Key Points
The MTD of POM for relapsed/refractory PCNSL is 5 mg orally daily for 21 days every 28 days.
POM and DEX combination has therapeutic activity against relapsed/refractory PCNSL.
Abstract
The combination of pomalidomide (POM) and dexamethasone (DEX) was evaluated for relapsed/refractory primary central nervous system lymphoma (PCNSL) and primary vitreoretinal lymphoma (PVRL) to determine the maximal tolerated dose (MTD) of POM as the primary objective, and overall response rate (ORR), progression-free survival (PFS), and safety profile as secondary objectives. A cohorts-of-3 study design was used with a dose-escalation schedule consisting of POM (3, 5, 7, or 10 mg) orally daily for 21 days every 28 days and DEX 40 mg orally every week. After 2 cycles, POM was continued alone until disease progression, intolerance, or subject withdrawal. Following MTD determination, the MTD cohort was expanded. Twenty-five of 29 patients with the median of 3 prior treatments were eligible for assessment as per international PCNSL collaborative group criteria. The MTD of POM was 5 mg daily for 21 days every 28 days. Whole-study ORR was 48% (12 of 25; 95% confidence interval [CI], 27.8%, 68.7%) with 6 complete response (CR), 2 complete response, unconfirmed (CRu), and 4 partial response (PR). MTD cohort ORR was 50% (8 of 16; 95% CI, 24.7%, 75.4%) with 5 CR, 1 CRu, and 2 PR. Median PFS was 5.3 months (whole study) and 9 months (for responders). One patient had pseudoprogression. Grade 3/4 hematologic toxicities included neutropenia (21%), anemia (8%), and thrombocytopenia (8%). Grade 3/4 nonhematologic toxicities included lung infection (12%), sepsis (4%), fatigue (8%), syncope (4%), dyspnea (4%), hypoxia (4%), respiratory failure (8%), and rash (4%). POM/DEX treatment is feasible with significant therapeutic activity against relapsed/refractory PCNSL and PVRL. This trial was registered at www.clinicaltrials.gov as #NCT01722305.
Introduction
Primary central nervous system (CNS) lymphoma (PCNSL) is predominantly a diffuse large B-cell lymphoma (DLBCL) confined to the CNS.1,2 It has an annual incidence in the United States of ∼1500 new cases each year.2 It represents ∼3% of brain tumors and 2% to 3% of non-Hodgkin lymphoma.3,4 The incidence rates in the 65 years and older age group have been steadily increasing in the last decades.5
Treatment of PCNSL is complicated by the fact that most antilymphoma agents do not cross the blood-brain barrier adequately. Use of standard chemotherapy for systemic DLBCL was associated with poor outcome.2 High-dose (HD) methotrexate (HD-MTX) chemotherapy alone or in combination with other agents has resulted in improvement in survival outcome.2,6,7 Consolidation with whole brain radiation (WBR), nonmyeloablative intensive chemotherapy or HD chemotherapy followed by autologous stem cell transplantation (ASCT) has further improved response rate and survival in the clinical trial setting.2,8-11 Current standard induction therapy for eligible patients consists of HD-MTX–based chemotherapy or chemoimmunotherapy followed by consolidation with intensive/high-dose chemotherapy or WBR.2,8,10 However, a significant number of patients, especially elderly, are not eligible for these intensive treatments. Once PCNSL has relapsed, treatment options are limited with dismal prognosis.2 Novel therapeutic agents with excellent CNS penetration, better efficacy, and tolerable toxicity profile are urgently needed.
Lenalidomide (LEN), a second-generation immunomodulatory agent (IMiD), has shown promising therapeutic activity as a single agent or when incorporated into standard chemoimmunotherapeutic regimen R2CHOP (lenalidomide [Revlimid], rituximab [Rituxan], cyclophosphamide, hydroxydaunorubicin, vincristine [Oncovin], and prednisone) against DLBCL, especially activated B-cell DLBCL (ABC-DLBCL) in phase 2 clinical trials.12-15 In a phase 3 trial testing maintenance LEN following induction with RCHOP (rituximab [Rituxan], cyclophosphamide, hydroxydaunorubicin, vincristine [Oncovin], and prednisone), improvement was seen in progression free survival (PFS) but not in overall survival (OS), especially in germinal center B-cell DLBCL (GCB-DLBCL).16 Preliminary reports of LEN in relapsed/refractory CNS lymphoma (CNSL) as a single agent or in combination with Rituximab have shown therapeutic efficacy.17,18
Pomalidomide (POM) is a novel, third-generation IMiD that has shown excellent CNS penetration and significant therapeutic activity against CNSL in our preclinical study.19 In an in vivo murine model, POM crossed the blood-brain barrier with a CNS penetration of ∼40%.19 Preclinical evaluation in 2 murine CNSL models revealed excellent single-agent therapeutic activity of POM with significant prolongation of survival and a major impact on tumor immune microenvironment (TIME) compared with no-treatment controls.19 It has a dual antilymphoma activity mediated via direct cytotoxicity against lymphoma cells and modulation of TIME.19 One striking feature of the impact of POM on TIME is the conversion of polarization status of tumor-associated macrophages from M2 to M1.19 Its effect on TIME may play an important role in tackling immune evasion which characterizes PCNSL.20 Moreover, addition of weekly dexamethasone (DEX) to POM treatment in our preclinical models resulted in further prolongation of survival compared with POM alone.19 Based upon these preclinical data, we initiated a phase 1 clinical trial of POM/DEX for relapsed/refractory PCNSL and primary vitreoretinal lymphoma (PVRL).
Methods
Eligibility
We enrolled relapsed/refractory PCNSL or PVRL patients with at least 1 prior line of systemic therapy, 18 years of age or older, with pathologic features consistent with DLBCL. Eligible patients had a CNS lesion, positive CSF (cerebrospinal fluid) cytology, or positive ocular tissue biopsy. All patients had biopsy-proven PCNSL or PVRL. Other inclusion criteria included an Eastern Cooperative Oncology Group (ECOG) performance status ≤3, absolute neutrophil count ≥ 1 × 109/L, platelets ≥ 100 × 109/L, total bilirubin ≤1.5× upper limit of normal (ULN) or if total bilirubin is >1.5× ULN, the direct bilirubin must be ≤1.5× ULN (≤0.45 mg/dL), AST (aspartate aminotransferase) ≤3× ULN, and creatinine ≤2.5× ULN. For thromboprophylaxis, all patients took an aspirin (81 or 325 mg orally daily) or an anticoagulant. Patients were required to follow standard guidelines with respect to fertility for the IMiD class of drugs. Females of childbearing potential (FCBP) were required to have a negative serum or urine pregnancy test within 10 to 14 days prior to and again within 24 hours of starting POM. They had to either commit to continued abstinence from heterosexual intercourse or begin 2 acceptable methods of birth control, 1 highly effective method and 1 additional effective method. Men were required to agree to use a latex condom during sexual contact with a FCBP even if they had had a vasectomy. All patients were counseled at a minimum of every 28 days about pregnancy precautions and risks of fetal exposure. Pregnant or nursing women, and men or women of childbearing potential who were unwilling to use adequate contraception, were excluded. Other exclusion criteria included uncontrolled infection, previous development of erythema nodosum while on thalidomide or similar drugs, therapy with myelosuppressive chemotherapy or biologic therapy within 21 days prior to registration, history of thromboembolic episodes within 3 months prior to registration, immunodeficiency states including HIV infection, inability to swallow or absorb treatment medications, major surgery within 4 weeks of registration, uncontrolled active hepatitis B or C, or New York Heart Association classification of III or IV.
Study design and treatment
The study was a phase 1, investigator-initiated, clinical trial conducted at Mayo Clinic Cancer Center, Memorial Sloan Kettering Cancer Center, and the University of Virginia. It was conducted in accordance with the Declaration of Helsinki and good clinical practice guidelines and was approved by the institutional review board at each participating institution. All accrued patients provided written informed consent. The primary objective was to determine the maximal tolerated dose (MTD) of POM with weekly DEX. The secondary objectives included overall response rate (ORR), progression-free survival (PFS), and safety profile. Following the determination of the MTD of POM, the MTD cohort was expanded with additional 10 patients. All patients who met eligibility criteria and received at least 1 dose of POM were considered evaluable for study end points.
The MTD of POM was determined using the standard cohorts-of-3 study design.21 The dose-escalation schedule consisted of POM (3, 5, 7, or 10 mg) orally daily for 21 days every 28 days and DEX 40 mg orally every week. After 2 cycles, POM was continued alone until disease progression, intolerance of side effects, or decline by the patient to continue. Three patients were treated at each dose level and monitored for a minimum of 4 weeks (1 cycle) to assess dose-limiting toxicities (DLTs), before new patients were treated. If toxicity assessment could not be completed in a patient as required by the protocol, replacement of that patient was required. The MTD of POM was defined as the dose level below the lowest dose that induced DLT in at least one-third of patients (at least 2 of a maximum of 6 patients). This determination was based on the data from the first cycle of treatment; however, toxicity data were collected during all cycles and monitored for cumulative toxicity. DLT was defined as an adverse event in the first cycle attributed (definitely, probably, or possibly related) to the study treatment and meeting the criteria that included grade 4 thrombocytopenia of any duration, grade 3 thrombocytopenia with active bleeding of any duration, febrile neutropenia lasting 7 days or more, all grade 4 hematological toxicities, and any grade 3 or 4 nonhematologic toxicity including diarrhea, nausea, and vomiting that did not respond to supportive therapy.
Assessments
Magnetic resonance imaging (MRI) of brain and whole spine, ophthalmologic examination, and lumbar puncture were performed at baseline to evaluate the extent of CNS involvement. Computed tomogram (CT) of chest, abdomen, and pelvis was performed at baseline to rule out lymphoma outside the CNS. To assess therapeutic response, MRI scans were repeated on positive areas with measurable lesions or to examine areas suggested by new neurologic symptoms or signs. MRI scans were performed every 2 cycles until complete remission at which point the frequency was reduced to every 3 cycles. Imaging scans were interpreted by site principal investigator/radiologists. Detailed ophthalmologic evaluation by dilated fundus examination was mandatory for all patients at study entry and was repeated as part of the restaging in patients with positive findings at baseline or presence of ocular symptoms. Lumbar puncture was performed at baseline if there was no contraindication and was repeated at each restaging if initial CSF cytology was positive or as indicated by new clinical findings. Therapeutic responses were determined per the modified International PCNSL Collaborative Group (IPCG) criteria.22 We complied with the IPCG criteria with the exception that the first restaging MRI was done within 1 to 7 days after the last weekly DEX. Subsequent MRI scans were done without any prior DEX. Adverse events were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0. Matched CSF and blood samples were collected from consenting patients during cycle 1 to determine the CNS penetration of POM.
Statistical analyses
The primary end point of this trial was the determination of the MTD of POM. Secondary end points were ORR, PFS, and safety profile. ORR was defined as the number of patients with an objective status of complete response (CR), complete response unconfirmed (CRu), and partial response (PR) divided by the total number of evaluable patients. Exact binomial 95% confidence intervals (CIs) for the true ORR were calculated. All patients who received at least 1 dose of POM were considered in the evaluation of ORR. PFS was defined as the time from study registration to any 1 of the following events: progressive disease, relapse after response, or death due to PCNSL or PVRL. Patients who died due to any other cause or who received subsequent treatment of lymphoma prior to progressive disease (PD) were censored on the date of last disease evaluation. Duration of response (DOR) was defined as the time from the first response (CR, CRu, or PR) to the event of relapse/death or censored on the last disease evaluation. The distributions of time-to-event measures were estimated using the Kaplan-Meier method.23 Median follow-up was calculated using the reverse Kaplan-Meier method.
The toxicity profile was defined in a descriptive manner by documenting all the adverse events including those at least possibly related to the treatment. All eligible patients who had initiated treatment were considered evaluable for assessing toxicity. The maximum grade for each type of adverse event was recorded for each patient, and frequency tables were reviewed to determine the patterns.
The Cancer Center Statistics unit of the Mayo Clinic Health Sciences Research division was responsible for data collection, data storage, and statistical analysis. Statistical analyses were performed by a Mayo Clinic statistician. The study statistician reviewed the study weekly to identify and address any accrual, adverse event, or end point concerns. The Mayo Clinic Cancer Center (MCCC) Data Safety Monitoring Board (DSMB) reviewed accrual and safety data for this trial at least twice a year.
The investigative team designed the study, collected, analyzed, and interpreted the data and wrote the manuscript. Celgene Pharmaceutical approved and funded the study, provided POM free of charge, and was provided access to the data and results. Statistical analyses were carried out using SAS software version 9.4M3.
Results
Patients
This study accrued 29 patients between April 2013 and June 2017. Four patients in the dose-escalation phase of the trial were found to be ineligible as per the study requirements. Two patients at the 7-mg dose level were found to be ineligible after registration as they had systemic lymphoma with secondary CNS involvement; 1 patient at the 7-mg dose level rapidly deteriorated soon after registration before treatment and was cancelled by the accruing physician; and 1 patient at the 5-mg dose level had a major protocol violation as he was incorrectly prescribed 7 mg daily instead of 5 mg daily (was not allowed to complete the first cycle) and was replaced. The characteristics of the 25 eligible patients are listed in Table 1. Eighteen patients (72%) were 60 years of age or older and 7 patients (28%) were younger than 60 years of age. Thirteen patients (52%) were women and 12 (48%) were men. Twenty-three patients (92%) had relapsed/refractory PCNSL and 2 (8%) had relapsed/refractory PVRL. Twenty patients (80%) had relapsed disease; 5 patients (20%) had refractory disease. Twenty-one patients (84%) had disease with involvement of the brain parenchyma and 4 (16%) had disease outside of the brain parenchyma (eyes or leptomeningeal). All patients were previously treated; 24 patients (96%) had prior HD-MTX–based chemotherapy or chemoimmunotherapy and 22 (88%) had prior treatments containing rituximab. Ten patients (40%) had radiation previously. Five patients (20%) had previously undergone ASCT. The patients had been heavily treated before the trial with the median of 3 prior regimens (range, 1-11). Radiation therapy or ASCT were counted as treatment. The duration between the end of previous treatment and the study treatment was <6 months (52%), between 6 and 12 months (20%), and >12 months (28%). No patient had previous treatment with lenalidomide. There were 15 patients in the dose-escalation phase (3 at 3-mg dose level, 6 at 5-mg dose level, and 6 at 7-mg dose level) to determine MTD. Ten additional patients were enrolled at the MTD as an expansion cohort, resulting in a total of 16 patients at the MTD dose level.
Characteristics of study patients
| . | Total = 25, n (%), or median (range) . |
|---|---|
| Study groups | |
| Cohort I (dose escalation) | 15 (60) |
| Cohort II (MTD expansion) | 10 (40) |
| Pomalidomide dose level, mg/d | |
| 3 | 3 (12) |
| 5 | 16 (64) |
| 7 | 6 (24) |
| Age, y | |
| <60 | 7 (28) |
| ≥60 | 18 (72) |
| Sex | |
| Male | 13 (52) |
| Female | 12 (48) |
| Diagnosis | |
| PCNSL | 23 (92) |
| PVRL | 2 (8) |
| Relapsed vs refractory | |
| Relapsed | 20 (80) |
| Refractory | 5 (20) |
| Brain parenchymal involvement | 21 (84) |
| Eye involvement | 7 (28) |
| CSF involvement | 3 (12) |
| No. of prior treatments | 3 (1-11) |
| Time between previous treatment and study treatment, mo | |
| <6 | 13 (52) |
| 6-12 | 5 (20) |
| >12 | 7 (28) |
| Prior autologous stem cell transplant | 5 (20) |
| Prior high-dose methotrexate | 24 (96) |
| Prior rituximab | 22 (88) |
| Prior radiotherapy | 10 (40) |
| Best response to study treatment | |
| CR | 6 (24) |
| CRu | 2 (8) |
| PR | 4 (16) |
| SD | 7 (28) |
| Off-treatment reason, n = 19 | |
| Refused further treatment | 1 (5.3) |
| Adverse event | 1 (5.3) |
| Disease progression | 15 (79) |
| Died on study | 1 (5.3) |
| Other: cognitive decline | 1 (5.3) |
| Cause of death, n = 11 | |
| Aspiration pneumonia | 1 (9.1) |
| Due to PCNSL or PVRL | 10 (90.9) |
| . | Total = 25, n (%), or median (range) . |
|---|---|
| Study groups | |
| Cohort I (dose escalation) | 15 (60) |
| Cohort II (MTD expansion) | 10 (40) |
| Pomalidomide dose level, mg/d | |
| 3 | 3 (12) |
| 5 | 16 (64) |
| 7 | 6 (24) |
| Age, y | |
| <60 | 7 (28) |
| ≥60 | 18 (72) |
| Sex | |
| Male | 13 (52) |
| Female | 12 (48) |
| Diagnosis | |
| PCNSL | 23 (92) |
| PVRL | 2 (8) |
| Relapsed vs refractory | |
| Relapsed | 20 (80) |
| Refractory | 5 (20) |
| Brain parenchymal involvement | 21 (84) |
| Eye involvement | 7 (28) |
| CSF involvement | 3 (12) |
| No. of prior treatments | 3 (1-11) |
| Time between previous treatment and study treatment, mo | |
| <6 | 13 (52) |
| 6-12 | 5 (20) |
| >12 | 7 (28) |
| Prior autologous stem cell transplant | 5 (20) |
| Prior high-dose methotrexate | 24 (96) |
| Prior rituximab | 22 (88) |
| Prior radiotherapy | 10 (40) |
| Best response to study treatment | |
| CR | 6 (24) |
| CRu | 2 (8) |
| PR | 4 (16) |
| SD | 7 (28) |
| Off-treatment reason, n = 19 | |
| Refused further treatment | 1 (5.3) |
| Adverse event | 1 (5.3) |
| Disease progression | 15 (79) |
| Died on study | 1 (5.3) |
| Other: cognitive decline | 1 (5.3) |
| Cause of death, n = 11 | |
| Aspiration pneumonia | 1 (9.1) |
| Due to PCNSL or PVRL | 10 (90.9) |
Maximal tolerated dose
The 5-mg dose level (POM 5 mg orally daily for 21 days every 28 days with DEX orally 40 mg weekly) was determined to be the MTD. No DLTs were seen at the 3-mg dose level. Two patients had DLTs at the 7-mg dose level with 1 experiencing grade 3 dyspnea and another having grade 4 thrombocytopenia. One patient had grade 4 neutropenia at the expanded 5-mg dose level.
Efficacy
As of November 2017, 15 patients have progressed, and 11 patients have died. Median follow-up was 16.5 months (95% CI, 6.9-39.3 months). The 15 patients who progressed received a median of 4 cycles of treatment (range, 1-18 cycles). The 10 patients who did not progress received a median of 4.5 cycles of treatment (range, 1-42 cycles); 6 patients are still receiving therapy. Besides disease progression (n = 15), reasons for going off treatment included: adverse events in cycle 2 (n = 1), cognitive decline after 3 cycles (n = 1), refusal (n = 1), and death (n = 1).
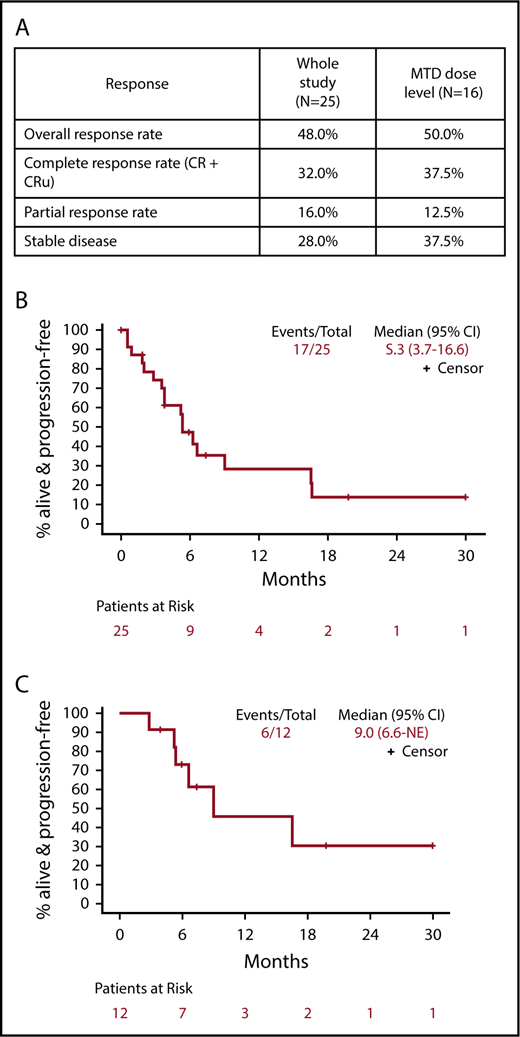
The ORR for all 25 eligible patients was 48% (12 of 25; 95% CI, 27.8%, 68.7%) with 6 CR, 2 CRu, and 4 PR (Figure 1). The ORR for the MTD dose level was 50% (8 of 16; 95% CI, 24.7%, 75.4%) with 5 CR, 1 CRu, and 2 PR. The ORR for the 23 PCNSL patients was 48% (11 of 23; 95% CI, 26.8%, 69.4%) with 6 CR, 2 CRu, and 3 PR. The 12 responders completed a median of 6.5 cycles (range, 2-42 cycles). Median DOR was 4.7 months (95% CI, 4.5-NE; range, 1.1-28.8 months). Median PFS was 5.3, 9, and 5.3 months for the whole study (Figure 1B), responders (Figure 1C) and PCNSL patients, respectively. The outcome events and total number of treatment cycles for each patient are summarized in a swimmer’s plot (Figure 2). Brain MRI of a PCNSL patient who achieved CR is shown in Figure 3. Two patients with PVRL were treated at the MTD dose level. One patient had disease progression after cycle 18. The other patient had PR after cycle 2 and remains on treatment after cycle 7.
Response to study treatment. (A) Response table showing response parameters for the whole study and the MTD dose level (median follow-up, 14.2 months). (B) PFS for all patients. (C) PFS for the subset of patients that experienced a CR, CRu, or PR. CR, complete response; CRu, unconfirmed complete response; NE, not estimable; PR, partial response.
Response to study treatment. (A) Response table showing response parameters for the whole study and the MTD dose level (median follow-up, 14.2 months). (B) PFS for all patients. (C) PFS for the subset of patients that experienced a CR, CRu, or PR. CR, complete response; CRu, unconfirmed complete response; NE, not estimable; PR, partial response.
Swimmer’s plot showing clinical course, outcome events, and number of treatment cycles of each patient in the clinical trial. PD, progressive disease.
Swimmer’s plot showing clinical course, outcome events, and number of treatment cycles of each patient in the clinical trial. PD, progressive disease.
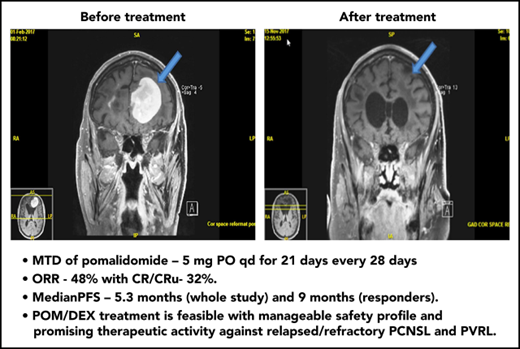
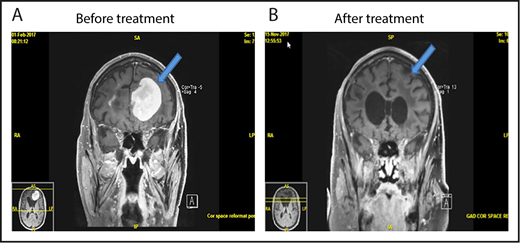
MRI of brain showing CR of relapsed PCNSL to the study treatment. This patient with relapsed PCNSL (A) achieved CR after cycle 8 (B). He achieved PR after cycle 4. This case corresponds to patient 21 in Figure 2.
MRI of brain showing CR of relapsed PCNSL to the study treatment. This patient with relapsed PCNSL (A) achieved CR after cycle 8 (B). He achieved PR after cycle 4. This case corresponds to patient 21 in Figure 2.
One patient had stable disease after 2 cycles and developed radiologic progression on brain MRI after 4 cycles without any significant clinical deterioration. She was taken off the study as per protocol. Brain MRI 1 month later without steroid or any other treatment showed improvement (Figure 4). This appears to be a case of pseudoprogression. She went on to have treatment with single-agent ibrutinib followed by ASCT and is currently in complete remission.
Pseudoprogression in a PCNSL patient treated with pomalidomide. (A-B) The patient had stable disease after cycle 2 and (C) showed radiologic findings suggestive of progression of disease on restaging MRI of brain after cycle 4 without clinical deterioration. She was taken off study as per protocol. (D) Repeat MRI of brain 1 month later without any steroid or any other treatment showed radiologic improvement. This case is the patient 11 on Figure 2.
Pseudoprogression in a PCNSL patient treated with pomalidomide. (A-B) The patient had stable disease after cycle 2 and (C) showed radiologic findings suggestive of progression of disease on restaging MRI of brain after cycle 4 without clinical deterioration. She was taken off study as per protocol. (D) Repeat MRI of brain 1 month later without any steroid or any other treatment showed radiologic improvement. This case is the patient 11 on Figure 2.
Safety profile
Twenty-four of 25 patients were evaluable for adverse events (Table 2). One patient did not return after C1D8 and was lost to follow-up. Grade 3/4 toxicities were seen in 62.5% of patients. Grade 3/4 hematologic toxicities included neutropenia (21%), anemia (8%), and thrombocytopenia (8%). Grade 3/4 nonhematologic toxicities included lung infection (12%), sepsis (4%), fatigue (8%), syncope (4%), dyspnea (4%), hypoxia (4%), respiratory failure (8%), and rash (4%). Febrile neutropenia >7 days was not experienced by any patient. The most common nonhematologic toxicity for all grades was fatigue. One patient died on study after 2 cycles secondary to severe aspiration pneumonia and respiratory failure.
Incidence of adverse events related to treatment over the entire study period for all evaluable patients (n = 24)
| . | Maximum grade per patient per event (at least possibly related), n (%) . | ||||
|---|---|---|---|---|---|
| Grade 1 . | Grade 2 . | Grade 3 . | Grade 4 . | Grade 5 . | |
| Hematologic adverse events | |||||
| Anemia | 10 (42) | 8 (33) | 2 (8) | 0 | 0 |
| Lymphopenia | 0 | 4 (17) | 1 (4) | 1 (4) | 0 |
| Neutropenia | 3 (13) | 12 (50) | 3 (13) | 2 (8) | 0 |
| Thrombocytopenia | 8 (33) | 1 (4) | 1 (4) | 1 (4) | 0 |
| Leukopenia | 5 (21) | 5 (21) | 3 (13) | 2 (8) | 0 |
| Nonhematologic adverse events | |||||
| Endocrine disorders | |||||
| Hypothyroidism | 0 | 1 (4) | 0 | 0 | 0 |
| Eye disorders | |||||
| Eyelid function disorder | 0 | 1 (4) | 0 | 0 | 0 |
| Gastrointestinal disorders | |||||
| Constipation | 0 | 1 (4) | 0 | 0 | 0 |
| Diarrhea | 2 (8) | 0 | 0 | 0 | 0 |
| Dyspepsia | 0 | 1 (4) | 0 | 0 | 0 |
| Mucositis oral | 0 | 1 (4) | 0 | 0 | 0 |
| Nausea | 1 (4) | 0 | 0 | 0 | 0 |
| General and administration site disorders | |||||
| Fatigue | 1 (4) | 7 (29) | 2 (8) | 0 | 0 |
| Infections and infestations | |||||
| Bronchial infection | 0 | 2 (8) | 0 | 0 | 0 |
| Infections and infestations | 0 | 1 (4) | 0 | 0 | 0 |
| Lung infection | 0 | 2 (8) | 2 (8) | 1 (4) | 0 |
| Mucosal infection | 0 | 1 (4) | 0 | 0 | 0 |
| Sepsis | 0 | 0 | 0 | 1 (4) | 0 |
| Upper respiratory infection | 0 | 1 (4) | 0 | 0 | 0 |
| Investigations | |||||
| AST increased | 0 | 1 (4) | 0 | 0 | 0 |
| Blood bilirubin increased | 0 | 1 (4) | 0 | 0 | 0 |
| Metabolism and nutrition disorders | |||||
| Anorexia | 0 | 1 (4) | 0 | 0 | 0 |
| Hyperglycemia | 0 | 1 (4) | 0 | 0 | 0 |
| Hypocalcemia | 0 | 1 (4) | 0 | 0 | 0 |
| Hypophosphatemia | 0 | 1 (4) | 0 | 0 | 0 |
| Nervous system disorders | |||||
| Peripheral sensory neuropathy | 1 (4) | 1 (4) | 0 | 0 | 0 |
| Syncope | 0 | 0 | 1 (4) | 0 | 0 |
| Psychiatric disorders | |||||
| Delirium | 0 | 1 (4) | 0 | 0 | 0 |
| Respiratory, thoracic, mediastinal disorders | |||||
| Dyspnea | 0 | 0 | 1 (4) | 0 | 0 |
| Hypoxia | 0 | 0 | 1 (4) | 0 | 0 |
| Respiratory failure | 0 | 0 | 0 | 2 (8) | 0 |
| Skin and subcutaneous tissue disorders | |||||
| Photosensitivity | 0 | 1 (4) | 0 | 0 | 0 |
| Rash, maculopapular | 0 | 1 (4) | 1 (4) | 0 | 0 |
| Vascular disorders | |||||
| Thromboembolic event | 0 | 2 (8) | 0 | 0 | 0 |
| . | Maximum grade per patient per event (at least possibly related), n (%) . | ||||
|---|---|---|---|---|---|
| Grade 1 . | Grade 2 . | Grade 3 . | Grade 4 . | Grade 5 . | |
| Hematologic adverse events | |||||
| Anemia | 10 (42) | 8 (33) | 2 (8) | 0 | 0 |
| Lymphopenia | 0 | 4 (17) | 1 (4) | 1 (4) | 0 |
| Neutropenia | 3 (13) | 12 (50) | 3 (13) | 2 (8) | 0 |
| Thrombocytopenia | 8 (33) | 1 (4) | 1 (4) | 1 (4) | 0 |
| Leukopenia | 5 (21) | 5 (21) | 3 (13) | 2 (8) | 0 |
| Nonhematologic adverse events | |||||
| Endocrine disorders | |||||
| Hypothyroidism | 0 | 1 (4) | 0 | 0 | 0 |
| Eye disorders | |||||
| Eyelid function disorder | 0 | 1 (4) | 0 | 0 | 0 |
| Gastrointestinal disorders | |||||
| Constipation | 0 | 1 (4) | 0 | 0 | 0 |
| Diarrhea | 2 (8) | 0 | 0 | 0 | 0 |
| Dyspepsia | 0 | 1 (4) | 0 | 0 | 0 |
| Mucositis oral | 0 | 1 (4) | 0 | 0 | 0 |
| Nausea | 1 (4) | 0 | 0 | 0 | 0 |
| General and administration site disorders | |||||
| Fatigue | 1 (4) | 7 (29) | 2 (8) | 0 | 0 |
| Infections and infestations | |||||
| Bronchial infection | 0 | 2 (8) | 0 | 0 | 0 |
| Infections and infestations | 0 | 1 (4) | 0 | 0 | 0 |
| Lung infection | 0 | 2 (8) | 2 (8) | 1 (4) | 0 |
| Mucosal infection | 0 | 1 (4) | 0 | 0 | 0 |
| Sepsis | 0 | 0 | 0 | 1 (4) | 0 |
| Upper respiratory infection | 0 | 1 (4) | 0 | 0 | 0 |
| Investigations | |||||
| AST increased | 0 | 1 (4) | 0 | 0 | 0 |
| Blood bilirubin increased | 0 | 1 (4) | 0 | 0 | 0 |
| Metabolism and nutrition disorders | |||||
| Anorexia | 0 | 1 (4) | 0 | 0 | 0 |
| Hyperglycemia | 0 | 1 (4) | 0 | 0 | 0 |
| Hypocalcemia | 0 | 1 (4) | 0 | 0 | 0 |
| Hypophosphatemia | 0 | 1 (4) | 0 | 0 | 0 |
| Nervous system disorders | |||||
| Peripheral sensory neuropathy | 1 (4) | 1 (4) | 0 | 0 | 0 |
| Syncope | 0 | 0 | 1 (4) | 0 | 0 |
| Psychiatric disorders | |||||
| Delirium | 0 | 1 (4) | 0 | 0 | 0 |
| Respiratory, thoracic, mediastinal disorders | |||||
| Dyspnea | 0 | 0 | 1 (4) | 0 | 0 |
| Hypoxia | 0 | 0 | 1 (4) | 0 | 0 |
| Respiratory failure | 0 | 0 | 0 | 2 (8) | 0 |
| Skin and subcutaneous tissue disorders | |||||
| Photosensitivity | 0 | 1 (4) | 0 | 0 | 0 |
| Rash, maculopapular | 0 | 1 (4) | 1 (4) | 0 | 0 |
| Vascular disorders | |||||
| Thromboembolic event | 0 | 2 (8) | 0 | 0 | 0 |
One patient was lost to follow-up.
Three patients experienced grade 3 or 4 respiratory AEs. The first patient had grade 3 hypoxia, grade 4 respiratory failure, grade 3 lung infection, and grade 4 sepsis during cycle 3 (all probably related). The respiratory AEs were likely related to infection as the patient recovered and has currently completed 34 more cycles of treatment. The second patient had grade 4 respiratory failure and grade 4 lung infection possibly related to treatment in cycle 2. At the same time, the patient was also diagnosed with congestive heart failure secondary to severe aortic stenosis and the spouse was suffering from severe flu. The third patient had grade 3 dyspnea and grade 3 lung infection in cycle 1 possibly related to treatment. He declined to continue treatment after cycle 1.
One patient had grade 3 syncope of unclear etiology in cycle 2 possibly related to treatment. The patient made a complete recovery and completed 7 more cycles.
Incidence of adverse events related to treatment over the entire study period per cohort and dose level is shown in supplemental Table 1 (available on the Blood Web site).
CNS pharmacokinetic analysis
CNS pharmacokinetic analysis was undertaken in 1 patient treated at the 3-mg dose level. The CSF-to-plasma ratio of POM was 19% and 17% based on the drug levels in matched CSF and plasma samples collected 3.5 hours after administration of POM on days 1 and 14 of cycle 1.
Discussion
Our study indicates that POM/DEX combination is feasible with therapeutic activity against heavily pretreated relapsed/refractory PCNSL and PVRL. The MTD of POM in PCNSL (5 mg daily for 21 of 28 days) is higher than in multiple myeloma (4 mg daily) and myelofibrosis (3 mg daily).24,25 We chose to combine low-dose weekly DEX with POM for first 2 cycles because the improvement in survival was seen with the combination of POM and DEX compared with POM alone in our preclinical study.19 DEX has been used in other clinical trials for rapid alleviation of symptoms related to cerebral edema.18 In real-life situations, a significant number of patients with relapsed PCNSL require steroid for control of neurological symptoms. Prednisone is an integral component of standard induction chemoimmunotherapy (RCHOP) for systemic DLBCL. Responses extending beyond 6 months were seen in our study, suggesting single-agent therapeutic activity of POM because DEX was discontinued after 2 cycles (56 days). Significant CSF penetration of POM in 1 study patient correlates well with the excellent CNS penetration observed in our preclinical testing.19
In terms of safety, side effects were comparable to those observed in myeloma clinical trials testing POM/DEX.26 In our trial, we used a higher dose of POM and included weekly DEX only for 2 months compared with the myeloma studies.26 Some differences in safety profile between PCNSL and myeloma patients are noteworthy. The incidence of grade 3/4 hematologic events was more favorable in our study likely because bone marrow is not involved by cancer in our patients whereas myeloma involves bone marrow. A relatively normal marrow may also explain why a higher MTD was achieved in our patients. Additionally, no grade 3 or 4 thromboembolic events were seen in the study patients.
We report the first apparent case of pseudoprogression/tumor flare in PCNSL related to IMiD. Although this was not confirmed by biopsy, the subsequent improvement on neuroimaging without steroid or any other treatment was consistent with this diagnosis. The possibility of pseudoprogression now needs to be considered in PCNSL patients after receiving IMiD. Future studies of immunotherapeutic agents such as IMiDs or checkpoint inhibitors should include option for continuation of treatment in these patients who are asymptomatic for additional 1 to 2 cycles before ascertaining true progression. Tumor flare has been previously reported in chronic lymphocytic leukemia and mantle cell lymphoma associated with lenalidomide.27,28
Another IMiD, LEN has been evaluated in treatment of relapsed/refractory CNSL in 2 studies that have been the subject of preliminary reports. In a phase 1 study, 8 of 13 subjects with CNSL (8 PCNSL and 5 secondary CNSL) responded to LEN monotherapy.17 In the same study, an independent cohort of 12 patients with recurrent CNSL received LEN monotherapy as maintenance after salvage with 5 patients maintaining remissions >2 years.17 In a phase 2 study, patients with relapsed/refractory PCNSL or PVRL were treated with rituximab and LEN for a total of 8 cycles followed by LEN alone for 12 cycles.18 Corticosteroids were allowed in the first cycle for treatment of cerebral edema. Forty-five of 50 patients (41 with relapsed PCNSL and 9 with isolated ocular relapse) were evaluable. ORR was 63% during the induction phase and 39% at the end of induction phase. With the median follow-up of 9 months, median OS and PFS were 15.3 and 8.1 months.18
It is impossible to compare POM to LEN for treatment of PCNSL based on the current data as our study differs significantly from the 2 LEN studies in terms of study treatments, characteristics of patients, and duration of follow-up. POM was shown to have higher CNS penetration than lenalidomide (∼40% vs 11%, respectively) based on pharmacokinetic studies in in vivo models.19,29 However, CNS penetration appears to be about the same for POM and LEN in human patients. It has been recently reported that CNS penetration was 20% or greater for LEN based on a phase 1 study.30 In 1 preclinical study for systemic lymphoma, POM was more potent than LEN in modulating the therapeutic activity of rituximab with the combination of rituximab plus POM showing significant improvement in survival compared with rituximab alone.13 In multiple myeloma, 30% of patients resistant/refractory to LEN-DEX responded to POM-DEX.31
It is an exciting time for PCNSL research as targeted therapeutic agents are being tested in clinical trials. In addition to IMiDs, inhibitors of B-cell receptor signaling and immune-checkpoint pathways are undergoing clinical trials. Ibrutinib has been tested in 2 studies. In 1 study,32,33 20 patients were evaluated with ORR of 75% and median PFS of 7.29 months. In another study,34 ibrutinib monotherapy demonstrated a high disease control rate of 83%, including 56% objective responses. Immune-checkpoint inhibition by anti-PD1 monoclonal antibody has shown excellent therapeutic activity in an immunocompetent preclinical CNS lymphoma model.35 Nivolumab, an anti-PD1 monoclonal antibody, has been reported to have therapeutic activity against CNSL in a case series with 4 CRs and 1 PR in 5 patients (4 relapsed/refractory PCNSL and 1 CNS relapse of primary testicular lymphoma) but it has to be noted that 2 patients also received radiation immediately prior to nivolumab.36 It is currently being evaluated in a phase 2 clinical trial (NCT02857426).
In conclusion, POM/DEX treatment is feasible with an acceptable toxicity profile and therapeutic activity against relapsed/refractory PCNSL and PVRL. Further evaluation of POM in combination with other therapeutic agents is warranted. Biomarkers that can guide therapeutic use of IMiDs also need to be elucidated.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Karen L. Best, Mayo Clinic (Rochester, MN), for administrative support.
The work was supported by a research grant from Celgene.
Authorship
Contribution: H.W.T., P.B.J., K.A.J., and T.E.W. conceived and designed the study; H.W.T., P.B.J., L.M.D., C.B.R., A.M.P.O., D.S., B.O., J.P., K.A.J., C.G., and T.E.W. accrued patients; H.W.T., P.J.A., L.D.P., and P.A.K. collected and compiled data; H.W.T., P.J.A., L.D.P., and T.E.W. analyzed and interpreted data; and H.W.T., P.J.A., and T.E.W. wrote the initial manuscript draft with editing and finalizing by all the authors.
Conflict-of-interest disclosure: L.M.D. has consulting/advisory roles with Roche and Sapience Therapeutics. K.A.J. has consulting/advisory roles with Bristol-Myers Squibb and holds stock in Entegrion Inc. A.M.P.O. has consulting/advisory roles with Bristol-Myers Squibb, Merck, Inovio, Stemline Therapeutics, and Novocure. D.S. has consulting/advisory roles with Genentech. C.G. received research funding from Pharmacyclics. C.B.R. received research funding from Celgene, Novartis, and Millennium (Takeda). H.W.T. received research funding from Celgene, Mundipharma, Bristol-Myers Squibb, TG Therapeutics, and Seattle Genetics. T.E.W. received research funding from Celgene. J.P. has a leadership role in, and holds stock in, LAgen Labs (iPSC derived RPE for laboratory use only). The remaining authors declare no competing financial interests.
Correspondence: Han W. Tun, Hematology/Oncology, Mayo Clinic, 4500 San Pablo Rd, Jacksonville, FL 32224; e-mail: tun.han@mayo.edu.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal