Abstract
Among adult lymphoma survivors, radiation treatment techniques that increase the excess radiation dose to organs at risk (OARs) put patients at risk for increased side effects, especially late toxicities. Minimizing radiation to OARs in adults patients with Hodgkin and non-Hodgkin lymphomas involving the mediastinum is the deciding factor for the choice of treatment modality. Proton therapy may help to reduce the radiation dose to the OARs and reduce toxicities, especially the risks for cardiac morbidity and second cancers. Because proton therapy may have some disadvantages, identifying the patients and the circumstances that may benefit the most from proton therapy is important. We present modern guidelines to identify adult lymphoma patients who may derive the greatest benefit from proton therapy, along with an analysis of the advantages and disadvantages of proton treatment.
Introduction
Reducing treatment-related toxic effects among survivors of Hodgkin and non-Hodgkin lymphomas has been the cornerstone of recent advances in the treatment of hematologic malignancies. Long-term follow-up studies of patients with hematologic malignancies have produced strong and reliable evidence that the benefits from ionizing radiation may be tempered by increased mortality and morbidity, particularly from secondary malignancies and cardiac complications.1 These findings fueled an immense effort to find ways to minimize collateral damage from radiation to adjacent thoracic organs at risk (OARs). Some of those ways included 3-dimensional (3D) conformal radiotherapy and intensity-modulated radiotherapy (IMRT).2-5 Proton therapy, with its unique characteristics of lower entrance dose, high-dose peak, and precipitous fall-off near the end of beam range, presents another opportunity for more conformal dose distribution and better OAR sparing.1,6,7
The guidelines represent a set of consensus recommendations by expert radiation oncologists and physicists from different international academic centers. Best practice recommendations, including potential benefits or harms, were based on an extensive review of the published literature. Every recommendation included was reached by consensus of more than 80% of the authors in the document; the article provides a reasoning for the recommendations, as well as an extensive explanation for recommendations that were a matter of much debate among the authors.
The guidelines presented here include an overview of proton therapy for adult lymphomas involving the mediastinum, an in-depth discussion of best practices along with examples of cases in which protons offer an advantage, and reference to areas in which further research is required. Although proton therapy is useful for treating other anatomic locations, we focus on the mediastinal location, because of the abundant data available. Table 1 lists helpful definitions of technical terms used in the article.
Definitions
| Term . | Definition . |
|---|---|
| Gross tumor volume (GTV) | Volume that contains the visible extent of the tumor that can be identifiable to contour and target using a CT scan |
| Clinical tumor volume (CTV) | Volume that contains a suspected microscopic disease; this could be around the gross tumor or at the original site after achieving complete remission to chemotherapy |
| Planning target volume | Volume that contains GTV and CTV; in addition, it accounts for all uncertainties like internal organ movement and daily patient’s setup errors |
| 3D planning radiation therapy | Forward planning, define anatomy on a CT scan, identify the target, and create a radiation beam arrangement to best encompass the target; generate the radiation dose that the operator judges acceptable |
| IMRT | The operator feeds the planning system the desired dose to the target and organs at risk upfront and what the end result dose distribution should look like, then the software will invert the problem and let the computer through a mathematical iteration search for the best and optimized solution; the software will keep trying until the operator judges the plan as acceptable |
| Linear energy transfer (LET) | Total amount of energy deposited per unit distance in biological materials by ionizing radiation |
| Radiobiological equivalent | Radiation relative biological effectiveness depending on LET, type of radiation particle, total dose, and dose fractionation |
| Proton active scanning | Proton beam delivery using a pencil beam that is precisely steered through the treatment volume, using a magnet in the beam line; the dose is then deposited layer by layer, allowing for a good conformation |
| Proton passive scattering | Spatially uniform dose distribution achieved by shaping the beam through a set of devices at the beam path |
| Intensity modulated proton therapy | Further shapes the active scanning to the distal tumor for irregularly shaped tumors |
| Term . | Definition . |
|---|---|
| Gross tumor volume (GTV) | Volume that contains the visible extent of the tumor that can be identifiable to contour and target using a CT scan |
| Clinical tumor volume (CTV) | Volume that contains a suspected microscopic disease; this could be around the gross tumor or at the original site after achieving complete remission to chemotherapy |
| Planning target volume | Volume that contains GTV and CTV; in addition, it accounts for all uncertainties like internal organ movement and daily patient’s setup errors |
| 3D planning radiation therapy | Forward planning, define anatomy on a CT scan, identify the target, and create a radiation beam arrangement to best encompass the target; generate the radiation dose that the operator judges acceptable |
| IMRT | The operator feeds the planning system the desired dose to the target and organs at risk upfront and what the end result dose distribution should look like, then the software will invert the problem and let the computer through a mathematical iteration search for the best and optimized solution; the software will keep trying until the operator judges the plan as acceptable |
| Linear energy transfer (LET) | Total amount of energy deposited per unit distance in biological materials by ionizing radiation |
| Radiobiological equivalent | Radiation relative biological effectiveness depending on LET, type of radiation particle, total dose, and dose fractionation |
| Proton active scanning | Proton beam delivery using a pencil beam that is precisely steered through the treatment volume, using a magnet in the beam line; the dose is then deposited layer by layer, allowing for a good conformation |
| Proton passive scattering | Spatially uniform dose distribution achieved by shaping the beam through a set of devices at the beam path |
| Intensity modulated proton therapy | Further shapes the active scanning to the distal tumor for irregularly shaped tumors |
Consensus recommendations
Lymphoma patients who can greatly benefit from proton therapy include (1) patients with mediastinal disease that spans below the origin of the left main stem coronary artery and is anterior to, posterior to, or on the left side of the heart; (2) young female patients for whom proton therapy can reduce breast dose and risk for secondary breast cancer; and (3) heavily pretreated patients who are at higher risk for radiation-related toxicity to the bone marrow, heart, and lungs.
When using proton therapy, treating physicians should (1) demonstrate by calculation that it provides greater benefit to the patient compared with optimally planned photon therapy; (2) document the medical necessity of proton therapy, including consideration of issues for long-term survivors and the risks for radiogenic late effects; (3) understand the complexities of lymphoma proton planning, including the need to manage uncertainties, and the evolving nature of the technology with the development of pencil beam scanning, in-room volumetric imaging, and robustness optimization; and (4) use deep inspiration breath hold (DIBH) when it further minimizes doses to the OARs, with an understanding of the increased complexity of using DIBH with proton therapy compared with photon therapy.
Different techniques in delivering proton therapy
Several dosimetric studies comparing proton with photon plans8-21 have been conducted in an effort to establish the benefit and indications for the use of proton therapy in lymphoma, with the vast majority focusing on mediastinal involvement. They showed that there is an individualized and potential benefit in patients with dose reduction to the heart, lungs, and breasts with proton therapy compared with 3D conformal radiotherapy or IMRT.1 Additionally, the lack of marginal relapses that are feared when using a highly conformal therapy like proton treatment has made it an appealing treatment.22-24
Because the magnitude of dosimetric benefit varies significantly depending on the specific case, each case must be considered individually, and the potential benefit from proton use should be weighed against the availability of the treatment (need to travel), out-of-pocket costs if insurance coverage is denied, resources required in terms of medical and physics staff, and the potential uncertainties associated with proton therapy.
Properties of the proton beam
Protons are charged particles that deposit radiation through linear energy transfer (LET), losing little energy when entering tissue and depositing most when slowing down just before stopping, resulting in the Bragg peak effect.25 Because the position of the peak strongly depends on the tissue density along the beam path, proton therapy suffers from range uncertainties that clinicians need to understand to best use this technology.
Proton therapy uncertainties and ways to mitigate them
Some of the challenges associated with proton therapy are the uncertainties arising from the beam-penetration range in tissues and the change in magnitude of biological effects along the proton beam path. Uncertainties in calculating the range of proton penetration in tissue have 2 major potential sources: (1) range errors caused by changes in tissue density from errors in setup (patient positioning), organ motion (eg, respiratory or cardiac related), or anatomic changes (eg, tissue deformation and tumor shrinkage), which collectively cause variations in beam path and range that could cause inconsistencies between the planned and delivered treatments, and (2) the input data used to plan the beam range (ie, conversion of computed tomography [CT] number to proton linear stopping power). The other major source of uncertainties is the change in magnitude of biological effect (described in terms of relative biological effectiveness [RBE]) of protons along the beam path, which affects targets and normal tissues.
Range uncertainties due to density variations
One potentially large source of range uncertainty is introduced by patient anatomic variations. Specifically, the current standard of care uses volumetric images as a basis to create a radiation treatment plan for use days or even weeks later. At the time of treatment, the delivered dose distribution will deviate from the planned distribution due to changes in position or size of the patient and tumor. These changes are not that important with regard to photon therapy, but they can have a profound impact on proton therapy dose distribution in the axial and lateral directions of the beam. Of these, the axial deviations are synonymous with range uncertainties; if the linear stopping power (closely related to the mass density) of any voxel in the patient changes (relative to its value used for treatment planning), then the range of all protons passing through that voxel will also change.26
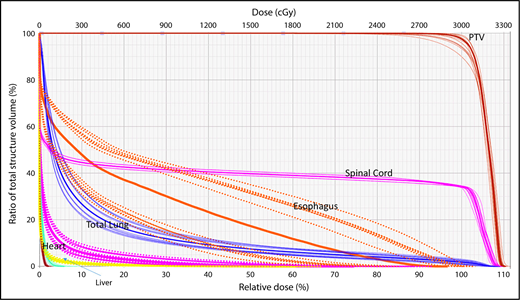
A wide variety of strategies have been developed to deal with dosimetric uncertainties associated with anatomical changes.27,28 Robust optimization tools now allow users to include range uncertainties, setup errors, and physiological motion by incorporating findings from multiple CT scans (such as 4-dimensional [4D] CT) in the plan optimization and dose-calculation processes. The effectiveness of these tools can be evaluated with robustness analysis, which involves calculating dose distributions for various error scenarios. Families of dose-volume histograms (DVHs) of clinical target volumes (CTVs) and OARs for several uncertainties scenarios (ie, shifts along the x, y, and z directions and the associated range uncertainties) are reviewed before treatment delivery to ensure the acceptability of the plan. Typically, the “worst-case” DVH of the band is used for evaluation (Figure 1).29,30
Robustness analysis based on DVHs showing multiple scenarios representative of different uncertainties and their resulting effect on the dose distribution for a specific organ at risk or target.
Robustness analysis based on DVHs showing multiple scenarios representative of different uncertainties and their resulting effect on the dose distribution for a specific organ at risk or target.
In addition to dose perturbations in the periphery of a proton field, interference between the dynamic pencil beam with motion of the target and other surrounding anatomy results in local dose heterogeneities within the target. This interplay between respiration-induced anatomical motion and spot scanning can lead to “hot” and “cold” regions of dose within the target volume. Margins cannot compensate for the interplay effect, but other solutions, including one or more of the following, can help: spot repainting, increasing spot size, gating, or motion-reduction techniques.8 Some 4D robust optimization algorithms are available that can take into account density variations, but the timing information required for interplay calculation is not included in these algorithms.
Discrepancies between calculated and actual proton ranges in tissue
This source of uncertainty results from conversion of Hounsfield units obtained from CT scans to tissue relative proton stopping powers, proton beam reproducibility, uncertainties in water measurement during beam commissioning, and errors in range compensator fabrication.31 Although the magnitude of these types of uncertainties is specific to each proton system (including the delivery, imaging, and calculation components), individual institutions can use “margin recipes” to ensure target coverage. Such recipes are commonly based on the formulation introduced by Moyers et al32 : distal margin = α % of depth + β mm, where α is related to uncertainties in dose calculation, and β is related to errors independent of the dose calculation.
Uncertainties in RBE
In current clinical practice, the RBE of protons compared with high-energy photons is assumed to be a constant value of 1.1. In reality, the RBE is variable and is a complex function of several quantities, including LET from the protons to the local medium, dose per fraction, tissue and cell type, oxygenation, biological or clinical end points, and other factors.33 Estimates of the effects of these factors on RBE are available from in vitro studies, but in vivo data in humans are still lacking.26 Nevertheless, the available data suggest that proton RBE can increase at the Bragg peak, perhaps more rapidly and nonlinearly at the distal edge. Because LET increases as the proton energy decreases with depth, leading to elevated LET and associated RBE values at the end of proton range, this raises concerns when the distal end of a treatment field is directed toward sensitive OARs. There is controversy about the extent of the region over which the RBE deviates from 1.1, and it might be >1.1. It is accepted that the higher the energy and range straggling, the more range mixing we see, explaining the variability in the dose at the end of the Bragg peak.34,35 On the other hand, this deviation is believed by others investigators to be small (a few millimeters around the Bragg peak). Because RBE-based or even LET-based planning is not commercially available, empirical methods to spare structures from increases in RBE are used, including reduced physical dose to the OAR at the distal edge of the beam, use of multiple fields to spatially dilute the effect, range feathering of the same field, irradiating past the sensitive structures, or allowing respiratory/cardiac motion to feather out the dose for moving OARs.
Clinical presentations and applications of proton beam therapy
Patients presenting with mediastinal disease, especially young women, pose a treatment challenge in which proton therapy can be very useful. This section addresses the general goals and challenges in specific clinical situations.
Regardless of the type of radiation to be used, the basic rules in clinically evaluating a radiation treatment plan are achievement of the following: (1) cover the planning target volume (PTV) with ≥95% of the dose; (2) minimize the dose to heart substructures, including coronary arteries, left ventricle, and valves,36 as much as reasonably possible; (3) minimize radiation to the breasts, with high priority assigned to avoiding irradiation of large volumes of the breasts in young women (<35 years old)37 ; and (4) minimize dose to the lungs, including V5, V20, V30, and mean dose.38 Table 2 summarizes these points and can be useful as a guide for fulfilling these requirements.38-41
Guide to acceptable dose, volume, and field considerations
| Structures . | Ideal . | Optimize technique . | Optimize field (consider field reduction) . | Unacceptable . | Avoid maximum dose landing in . |
|---|---|---|---|---|---|
| Heart: left ventricle, coronary arteries, valves39-41 | Mean < 5 Gy | Mean, 5-15 Gy | Mean > 15 Gy | Mean > 30 Gy | Coronary vessels |
| Breast (age dependent)* | Mean < 4 Gy | Mean, 4-15 Gy | Mean > 15 Gy | Mean > 30 Gy | Glandular tissue |
| Lung38 | V5 < 55% | V5, 55-60% | — | V5 > 60% | |
| V20 < 30% | Mean, 10-13.5 Gy | Mean > 13.5 Gy | |||
| Mean < 10 Gy | |||||
| Thyroid62 | V25 < 62.5% | V25 < 62.5% | Whole thyroid |
| Structures . | Ideal . | Optimize technique . | Optimize field (consider field reduction) . | Unacceptable . | Avoid maximum dose landing in . |
|---|---|---|---|---|---|
| Heart: left ventricle, coronary arteries, valves39-41 | Mean < 5 Gy | Mean, 5-15 Gy | Mean > 15 Gy | Mean > 30 Gy | Coronary vessels |
| Breast (age dependent)* | Mean < 4 Gy | Mean, 4-15 Gy | Mean > 15 Gy | Mean > 30 Gy | Glandular tissue |
| Lung38 | V5 < 55% | V5, 55-60% | — | V5 > 60% | |
| V20 < 30% | Mean, 10-13.5 Gy | Mean > 13.5 Gy | |||
| Mean < 10 Gy | |||||
| Thyroid62 | V25 < 62.5% | V25 < 62.5% | Whole thyroid |
The importance of adhering to breast-dose restrictions is inversely related to patient age.
It should be kept in mind that current clinical treatment-planning systems lack a few key capabilities needed to create, optimize, or evaluate candidate treatment plans. Specifically, stray radiation exposure is typically underestimated beyond a few centimeters outside the treatment field, precluding routine risk projections based on radiation exposure.42 Similarly, their risk-modeling capabilities are limited, if they are present at all.43,44
Consideration of heart dose
The relation of disease to OARs determines the situations in which proton therapy is most beneficial. Specifically, with regard to disease in the heart and its substructures, published findings suggest that mean heart doses > 15 Gy are associated with increased risk for cardiac morbidity.39 Mean heart dose is a rough estimate used quite often45 ; mechanistically, however, ischemic heart disease likely depends on mean heart dose, as well as on the maximum dose to the heart substructures,46,47 especially with regard to atherosclerosis of the coronary vessels. Consequently, one must remain mindful of the high-dose volume, especially with regard to potential RBE uncertainty for beams that end within a few millimeters of the coronary vessels.21
For the purposes of this article, proton and photon plans in all of the illustrations shown use DIBH, inclined board for females to further avoid the breasts and, for IMRT, a butterfly technique.48-51
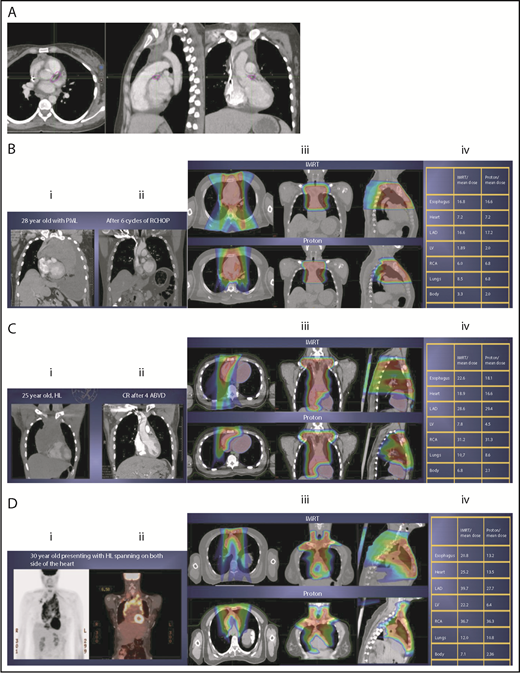
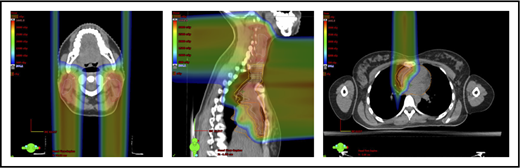
Scenario 1: mediastinal target is completely above the heart with no axillary involvement
The take-off of the left main stem coronary artery is used to define whether the target is above or below the heart (Figure 2A). In this example (Figure 2B), the exposure of the heart is quite comparable, regardless of which technique is used. Differences in DVHs, as well as the mean dose to other structures, are similar to the proton and IMRT plans.
Three scenarios of the relation between mediastinal disease and the heart. (A) Showing how to use the takeoff of the left main stem coronary artery (outlined in pink) to determine the upper and lower mediastinal locations. (B) Scenario 1: coronal CT images of a 28-year-old man with primary mediastinal lymphoma before (i) and after (ii) 6 cycles of rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone (RCHOP) chemotherapy presenting for consolidation with radiation. (Biii) Axial, coronal, and sagittal views of an IMRT plan (upper panels) and a proton plan (lower panels). (Biv) Corresponding mean doses to critical structures using IMRT vs protons. (C) Scenario 2: coronal CT images of a 25-year-old man with Hodgkin lymphoma before (i) and after (ii) 4 cycles of doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) chemotherapy presenting for consolidation with radiation. (Ciii) Axial, coronal, and sagittal views of an IMRT plan (upper panels) and a proton plan (lower panels). (Civ) Corresponding mean doses to critical structures using IMRT vs protons. (D) Scenario 3: coronal CT images of a 30-year-old man with recurrent Hodgkin lymphoma as shown in the coronal images of a PET/CT scan (i-ii) presenting for definitive radiation. (Diii) Axial, coronal, and sagittal views of an IMRT plan (upper panels) and a proton plan (lower panels). (Div) Corresponding mean doses to critical structures using IMRT vs protons.
Three scenarios of the relation between mediastinal disease and the heart. (A) Showing how to use the takeoff of the left main stem coronary artery (outlined in pink) to determine the upper and lower mediastinal locations. (B) Scenario 1: coronal CT images of a 28-year-old man with primary mediastinal lymphoma before (i) and after (ii) 6 cycles of rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone (RCHOP) chemotherapy presenting for consolidation with radiation. (Biii) Axial, coronal, and sagittal views of an IMRT plan (upper panels) and a proton plan (lower panels). (Biv) Corresponding mean doses to critical structures using IMRT vs protons. (C) Scenario 2: coronal CT images of a 25-year-old man with Hodgkin lymphoma before (i) and after (ii) 4 cycles of doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) chemotherapy presenting for consolidation with radiation. (Ciii) Axial, coronal, and sagittal views of an IMRT plan (upper panels) and a proton plan (lower panels). (Civ) Corresponding mean doses to critical structures using IMRT vs protons. (D) Scenario 3: coronal CT images of a 30-year-old man with recurrent Hodgkin lymphoma as shown in the coronal images of a PET/CT scan (i-ii) presenting for definitive radiation. (Diii) Axial, coronal, and sagittal views of an IMRT plan (upper panels) and a proton plan (lower panels). (Div) Corresponding mean doses to critical structures using IMRT vs protons.
Scenario 2: target spans the right side of the heart
For the targets on the right side of the heart, IMRT often provide comparable doses to the heart and other structures as proton therapy. Notably, however, even when proton plans give a dosimetric advantage over IMRT plans, the magnitude of advantage could vary between cases, and it needs to be judged individually. For example, for the patient shown in Figure 2C, the mean doses to critical structures from proton therapy are sufficiently lower than those from IMRT to suggest that proton therapy may be preferred because of the large volume spanning the right side of the heart. This example illustrates the need for careful consideration of individual cases before deciding on treatment.
Scenario 3: target is on both sides of the heart
Disease that spans significantly in front of the heart anteriorly, posteriorly, or to the left side poses a particular challenge for IMRT; therefore, proton therapy may be the superior plan. Notably, toxicity to the heart and lungs is not eliminated by using proton therapy; rather, the dose to the heart may be lower than IMRT. Thus, for cases like in Figure 2D, clinicians should carefully weigh the therapeutic benefit against the long-term risks for radiation-induced treatment toxicities before deciding on which technique should be used. Indeed, in this case, proton therapy can significantly avoid the heart and should be sought in an attempt to reduce the dose to the heart substructures.
Consideration for axillary involvement
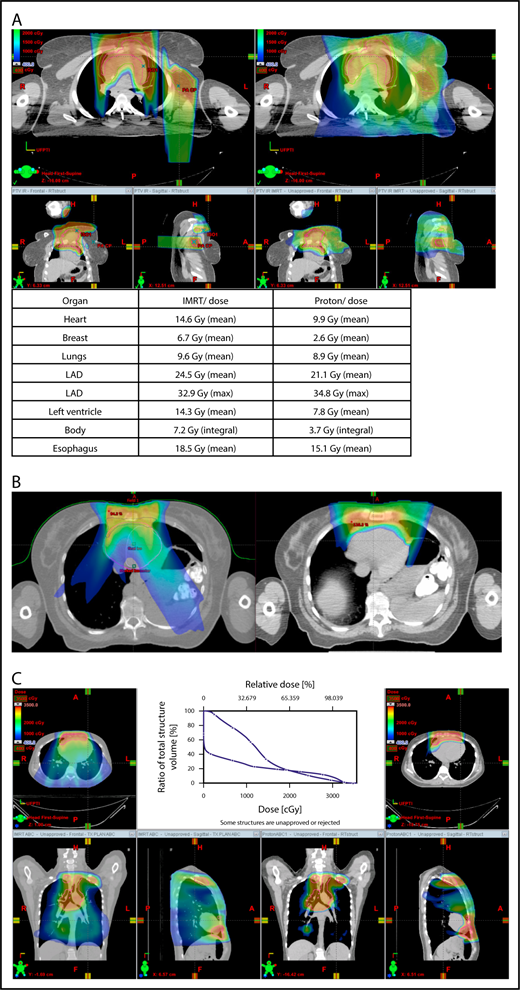
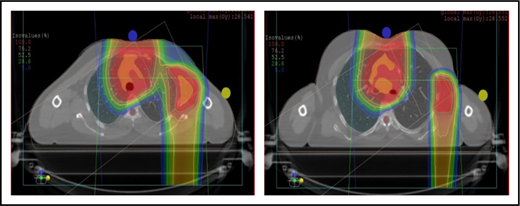
Proton therapy can significantly reduce the radiation dose to the breasts by specifically using fields that enter posteriorly and stop short of exiting through the breast. Alternatively, if protons cannot be used, other methods of displacing the breast can be used instead, such as using an inclined board or physically moving the breast out the beam path. Figure 3A shows how using proton therapy can spare the breast in a case with axillary involvement.
Axillary involvement at presentation. (A) Axial, coronal, and sagittal views of a proton plan (left) and an IMRT plan (right) for a patient presenting with axillary involvement. Use of proton therapy in this case spares the left breast. (B) Regardless of which treatment modality is chosen, IMRT (left panel) and proton (right panel), limiting the volume exposed to radiation should include attention to avoiding a low-dose bath. (C) Limiting lung dose. If avoiding the lung is the primary objective in a given patient, especially if the patient has received pulmonary toxic chemotherapy (eg, any combination of bleomycin, busulfan, gemcitabine, brentuximab, etc.), proton therapy may better spare the lungs by reducing the low-dose bath seen with photons.
Axillary involvement at presentation. (A) Axial, coronal, and sagittal views of a proton plan (left) and an IMRT plan (right) for a patient presenting with axillary involvement. Use of proton therapy in this case spares the left breast. (B) Regardless of which treatment modality is chosen, IMRT (left panel) and proton (right panel), limiting the volume exposed to radiation should include attention to avoiding a low-dose bath. (C) Limiting lung dose. If avoiding the lung is the primary objective in a given patient, especially if the patient has received pulmonary toxic chemotherapy (eg, any combination of bleomycin, busulfan, gemcitabine, brentuximab, etc.), proton therapy may better spare the lungs by reducing the low-dose bath seen with photons.
Consideration of breast dose
The long-term risk for developing breast cancer is related to exposure to high and low radiation doses.37,52 Especially when treating young patients, efforts must be directed to keeping the radiation dose to the breast tissue as low as possible. In most cases involving mediastinal disease, the axilla is uninvolved, and the radiation dose to the breast with IMRT or proton therapy will be small. Although IMRT leads to greater volumes of breast tissue receiving low-dose radiation, in some cases proton therapy can lead to greater volumes of breast tissue receiving high-dose radiation. It is unknown whether it is more important to minimize the low-dose portion vs the high-dose portion; subsequently, mean breast dose continues to be the best factor for evaluating different plans. However, at this time, a high dose to a small volume is generally favored over a low dose to a large volume based on findings suggesting that doses as low 4 Gy37 can be associated with a risk for long-term second malignancies, especially in patients younger than 24 years of age. Although the dose calculation by Travis et al37 refers to a point dose (not a mean), we still recommend erring on the side of caution in an attempt to keep the mean breast dose ≤ 4 Gy.
When hilar disease needs to be covered, the dose to the breasts can increase, and avoiding the breasts becomes difficult with either modality (proton or photon). The choice of treatment modality for such cases must consider the doses received by other critical structures, such as the heart and lungs, especially in previously and heavily treated patients. However, in considering how to best limit the volumes exposed to radiation, avoiding “low-dose baths” is equally important (ie, irradiation of large volumes with low doses), regardless of which modality is used (Figure 3B).
Consideration of lung dose
With the advent of CT-based planning for mediastinal lymphoma, the dose to the lungs can now be correlated with the risk for pneumonitis. Restrictions on lung dose are encouraged to be V5 < 55%, mean lung dose < 13.5 Gy, and V30 < 20%. These values are more attainable with the use of DIBH.38 Although a mean lung dose of 13.5 Gy has been associated with a lower risk for pneumonitis, it is advisable to aim for a lower dose, which is quite often attainable when strict constraints are used. For example, when IMRT is used, it is important to limit the beams to some variation of anteroposterior beams, avoiding lateral beams. If avoiding the lung is the primary objective for a given patient, especially if that patient has received pulmonary-toxic chemotherapy (eg, bleomycin, busulfan, gemcitabine, brentuximab), proton therapy may better spare the lungs by reducing the low-dose bath seen with photons (Figure 3C).
Current techniques for delivering proton beam therapy
Passive scattering proton therapy
Passive scattering proton therapy (PSPT) is the least complex proton-delivery technique; the beam is broadened laterally by placing scattering material in the beam path. Range modulator wheels or ridge filters are used to create the spread out Bragg peak, a region of flat dose distribution meant to cover the target laterally and longitudinally. The entire spread out Bragg peak is delivered nearly instantaneously (<0.1 second), so there is no interplay between the proton beam and physiological motion during delivery, making PSPT the most robust form of proton delivery.53
Challenges in planning PSPT
Conforming to target heterogeneity (eg, contiguous subvolumes of varying size, shape, and depth) is challenging; PSPT beams can only conform to 1 side of the target, either proximally or distally. Also, large targets often necessitate matching fields because of the limited maximum size of the scattered beams. Dose inhomogeneity produced by matching beams is alleviated by match-line changes, which increase the treatment complexity.
Motion management in PSPT requires the use of accurate motion analysis, adequate margin determination, and motion-reduction techniques. For free-breathing treatments, 4D CT is used to evaluate target displacement perpendicularly to the beam and to expand the width of the beam. Along the beam, motion is evaluated by the maximum difference in water-equivalent depth from the patient surface to the distal edge of the target for each beam, based on the density changes of the 4D CT, and is accounted for by compensator smearing. DIBH reduces the target motion and accounts for the tissue interfaces.
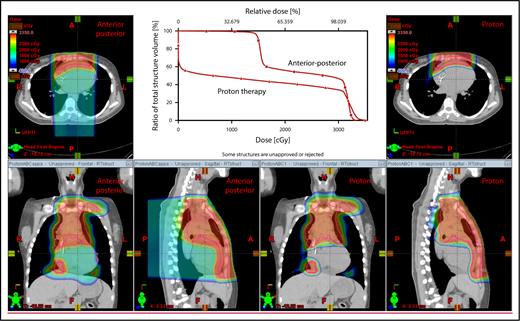
The plan target is the CTV; when the treatment area is affected by breathing motion, the plan target is the internal target volume (ITV), which encompasses the motion of the CTV depicted on 4D CT scans. Alternately, if treatment is to be given with DIBH, an ITV can be derived from the positions of the CTV reproduced from multiple DIBH scans. For lateral beam shaping, margin expansion to form the PTV accounts for setup uncertainty, and interfractional variability in anatomy is applied to the CTV/ITV. Patient-specific beam collimators conform the dose laterally to the PTV, with a margin for penumbra. Depending on the target depth and adjacent tissue, these margins vary from 5 to 10 mm. Range compensators are designed for each beam to conform the dose distally to the CTV/ITV. Range compensator smearing within a radius appropriate for setup tolerances and tissue motion is applied to account for proton range changes caused by density changes in the beam path. In addition, along each beam, distal and proximal margins are set to the CTV/ITV to compensate for proton range uncertainties, as described under “Range uncertainties due to density variations.” Multiple beams (compared with 1 beam) can be used to increase dose conformality and reduce dose uncertainties by spreading the beams in various directions. The preference is to use anterior or posterior fields, rather than both, because of the need to avoid unnecessary beam through the heart (Figure 4).
An example of an approach using 2 anterior fields with proton therapy, which can better spare the heart and esophagus (right) compared with an anterior/posterior approach (left).
An example of an approach using 2 anterior fields with proton therapy, which can better spare the heart and esophagus (right) compared with an anterior/posterior approach (left).
Pencil beam scanning proton therapy
Compared with PSPT, active-mode pencil beam scanning proton therapy (PBSPT) offers the potential for better conformality and OAR sparing.54 Because treatment involves delivery of individually controlled spots, inhomogeneous doses can be created deliberately. PBSPT has the potential to deliver lower doses to OARs by its ability to conform the dose proximal to the target, by its improved conformality distal to the target, and by reducing the formation of secondary neutrons produced in the treatment apparatus.55,56 However, lateral penumbral widths of some uncollimated scanned beams may be larger than those achieved with collimated passively scattered proton beams. When each scanned treatment field is used to treat the entirety of the target, the techniques is termed “single-field uniform dose.” Conformality can be improved further with the use of an intensity-modulated approach (ie, intensity-modulated proton therapy), in which each beam is used to treat only part of the target volume; this technique is analogous to step-and-shoot IMRT. Compared with PSPT beams, PBSPT fields are larger, so larger targets can be treated without matched fields. However, when matches are required, PBSPT allows “gradient matching,”57,58 which obviates the need for match-line changes. Gradient matching can be used for opposing beams targeting different regions of complicated target volumes, as described below.
Challenges of PBSPT
Compared with PSPT, PBSPT is more sensitive to density changes in the beam path; thus, motion management is of prime importance. Whereas interfractional changes and tumor shrinkage can require adaptive planning when PSPT, the increased sensitivity to beam path variations makes this even more important when PBSPT is used.
Motion management
Use of PBSPT requires close attention to evaluation of intrafractional movement, which is usually connected with the breathing cycle. A free-breathing technique can be safely used when specific conditions are fulfilled.54 Use of PBSPT with free breathing requires 4D CT scanning to evaluate the motion of internal structures during the breathing cycle. Robustness is improved by using a repainting strategy, which reduces the potential for the interplay effect. For patients with target motion than exceeds 5 mm over the breathing cycles, alternate strategies can be used, such as DIBH, respiratory gating, abdominal compression, or repositioning.
With regard to patient positioning, it is essential to ensure reproducibility between fractions. Compared with PSPT, PBSPT requires even more stringent reproducibility because of its greater sensitivity to density changes in the beam path.
With regard to field arrangements, the PBSPT technique for an anterior upper mediastinal and lower neck target usually requires 1 (repainted) or 2 anterior fields. For more complex target volumes, targets can be divided into 2 or more parts (eg, neck CTV, mediastinal CTV, and axillary CTV), and a multifield plan can be used (Figure 5). For cases that involve lower (posterior) and upper (anterior) mediastinal targets, a combination of posterior and anterior fields can maximally spare the heart and lungs (Figure 6). For upper neck targets, lateral or posterior fields can avoid the oral cavity/salivary structures. For axillary targets, a posterior field can help to spare breast tissue. Although these various field arrangements can be used in PSPT, gradient matching is simpler in PBSPT when the fields overlap or oppose, obviating the need for feathering.
Plans for PBSPT with a single-field uniform dose and a gradient match, with anterior and posterior beams used to treat disease that involves the bilateral upper neck and the mediastinum (disease anterior to the right heart).
Plans for PBSPT with a single-field uniform dose and a gradient match, with anterior and posterior beams used to treat disease that involves the bilateral upper neck and the mediastinum (disease anterior to the right heart).
Scans for a young woman in whom the target included mediastinal, left parasternal, and left axillary regions. One anterior field was used for the mediastinum, and a separate posterior field was used for the axillary region.
Scans for a young woman in whom the target included mediastinal, left parasternal, and left axillary regions. One anterior field was used for the mediastinum, and a separate posterior field was used for the axillary region.
Verification of patient position and dose distribution in PSPT or PBSPT
Proton-delivery systems are equipped with orthogonal-kV imaging systems used for daily patient setup. Newer systems have cone beam CT capabilities. Although PSPT and PBSPT plans can be made to account for setup errors and breathing motion, dose verification is recommended. The previously described margins do not protect against unpredictable changes that occur during the course of treatment, such as lymphoma progression, pleural effusion, pneumonia, or weight loss. If image-guided treatment setup is not based on 3D imaging, repeat verification CT imaging and dose recalculation are recommended to verify the accuracy of the treatment. Adaptive planning is advised (eg, in refractory-relapsed disease) when the target coverage is not maintained or the OAR dose constraints are violated during the course of treatment
Treatment planning
Target delineation and margins
Target definition follows the modern definition of involved-sites radiation previously published by the International Lymphoma Radiation Oncology Group (ILROG).3 The CTV/ITV and normal structures are delineated in the same way for proton therapy as for photon therapy. Because of uncertainties in proton range, the validity of the concept of PTV is questionable. In the current practice of PSPT, margins to the CTV are assigned per beam, and lateral margins are the same as for photons; however, distal and proximal margins depend on the depth of the distal and proximal edges of the target. This results in what is called the “beam-specific PTV.” This practice is not applicable to intensity-modulated proton therapy, in which robust optimization is the recommended solution.
Motion management
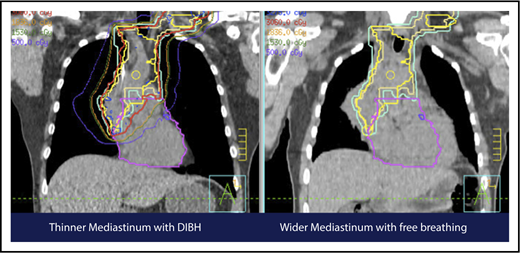
A 4D CT scan can help to establish the extent of respiration-induced anatomic motion and the appropriate ITV expansion. However, this introduces an additional uncertainty; to mitigate the latter, the radiation volume has to be increased. Thus, use of DIBH is ideal to reduce the motion of the mediastinum and its respiration-dependent change in shape (thinner during inspiration, wider during free breathing) (Figure 7).59 It is accomplished by acquisition of the anatomical data in a fixed respiratory phase. DIBH can have the additional benefit of displacing the heart inferiorly, as well as expanding the lungs away from the target, potentially reducing the dose received by these OARs.
Use of DIBH can help to manage some of the uncertainties associated with the use of proton therapy. Compared with free breathing (right panel), DIBH expands the lungs, moves the heart downward, and causes the mediastinum to become thinner (left panel).
Use of DIBH can help to manage some of the uncertainties associated with the use of proton therapy. Compared with free breathing (right panel), DIBH expands the lungs, moves the heart downward, and causes the mediastinum to become thinner (left panel).
DIBH can be accomplished in several ways, such as by actively blocking airflow, voluntary breath holding, or synchronization (respiratory gating). Each of these methods has advantages and limitations when used with proton therapy, particularly PBSPT, which tends to have longer “beam-on” time than PSPT. Interfractional reproducibility of the diaphragm position during the course of the radiation fraction should be evaluated.60,61 Regardless of the breath-hold technique used, on-board imaging can be useful to verify and check the daily reproducibility of the lung volume against that in the treatment-planning CT.
Summary and future directions
The use of proton therapy for lymphomas engaging the mediastinum is promising, and treatment techniques continue to evolve. The dosimetric advantage of reducing the dose to OARs in certain disease distributions can be significant and highly desirable. However, the limited availability of proton therapy calls for case selection based on a clear understanding of which cases will derive the most benefit from protons therapy compared with advanced photon techniques.
Each proton therapy center has different capacities and techniques, and no unified technique for proton radiotherapy for patients with lymphoma has been widely adopted. Ultimately, key tools and concepts should be applied, and a minimum requirement for competency should be acquired for those who intend to use proton therapy.
The online version of this article contains a data supplement.
Authorship
Contribution: All authors contributed to the creation of the manuscript; differences in opinion were resolved by majority consensus.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
A list of the steering committee members of the International Lymphoma Radiation Oncology Group appears in the online appendix.
Correspondence: Bouthaina Shbib Dabaja, Department of Radiation Oncology, University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Houston, TX 77030; e-mail: bdabaja@mdanderson.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal