discovered that the BCR of the PCNSL cells recognize the hyper-N-glycosylated forms of SAMD14 and neurabin-1, providing an attractive explanation that they have a canonical role in CNS tropism of this disease. (C) Concept of BCR-directed targeted therapy. A toxin is bound to the epitope of neurabin-1 that then bound specifically the tumor cells.
Hyper-N-glycosylated autoantigen-mediated CNS tropism. (A) PCNSL cells are derived from germinal center exit B cells that preferentially express BCR using the IGVH-4-34 genes that are known to produce antibodies with increased autoreactivity. Besides uniform activation of the BCR with CD79B mutations, PCNSL cells exhibit frequent cooccurring MYD88L265P and infrequent CARD11 mutations, both known genetic alterations associated with tolerance failure and survival of autoantigen recognizing B cells (lower). In this issue of Blood, Thurner et al identify that PCNSL patients exhibit a hyper-N-glycosylated form of SAMD14 and neurabin-1 with high expression in the CNS tissue (upper). (B) Thurner et al discovered that the BCR of the PCNSL cells recognize the hyper-N-glycosylated forms of SAMD14 and neurabin-1, providing an attractive explanation that they have a canonical role in CNS tropism of this disease. (C) Concept of BCR-directed targeted therapy. A toxin is bound to the epitope of neurabin-1 that then bound specifically the tumor cells.
PCNSL is a rare extranodal variant of a B-cell non-Hodgkin lymphoma that presents as infiltration of Epstein-Barr virus negative (EBV−) lymphoma cells in the brain parenchyma, leptomeninges, spinal cord, or the eye without evidence of systemic disease. The most frequent histology of PCNSL is a diffuse large B-cell lymphoma (DLBCL). An etiologically different EBV+ PCNSL occurs in immunocompromised patients. Despite its limited state, clinically PCNSL is an aggressive disease with dismal outcomes due to inferior responses to empirically derived treatment regimens. A better mechanistic understanding of the disease pathogenesis might lead to novel targeted therapeutic options for patients with unmet clinical needs.
Genetically, PCNSLs exhibit frequent biallelic inactivation of the tumor suppressor CDKN2A, almost uniform activation of the BCR- and Toll-like receptor signaling pathways through cooccurring CD79B and MYD88 (L265P) mutations, and additional alterations in downstream regulators, such as inactivation of TNFAIP3 and oncogenic mutations in CARD11, in addition to frequent gain of both PD-1 ligands.2 The full coordinate genetic signature of PCNSLs has striking similarities to genetically defined ABC DLBCL subtypes (C5-DLBCL in Chapuy et al3 ; C5-DLBCL in Schmitz et al4 ).
Prior studies have established the origin of PCNSLs from germinal center experienced B cells with rearranged and hypermutated immunoglobulin genes, functional BCR-signaling despite ongoing somatic hypermutation, and a block in class switch recombination and subsequent restricted immunoglobulin M (IgM) surface expression.5,6 As brain tissue does not contain germinal centers, currently PCNSL is thought to derive from malignant B cells that transformed outside the CNS with subsequent migration to the brain. The mechanism of this CNS tropism is unknown.
Notably, PCNSL cells have a restricted immunoglobulin variable region heavy chain repertoire with the preferential use of a specific immunoglobulin heavy chain, encoded by the IGVH4-34 gene,7 that is also predominantly found in IgM-positive ABC DLBCLs and associated with autoreactivity.8 The frequent cooccurring MYD88L265P and infrequent CARD11 mutations in PCNSL3 provide additional mechanisms for tolerance failure to autoantigens in these transformed B cells (see figure panel A).9 These findings, paired with the knowledge that the BCR receptor is fully functional, prompted the speculation that CNS-derived autoantigen-mediated chronic antigen stimuli serve as central factors in the pathogenesis of PCNSL and let Thurner et al search for specific CNS-derived autoantigens that drive B-cell activation and might trigger CNS tropism of the disease (see figure panel B). Prior attempts to find specific autoantigens in PCNSLs have identified polyclonal autoreactive BCRs.10
Here, Thurner et al cloned and expressed Fab fragments of 12 PCNSL patient-derived BCRs and used these recombinant BCRs to screen a large protein array with >7300 proteins to identify hyper-N-glycosylated sterile α-motif domain-containing protein 14 (SAMD14) and neural-specific F-actin binding protein 1 (neurabin-1) as 2, highly in CNS tissue expressed, putative autoantigens in PCNSLs. Importantly, recombinant BCRs of 12 systemic DLBCL, 19 mantle cell, and 31 follicular lymphomas did not cross-react, underscoring the specificity of these antigens to PCNSL-derived BCRs. Interestingly, Thurner et al discovered that PCNSL patients harbored a hyper-N-glycosylated form of SAMD14 and neurabin-1, in contrast to control tissue, including the genetically related primary testicular lymphoma (PTL), secondary CNS lymphoma (SCNSL), or brain tissue of multiple sclerosis patients (see figure panel A). This prompted the authors to identify the atypical new glycosylation site in each of the respective proteins. Hyper-N-glycosylated SAMD14/neurabin-1 was not only found in lysates of PCNSL samples with SAMD14/neurabin-1 reactive BCR but also, to a lesser extent, found in circulating normal blood cells of other lineage, raising speculations regarding the founding cell that carried the genetic alteration causing the new glycosylation site. Through a series of in vitro studies, the authors confirmed antigen specificity, antigen-dependent BCR pathway activation, and demonstrated that ligand binding provided a proliferation advantage. Notably, one-third of PCNSL patients had anti-SAMD14/neurabin-1–specific antibodies in their sera and cerebrospinal fluid. Last, Thurner et al explored the idea of coupling the BCR-binding epitope of neurabin-1 to a toxin and test the efficacy and specificity of this BCR-directed therapy in cell-line models of related ABC-type DLBCLs (see figure panel C). These in vitro studies are currently just a proof-of-concept study, but might pave the way for an interesting targeted therapeutic paradigm leveraging toxin-bound ligands against specific BCRs in this dismal disease and related disorders.
This study by Thurner et al provides novel insights into the potential pathogenesis of a rare and dismal disease. Particularly, the proof of specific antigens provides an attractive explanation for the tropism of B cells with genetically induced tolerance failure by recognizing the hyper-N-glycosylated SAMD14/neurabin-1 because the CNS had the highest expression of these autoantigens (see figure panel B). Subsequent mechanistic studies in mice need to follow and should aim to provide experimental evidence of the postulated lymphomagenesis. Despite these findings being a step forward, we still do not have a comprehensive understanding of the probably multifactorial molecular bases of CNS tropism. Even if mechanistic studies in mice confirm certain aspects of the postulated lymphomagenesis, we do not know what the putative antigens are in the remaining PCNSL cases. The absence of the hyper-N-glycosylated form of SAMD14 and neurabin-1 expression in SCNSL and PTL that often relapsed in the brain, and likely other B-cell diseases with CNS tropism such as Burkitt lymphoma or B-cell acute lymphoblastic leukemia, suggests that different autoantigens and/or mechanisms must also play a role that warrants further studies.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal