Key Points
HS chains decorating syndecan-1 promote autocrine and paracrine Wnt signaling in MM.
Loss of HS inhibits MM cell growth by attenuating Wnt signaling.
Abstract
Multiple myeloma (MM) is characterized by the expansion of malignant plasma cells in the bone marrow (BM). Most MMs display aberrant Wnt/β-catenin signaling, which drives proliferation; however, they lack oncogenic Wnt pathway mutations, suggesting activation by autocrine Wnt ligands and/or paracrine Wnts from the BM microenvironment. Expression of the heparan sulfate (HS) proteoglycan syndecan-1 is a hallmark of MM. Syndecan-1 is a critical player in the complex reciprocal interaction between MM cells and their BM niche, mediating growth factor/cytokine binding and signaling by its HS chains. Here, by means of CRISPR/Cas9-mediated knockout and doxycycline-inducible short hairpin RNA–mediated knockdown of EXT1, a critical enzyme for HS polymerization, we demonstrate that the HS chains decorating syndecan-1 mediate aberrant Wnt pathway activation in MM. HS-deficient MM cells exhibited strongly decreased autocrine Wnt/β-catenin pathway activity and reduced Wnt pathway–dependent proliferation. In addition, we demonstrate that Wnts bind to the HS side chains of syndecan-1 and that this binding contributes to paracrine Wnt pathway activation through the Wnt receptor Frizzled (Fzd). Furthermore, in an HS-dependent fashion, syndecan-1 also binds osteoblast-produced R-spondin, which represses Fzd degradation by activation of LGR4, an R-spondin receptor aberrantly expressed on MM cells. Costimulation with R-spondin and its binding to HS chains decorating syndecan-1 are indispensable for optimal stimulation of Wnt signaling in MM. Taken together, our results identify syndecan-1 as a crucial component of the Wnt signalosome in MM cells, binding Wnts and R-spondins to promote aberrant Wnt/β-catenin signaling and cell growth, and suggest HS and its biosynthetic enzymes as potential targets in the treatment of MM.
Introduction
Multiple myeloma (MM) is, in most patients, an incurable hematologic neoplasm characterized by the accumulation of malignant plasma cells in the bone marrow (BM). Notwithstanding the diversity of underlying structural and numerical genomic abnormalities,1-3 virtually all MMs are highly dependent on a protective BM microenvironment for growth and survival.4 The membrane-bound heparan sulfate (HS) proteoglycan (HSPG) syndecan-1 has been identified as a critical player in this complex reciprocal interaction between MM cells and their BM niche.5,6 Consequently, targeting syndecan-1 could be employed as a strategy to disconnect MM cells from their BM niche.
Expression of the HSPG syndecan-1 is characteristic of plasma cells and their malignant MM counterparts7 and plays a crucial role in MM cell growth and survival.6,8,9 HSPGs are a class of extracellular matrix or cell membrane–bound glycoproteins designed to specifically bind and regulate the bioactivity of soluble protein ligands10,11 and play an essential role in the spatial control of extracellular signals regulating cell growth, survival, and differentiation, including tumorigenesis.11-13 They consist of a protein core with covalently attached polysaccharide HS chains. These HS chains undergo a series of processing reactions involving N-deacetylation/N-sulfation, epimerization, and O-sulfation.10,11 This endows HS chains with highly modified domains, which provide specific docking sites for a large number of bioactive molecules, such as growth factors and cytokines, and serve a variety of functions ranging from immobilization and concentration to distinct modulation of signaling.13-15 On MM cells, syndecan-1 is the principal, if not sole, HSPG.8,9 The HS chains decorating syndecan-1 mediate interaction between the tumor cells and growth factors from the BM microenvironment,6 including hepatocyte growth factor, epidermal growth factor family members, and APRIL.5,8,16 In addition, syndecan-1 may also regulate Wnt signaling,17 which has been implicated in driving MM proliferation and is associated with disease progression, dissemination, and drug resistance.18-22
Wnts are lipid-modified glycoproteins that function as typical niche factors because they are relatively unstable and insoluble as a result of their hydrophobic nature, which constrains long-range signaling.23 Binding of a Wnt ligand to its receptor Frizzled (Fzd) initiates a signaling cascade that ultimately results in stabilization and nuclear translocation of the Wnt effector β-catenin. In cooperation with TCF/LEF family transcription factors, this orchestrates a transcriptional program24,25 including c-Myc and cyclin D1, which play crucial roles in the pathogenesis of MM.26-28 The turnover of the Wnt receptor Fzd is critically regulated by LGR family receptors and their cognate R-spondin ligands. R-spondin/LGR binding alleviates the inhibitory effect of 2 homologous membrane-bound E3 ubiquitin ligases, ZNRF3 and RNF43, which promote Fzd internalization, thereby controlling the amplitude of Wnt signaling.29-32
Aberrant Wnt signaling in cancer typically results from mutations in APC, β-catenin (CTNNB1), or AXIN that drive constitutive, ligand-independent pathway activation.32 However, MMs displaying active Wnt signaling do not harbor these classic mutations. Instead, oncogenic Wnt pathway activity in MM involves autocrine and/or paracrine Wnt ligands19,21,32,33 and is potentiated by aberrant expression and turnover of Wnt (co)receptors and transcriptional activators, as well as by loss of negative Wnt pathway regulators. These activating events include aberrant expression of the R-spondin receptor LGR434 and of the transcriptional coactivator BCL9,35,36 epigenetic silencing of the feedback inhibitors secreted Fzd-related proteins and DKK1,21,33 and mutational inactivation of CYLD, a deubiquinating enzyme that negatively regulates Wnt.37 Given the ligand dependence of Wnt pathway activation in MM, targeting of Wnt receptors and coreceptors, disconnecting the MM cells from their growth-promoting microenvironment, is a potentially promising therapeutic strategy for MM. This prompted us to explore the specific contribution of syndecan-1 HS to Wnt signaling activation in MM.
Materials and methods
Cell culture and treatment
The human MM cell lines (HMCLs) LME-1, LP1, OPM-2, RPMI 8226 (RPMI), XG-1, RIES, and XG-3 were cultured in Iscove modified Dulbecco medium (Invitrogen Life Technologies) containing 10% fetal bovine serum (Invitrogen Life Technologies), 100 U/mL of penicillin (Sigma Aldrich), and 100 µg/mL of streptomycin (Sigma Aldrich). For XG-1, XG-3, LME-1, and RIES, medium was supplemented with 500 pg/mL of interleukin-6 (Prospec). Primary MM cell samples were derived from patients diagnosed at the Academic Medical Center, Amsterdam, The Netherlands. This study was conducted and approved by the AMC Medical Committee on Human Experimentation. Informed consent was obtained in accordance with the Declaration of Helsinki. For signal transduction experiments, recombinant hWnt3a (R&D Systems) was used at 100 ng/mL and recombinant hR-spondin1 at 50 ng/mL (R&D Systems) unless otherwise stated. Wnt inhibitors used were IWP-2 (2.5 µM; Stemgent) and LGK974 (2.5 µM; StemRD). For heparitinase treatment, 300 000 MM cells were treated with 5 mU of heparitinase (Amsbio) for 60 minutes at 37°C.
Cloning, transfection, and transduction
The dominant negative TCF4 (dnTCF4) constructs were obtained from Addgene (52961); S33Y β-catenin was generated by inserting S33Y β-catenin into the HPAI site of pHIV-H2BmRFP (Addgene 18982). pTRIPZ-shEXT1 was custom constructed. In short, a 97-mer template was inserted into the XhoI/EcoRI site of the pTRIPZ vector (Thermo Scientific, Waltham, MA). The targeting sequence was GCACATATCACGTAACAGT. pLentiCrispr-sgEXT1 was constructed by inserting sgEXT1 (GACCCAAGCCTGCGACCACG, chr8:118849372-118849391; GTCTGGTTCCTCGTGGTCGC, chr8:118849383-118849402) into pLentiCrispr (Addgene 52961) as previously described.38 TOP-GFP.mCh and FOP-GFP.mCh (Addgene 35491 and 35492) were kind gifts by David Horst.39 c-Myc constructs were obtained from Addgene (18770). Mutant R-spondin–∆thrombospondin protein (TSP) was constructed by inserting R-spondin1 lacking TSP domain and C-terminal basic amino acid–rich domain into pcDNA3. The control R-spondin-∆AA was constructed by inserting R-spondin1 lacking C-terminal basic amino acid–rich domain into pcDNA3. Lentiviral transduction was performed as described before.34 For retroviral transduction, constructs were transfected into Phoenix-GALV cells with genius DNA transfection reagent (Westburg). MM cells were spinfected for 60 minutes at 1800 RPM at 33°C in RetroNectin-coated plates. Transduced cells were sorted by flow cytometry.
In vitro cell growth and apoptosis
In vitro cell growth and apoptosis were performed as described before.6 For details, see supplemental Methods (available on the Blood Web site).
Confocal microscopy
The reduction in heparan sulfate expression after EXT-1 knockout (KO) was evaluated by confocal microscopy. For details, see supplemental Methods.
Cell-cycle analysis
Cells were incubated for 1 hour with 20 µM of BrdU (Sigma Aldrich) and subsequently fixed in ice-cold 70% ethanol. Cells were washed twice with phosphate-buffered saline (PBS)/0.1% bovine serum albumin (BSA) and incubated with 0.4 mg/mL of pepsin/0.2 mM of hydrochloric acid for 30 minutes at room temperature, spun down, and subsequently incubated in 2 M of hydrochloric acid for 25 minutes at 37°C. Cells were then washed with 0.05% Tween-20/0.5% BSA in PBS and stained for 30 minutes with anti-BrdU FITC (clone B44; BD). After washing, cells were stained with 0.1 µM of TO-PRO-3-iodide (Invitrogen Life Technologies) in PBS/0.5% BSA containing 500 µg/mL of RNAse-A (Bioke; Leiden, The Netherlands) for 15 minutes at 37°C. Cell-cycle distribution was analyzed by flow cytometry.
For cell-cycle analysis of Wnt-responsive cells (supplemental Figure 4), see supplemental Methods.
Immunoblotting
Immunoblotting were perform as describe before.34 For details, see supplemental Methods.
Flow cytometry
Cell staining was analyzed by flow cytometry. For details, see supplemental Methods.
Results
HMCLs display constitutive Wnt signaling
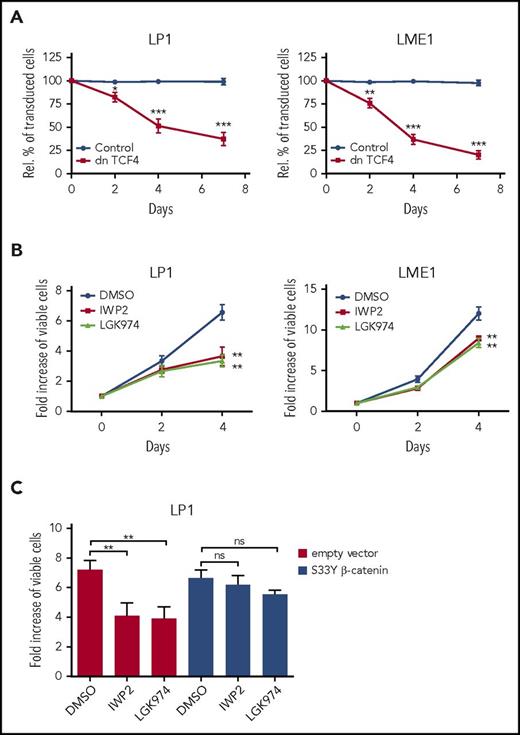
Previous studies have shown that activation of the canonical Wnt pathway is common in advanced primary MMs and in HMCLs.19-22 This aberrant Wnt signaling activity is driven by autocrine and/or paracrine Wnt ligands and promotes MM proliferation.19,22 To confirm and extend these findings, we expressed dnTCF4 from a lentiviral vector containing an mCherry marker in HMCLs and measured the percentage of transduced cells over time. dnTCF4-transduced myeloma cells were rapidly outcompeted, whereas the percentage of control vector–transduced cells was stable (Figure 1A; supplemental Figure 1A), confirming that growth of these HMCLs indeed depends on Wnt signaling.
HMCLs display constitutive Wnts signaling. (A) Percentage of transduced cells in HMCLs transduced with empty vector control or dnTCF4 expressed from bicistronic lentiviral vectors, relative to day 0. The mean ± standard deviation (SD) of 3 independent experiments in triplicate is shown. *P ≤ .05, **P ≤ .01, ***P ≤ .001 using unpaired Student t test. (B) Flow cytometry analysis of the effect of small-molecule Wnt secretion inhibitor IWP-2 (2.5 µM) and LGK974 (2.5 µM) on HMCL cell expansion in a 4-day time course, relative to day 0. The mean ± SD of 3 independent experiments in triplicate is shown. **P ≤ .01 using unpaired Student t test. (C) Flow cytometry analysis of the effect of small-molecule Wnt secretion inhibitor IWP-2 (2.5 µM) and LGK974 (2.5 µM) on the expansion of HMCL LP1 transduced with S33Y mutant β-catenin or the empty vector control in a 4-day time course, relative to day 0. The mean ± SD of 3 independent experiments in triplicate is shown. Not significant (ns) P > .05, **P ≤ .01 using 1-way analysis of variance with Bonferroni correction.
HMCLs display constitutive Wnts signaling. (A) Percentage of transduced cells in HMCLs transduced with empty vector control or dnTCF4 expressed from bicistronic lentiviral vectors, relative to day 0. The mean ± standard deviation (SD) of 3 independent experiments in triplicate is shown. *P ≤ .05, **P ≤ .01, ***P ≤ .001 using unpaired Student t test. (B) Flow cytometry analysis of the effect of small-molecule Wnt secretion inhibitor IWP-2 (2.5 µM) and LGK974 (2.5 µM) on HMCL cell expansion in a 4-day time course, relative to day 0. The mean ± SD of 3 independent experiments in triplicate is shown. **P ≤ .01 using unpaired Student t test. (C) Flow cytometry analysis of the effect of small-molecule Wnt secretion inhibitor IWP-2 (2.5 µM) and LGK974 (2.5 µM) on the expansion of HMCL LP1 transduced with S33Y mutant β-catenin or the empty vector control in a 4-day time course, relative to day 0. The mean ± SD of 3 independent experiments in triplicate is shown. Not significant (ns) P > .05, **P ≤ .01 using 1-way analysis of variance with Bonferroni correction.
To explore the possible involvement of autocrine Wnt ligands, we tested the effect of the Wnt secretion inhibitors IWP-2 and LGK974 on myeloma growth. These small molecules inhibit the activity of Porcupine, a membrane-bound acyltransferase that is essential for the secretion of Wnt proteins.40,41 We found that both inhibitors significantly inhibited the growth of LP1 and LME1 (Figure 1B) and that they indeed inhibited autocrine Wnt signaling (supplemental Figure 1B). Despite the significant reduction in cell numbers, both inhibitors hardly affected cell viability (supplemental Figure 1C). Importantly, downstream activation of Wnt signaling by an active mutant β-catenin (S33Y β-catenin) overcame the growth inhibition by IWP2 and LGK974 (Figure 1C). Taken together, these findings suggest that autocrine Wnt ligands are involved in Wnt pathway activation and cell growth in these HMCLs.
Deletion of heparan sulfate by EXT1 KO inhibits Wnt signaling in HMCLs
HSPGs, including syndecan-1, have previously been implicated in the regulation of Wnt signaling, including Wnt-mediated tumorigenesis.17 To explore the possible involvement of syndecan-1 HS in Wnt signaling in MM, we deleted the HS copolymerase EXT1 in HMCLs by CRISPR/Cas9-mediated KO. This completely abolished cell-surface HS expression (Figure 2A). To determine its effect on Wnt signaling, we measured the levels of nuclear and cytoplasmic β-catenin (Figure 2B), a key component of canonical Wnt signaling. In addition, TCF4-mediated Wnt reporter activity was assessed in LP1 cells stably transduced with a fluorescent Wnt reporter (TOP-GFP) and coexpressing a histone2B/mCherry fusion protein as transduction marker. As control, LP1 cells were transduced with FOP-GFP (Figure 2C). As shown in Figure 2B, KO of EXT1 resulted in reduced β-catenin levels in both the nucleus and cytoplasm in 3 HMCLs studied. In contrast, phosphorylation of AKT and the MAP kinases ERK1/2, typically reflecting activation of growth factor signaling, was not affected in LME1 or LP1 (supplemental Figure 2). Furthermore, deletion of EXT1 resulted in a strong reduction in the percentage of TOP-GFP+ cells in LP1 (Figure 2D). We also determined the effect of EXT1 KO on the expression of the Wnt target genes c-Myc and CCND1 (cyclin D1).20 As shown in Figure 2E, EXT1 KO resulted in reduced expression of c-Myc and cyclin D1. Taken together, these data show that loss of HS leads to reduced baseline Wnt pathway activity in HMCLs, suggesting that HSPGs mediate Wnt signaling in MM.
Deletion of heparan sulfate by EXT1 KO reduces Wnt signaling in HMCLs. (Ai) Flow cytometry analysis of cell-surface expression of HS in control-transduced cells (Cas9-empty control) or upon CRISPR/Cas9-mediated EXT1 KO (Cas9-sgEXT1) in HMCLs. (Aii) Confocal microscopy analysis of HS expression in control-transduced cells (EXT1 wild type [WT]) or upon CRISPR/Cas9-mediated EXT1 KO in HMCL LME1. Scale bars represent 2.5 μm. (B) Analysis of the nuclear and cytoplasmic distribution of β-catenin in HMCLs after EXT1 KO by western blot. Tubulin (cytoplasm) and TBP (nucleus) served as a fractionation and loading controls. For quantification, the expression level in WT cells was normalized to an arbitrary level of 100 units. (C) Flow cytometry analysis of Wnt reporter activity in LP1 cells transduced with Wnt signaling reporter TOP-GFP or control FOP-GFP. (D) Flow cytometry analysis of Wnt reporter activity in EXT1 WT or EXT1 KO LP1 cells. A representative plot is shown at left. Quantification of Wnt reporter activity is plotted as the percentage of mCherry/GFP double-positive live cells (right). The mean ± standard deviation of 3 independent experiments in triplicate is shown. **P ≤ .01 using 1-sample t test. (E) Analysis of c-Myc and cyclin D1 expression after EXT1 KO by western blot. Actin served as loading control. For quantification, the expression level in WT cells was normalized to an arbitrary level of 100 units.
Deletion of heparan sulfate by EXT1 KO reduces Wnt signaling in HMCLs. (Ai) Flow cytometry analysis of cell-surface expression of HS in control-transduced cells (Cas9-empty control) or upon CRISPR/Cas9-mediated EXT1 KO (Cas9-sgEXT1) in HMCLs. (Aii) Confocal microscopy analysis of HS expression in control-transduced cells (EXT1 wild type [WT]) or upon CRISPR/Cas9-mediated EXT1 KO in HMCL LME1. Scale bars represent 2.5 μm. (B) Analysis of the nuclear and cytoplasmic distribution of β-catenin in HMCLs after EXT1 KO by western blot. Tubulin (cytoplasm) and TBP (nucleus) served as a fractionation and loading controls. For quantification, the expression level in WT cells was normalized to an arbitrary level of 100 units. (C) Flow cytometry analysis of Wnt reporter activity in LP1 cells transduced with Wnt signaling reporter TOP-GFP or control FOP-GFP. (D) Flow cytometry analysis of Wnt reporter activity in EXT1 WT or EXT1 KO LP1 cells. A representative plot is shown at left. Quantification of Wnt reporter activity is plotted as the percentage of mCherry/GFP double-positive live cells (right). The mean ± standard deviation of 3 independent experiments in triplicate is shown. **P ≤ .01 using 1-sample t test. (E) Analysis of c-Myc and cyclin D1 expression after EXT1 KO by western blot. Actin served as loading control. For quantification, the expression level in WT cells was normalized to an arbitrary level of 100 units.
Loss of HS inhibits MM cell growth by attenuating Wnt signaling
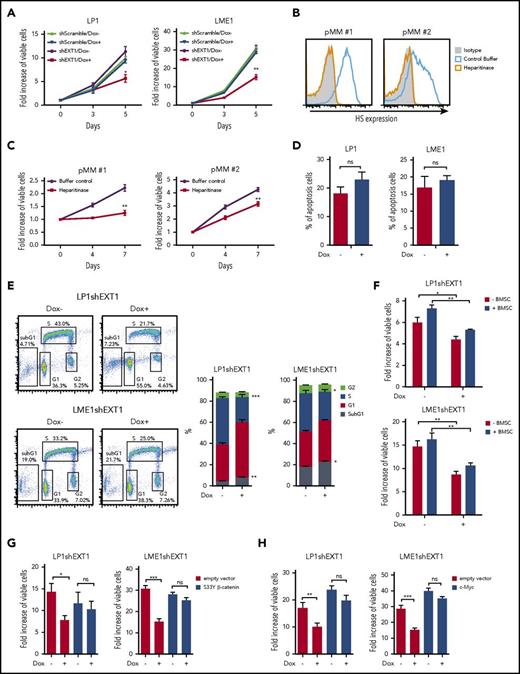
We have previously shown that knockdown (KD) of either EXT1 or syndecan-1 inhibits the growth of MM cells both in vitro and in vivo.6 To confirm and extend these findings, we performed EXT1 KD in a panel of HMCLs (supplemental Figure 3A). Loss of HS resulted in a significant inhibition of cell growth in 4 of 6 HMCLs (Figure 3A; supplemental Figure 3C). Importantly, similar results were also obtained in primary MM cells. Because the poor ex vivo viability and growth of these cells prohibited viral transduction and subsequent selection to (inducible) silence EXT1 by short hairpin RNA, we employed heparitinase to remove HS. This resulted in a complete depletion of HS chains from the MM cell surface (Figure 3B), resulting in significant growth inhibition (Figure 3C).
Loss of HS inhibits MM cell growth by attenuating Wnt signaling. (A) Flow cytometry analysis of the effect of doxycycline (Dox)–induced EXT1 KD on MM cell expansion at 5 days of culture, relative to day 0. Short hairpin Scramble (shScramble) was used as control. The mean ± standard deviation (SD) of 3 independent experiments in triplicate is shown. *P ≤ .05, **P ≤ .01 using unpaired Student t test. (B) Flow cytometry analysis of primary MM cell-surface HS expression after heparitinase treatment. (C) Flow cytometry analysis of primary MM cell expansion after heparitinase or control buffer treatment. A representative plot for 2 independent experiments is shown. **P ≤ .01 using unpaired Student t test. (D) Flow cytometry analysis of the effect of Dox-induced EXT1 KD on HMCL apoptosis. The mean ± SD of 3 independent experiments in triplicate is shown, not significant (ns) using unpaired Student t test. (E) Left: representative plot of cell-cycle analysis after BrdU incorporation upon Dox-induced EXT1 KD in HMCLs. Right: quantification of cell-cycle distribution after BrdU incorporation in Dox-induced EXT1 KD in HMCLs, The mean ± SD of 3 independent experiments in triplicate is shown. *P ≤ .05, **P ≤ .01, ***P ≤ .001 using unpaired Student t test. (F) Flow cytometry analysis of the effect of Dox-induced EXT1 KD on the expansion of HMCLs in the presence of BM stromal cells (BMSCs) in 4 days. The mean ± SD of 3 independent experiments each performed in triplicate is shown. *P ≤ .05, **P ≤ .01 using 1-way analysis of variance (ANOVA) with Bonferroni correction. (G) Flow cytometry analysis of the effect of Dox-induced EXT1 KD on the expansion of HMCLs transduced with S33Y β-catenin or empty control vector in 5 days. The mean ± SD of 3 independent experiments in triplicate is shown. ns P > .05, *P ≤ .05, ***P ≤ .001 using 1-way ANOVA with Bonferroni correction. (H) Flow cytometry analysis of the effect of Dox-induced EXT1 KD on the expansion of HMCLs transduced with c-Myc or empty control vector in 5 days. The mean ± SD of 3 independent experiments in triplicate is shown. ns P > .05, **P ≤ .01, ***P ≤ .001 using 1-way ANOVA with Bonferroni correction.
Loss of HS inhibits MM cell growth by attenuating Wnt signaling. (A) Flow cytometry analysis of the effect of doxycycline (Dox)–induced EXT1 KD on MM cell expansion at 5 days of culture, relative to day 0. Short hairpin Scramble (shScramble) was used as control. The mean ± standard deviation (SD) of 3 independent experiments in triplicate is shown. *P ≤ .05, **P ≤ .01 using unpaired Student t test. (B) Flow cytometry analysis of primary MM cell-surface HS expression after heparitinase treatment. (C) Flow cytometry analysis of primary MM cell expansion after heparitinase or control buffer treatment. A representative plot for 2 independent experiments is shown. **P ≤ .01 using unpaired Student t test. (D) Flow cytometry analysis of the effect of Dox-induced EXT1 KD on HMCL apoptosis. The mean ± SD of 3 independent experiments in triplicate is shown, not significant (ns) using unpaired Student t test. (E) Left: representative plot of cell-cycle analysis after BrdU incorporation upon Dox-induced EXT1 KD in HMCLs. Right: quantification of cell-cycle distribution after BrdU incorporation in Dox-induced EXT1 KD in HMCLs, The mean ± SD of 3 independent experiments in triplicate is shown. *P ≤ .05, **P ≤ .01, ***P ≤ .001 using unpaired Student t test. (F) Flow cytometry analysis of the effect of Dox-induced EXT1 KD on the expansion of HMCLs in the presence of BM stromal cells (BMSCs) in 4 days. The mean ± SD of 3 independent experiments each performed in triplicate is shown. *P ≤ .05, **P ≤ .01 using 1-way analysis of variance (ANOVA) with Bonferroni correction. (G) Flow cytometry analysis of the effect of Dox-induced EXT1 KD on the expansion of HMCLs transduced with S33Y β-catenin or empty control vector in 5 days. The mean ± SD of 3 independent experiments in triplicate is shown. ns P > .05, *P ≤ .05, ***P ≤ .001 using 1-way ANOVA with Bonferroni correction. (H) Flow cytometry analysis of the effect of Dox-induced EXT1 KD on the expansion of HMCLs transduced with c-Myc or empty control vector in 5 days. The mean ± SD of 3 independent experiments in triplicate is shown. ns P > .05, **P ≤ .01, ***P ≤ .001 using 1-way ANOVA with Bonferroni correction.
EXT1 KD only weakly affected cell viability in 2 of the HMCLs and did not induce apoptosis (supplemental Figure 3D; Figure 3D); however, it strongly decreased the percentage of cells in S phase and slightly increased the percentage of cells in the sub-G1 phase, as determined by measuring DNA content and BrdU incorporation (Figure 3E). Similarly, removal of HS in primary MM by heparitinase did not induce significant cell death (supplemental Figure 3E). These data indicate that loss of HS mainly affects MM growth by attenuating proliferation. Importantly, this growth inhibition could not be overcome by coculture of MM cells with BM stromal cells (Figure 3F).
We next studied whether the growth inhibition by EXT1 KD was causally related to inhibition of Wnt signaling. To this end, we transduced LP1 and LME1 cells with the active mutant β-catenin (S33Y β-catenin), which activates Wnt signaling in the absence of ligand. Interestingly, EXT1 KD did not cause growth inhibition in mutant β-catenin (S33Y β-catenin)–transduced cells, whereas EXT1 KD in empty vector–transduced cells resulted in strong growth reduction (Figure 3G). These findings suggest that the growth reduction by loss of HS is largely due to Wnt signaling inhibition. In view of our observation that EXT1 KO markedly reduces the expression of Wnt target c-Myc (Figure 2E), an established key player in B-cell42 and MM cell43 proliferation, we explored the possible role of c-Myc in the observed EXT1 KD–mediated growth reduction. To this end, we overexpressed c-Myc in LP1 and LME1 cells (supplemental Figure 3F). In line with a previous study,43 this enhanced MM cell growth. EXT1 KD in empty vector–transduced LP1 and LME1 cells resulted in strong growth reduction (supplemental Figure 3H). However, in c-Myc–overexpressing cells, it did not significantly inhibit growth (supplemental Figure 3H), suggesting that c-Myc downregulation plays an important role in the growth inhibition observed upon HS loss.
Loss of HS impairs Wnt pathway activation by paracrine Wnts
Although the Wnt pathway is aberrantly activated in MM cells, these cells can still respond to exogenous Wnt ligands.19 Within the BM microenvironment, stromal cells secrete Wnt ligands,19,22,44,45 whereas (pre)osteoblasts produce R-spondins, which can strongly potentiate Wnt signaling in MM cells.34 To model the response of MM cells to paracrine Wnts and/or R-spondins, we stimulated a panel of 6 HMCLs, containing the TOP-GFP Wnt reporter, with either Wnt3a, R-spondin, or Wnt3a plus R-spondin. As shown in Figure 4A, the HMCLs showed a heterogeneous response to these stimuli. Stimulation of OPM2 and XG3 with Wnt3a alone induced strong Wnt reporter activity. In LME1, XG1, and RIES, stimulation with Wnt3a alone resulted in minimal response, but the combination of Wnt3a and R-spondin led to a huge increase in the reporter activity. In line with our results presented in Figures 1 and 2, LP1 cells displayed high basal Wnt reporter activity as a consequence of high autocrine Wnt signaling and therefor showed a moderate response to Wnt3a and R-spondin stimulation (Figure 4A; supplemental Figures 6 and 7).
Loss of HS impairs Wnt pathway activation by paracrine Wnts. (A) Flow cytometry analysis of Wnt reporter activity in 6 TOP-GFP–transduced HMCLs treated with Wnt3a, R-spondin, or both. Wnt reporter activity is plotted as the percentage of mCherry/TOP-GFP double-positive live cells. The mean ± standard deviation (SD) of 3 independent experiments in triplicate is shown. (B) Western blot analysis of the nuclear and cytoplasmic distribution of β-catenin in control-transduced (wild-type [WT]) or EXT1 KO HMCLs after treatment with Wnt3a. Tubulin (cytoplasm) and TBP (nucleus) served as fractionation and loading controls. For quantification, the expression level in Wnt3a-treated WT cells was normalized to an arbitrary level of 100 units. (C) Flow cytometry analysis of Wnt reporter activity in control-transduced (EXT1 WT) or EXT1 KO HMCLs after treatment with Wnt3a. Left panel: representative picture of TOP-GFP reporter assay in HMCL OPM2 is shown. Right panel: Wnt reporter activity is plotted as the percentage of mCherry/TOP-GFP double-positive cells. The mean ± SD of 3 independent experiments in triplicate is shown. *P ≤ .05, **P ≤ .01 using 1-way analysis of variance with Bonferroni correction.
Loss of HS impairs Wnt pathway activation by paracrine Wnts. (A) Flow cytometry analysis of Wnt reporter activity in 6 TOP-GFP–transduced HMCLs treated with Wnt3a, R-spondin, or both. Wnt reporter activity is plotted as the percentage of mCherry/TOP-GFP double-positive live cells. The mean ± standard deviation (SD) of 3 independent experiments in triplicate is shown. (B) Western blot analysis of the nuclear and cytoplasmic distribution of β-catenin in control-transduced (wild-type [WT]) or EXT1 KO HMCLs after treatment with Wnt3a. Tubulin (cytoplasm) and TBP (nucleus) served as fractionation and loading controls. For quantification, the expression level in Wnt3a-treated WT cells was normalized to an arbitrary level of 100 units. (C) Flow cytometry analysis of Wnt reporter activity in control-transduced (EXT1 WT) or EXT1 KO HMCLs after treatment with Wnt3a. Left panel: representative picture of TOP-GFP reporter assay in HMCL OPM2 is shown. Right panel: Wnt reporter activity is plotted as the percentage of mCherry/TOP-GFP double-positive cells. The mean ± SD of 3 independent experiments in triplicate is shown. *P ≤ .05, **P ≤ .01 using 1-way analysis of variance with Bonferroni correction.
To assess whether the HS chains decorating syndecan-1 play a role in Wnt pathway activation by exogenous/paracrine Wnts, we employed OPM2 and XG3, the HMCLs with a high Wnt3a response largely independent of costimulation via R-spondin/LGR4. As shown in Figure 4B, Wnt3a stimulation resulted in cytoplasmic and nuclear accumulation of β-catenin, which were clearly inhibited in EXT1 KO cells. Also, Wnt3a-induced reporter activity in the EXT1 KO cells was strongly diminished compared with that in empty vector–transduced control cells (Figure 4C). Interestingly, in line with previous studies,46,47 the Wnt-responsive subpopulation of cells was enriched for cells in the G2/M phase of the cell cycle (supplemental Figure 4). Furthermore, enzymatic removal of cell-surface HS by heparitinase similarly attenuated Wnt/β-catenin signaling (supplemental Figure 5). Taken together, these results demonstrate that HS plays an important role in Wnt3a-induced Wnt pathway activation.
Loss of HS mitigates the potentiating effect of R-spondin on Wnt signaling
Recently, R-spondin family proteins have emerged as important positive regulators of Wnt signaling.29,30 Within the MM BM niche, R-spondins are abundantly expressed by (pre)osteoblasts.34 To study the role of HS in Wnt pathway activation by exogenous R-spondins, we employed the HMCLs XG1, LME1, and RIES, which require costimulation via R-spondin/LGR4 for substantial Wnt pathway activation (Figure 4A). Wnt signaling was assessed by measuring accumulation and nuclear translocation of β-catenin and by quantifying TCF4-mediated Wnt reporter activity. As shown in Figure 5A, simultaneous stimulation with R-spondin and Wnt3a resulted in robust β-catenin stabilization and nuclear translocation. These responses were dramatically impaired in EXT1 KO cells. In line with these findings, removal of HS from primary MM cells by heparitinase strongly attenuated Wnt3a and R-spondin–induced β-catenin accumulation (Figure 5B). EXT1 KO also significantly impeded Wnt reporter activation (Figure 5C; supplemental Figure 6). Taken together, these results demonstrate that HS plays a crucial role in Wnt3a and R-spondin–induced Wnt pathway activation.
Loss of HS mitigates the potentiating effect of R-spondin on Wnt signaling. (A) Western blot analysis of nuclear and cytoplasmic distribution of β-catenin in control-transduced (EXT1 wild-type [WT]) or EXT1 KO HMCLs after treatment with recombinant Wnt3a, R-spondin, or both. Tubulin (cytoplasm) and TBP (nucleus) served as fractionation and loading controls. For quantification, the expression level in Wnt3a and R-spondin–treated WT cells was normalized to an arbitrary level of 100 units. (B) Western blot analysis of β-catenin accumulation in control (buffer)– or heparitinase-treated primary MM cells stimulated with recombinant Wnt3a, R-spondin, or both. Actin served as a loading control. For quantification, the signal in Wnt3a and R-spondin–treated control cells was normalized to an arbitrary level of 100 units. (C) Flow cytometry analysis of Wnt reporter activity in control-transduced (EXT1 WT) or EXT1 KO HMCLs treated with Wnt3a, R-spondin, or both. Wnt reporter activity is plotted as the percentage of mCherry/TOP-GFP double-positive live cells. The mean ± standard deviation of 3 independent experiments in triplicate is shown. **P ≤ .01 using 1-way analysis of variance with Bonferroni correction.
Loss of HS mitigates the potentiating effect of R-spondin on Wnt signaling. (A) Western blot analysis of nuclear and cytoplasmic distribution of β-catenin in control-transduced (EXT1 wild-type [WT]) or EXT1 KO HMCLs after treatment with recombinant Wnt3a, R-spondin, or both. Tubulin (cytoplasm) and TBP (nucleus) served as fractionation and loading controls. For quantification, the expression level in Wnt3a and R-spondin–treated WT cells was normalized to an arbitrary level of 100 units. (B) Western blot analysis of β-catenin accumulation in control (buffer)– or heparitinase-treated primary MM cells stimulated with recombinant Wnt3a, R-spondin, or both. Actin served as a loading control. For quantification, the signal in Wnt3a and R-spondin–treated control cells was normalized to an arbitrary level of 100 units. (C) Flow cytometry analysis of Wnt reporter activity in control-transduced (EXT1 WT) or EXT1 KO HMCLs treated with Wnt3a, R-spondin, or both. Wnt reporter activity is plotted as the percentage of mCherry/TOP-GFP double-positive live cells. The mean ± standard deviation of 3 independent experiments in triplicate is shown. **P ≤ .01 using 1-way analysis of variance with Bonferroni correction.
Loss of HS interferes with upstream, receptor-proximal Wnt signaling
Previous studies have shown that HSPG localization and function are not restricted solely to the cell surface. Specifically, HSPGs localized in the nucleus have been implicated in the regulation of transcription factor binding and cell proliferation.48-52 Hence, HSPG/syndecan-1 might also play a role in downstream Wnt signaling in MM. To explore this possibility, EXT1 KO HMCLs were treated with CHIR99021, which activates Wnt signaling by stabilizing β-catenin. As is shown in Figure 6A, CHIR99021 induced similar levels of Wnt activation in EXT1 KO cells and empty vector control cells in both XG1 and LME1. In addition, we assessed activation of Wnt signaling at the receptor level by measuring phosphorylation of the Wnt coreceptor LRP6. As shown in Figure 6B, simultaneous stimulation with R-spondin and Wnt3a resulted in phosphorylation of LRP6 in both XG1 and LME1, which was clearly inhibited in EXT1 KO cells. These data indicate that loss of HS interferes with upstream, receptor-proximal Wnt signaling activation and that HSPGs most likely control Wnt signaling activity extracellularly, at the level of ligand/receptor interaction.
Loss of HS interferes with upstream, receptor-proximal Wnt pathway activation. (A) Flow cytometry analysis of Wnt reporter activity in control-transduced (EXT1 wild-type [WT]) or EXT1 KO HMCLs XG1 and LME1 treated with the GSK3 inhibitor CHIR99021 or DMSO. Wnt activity is plotted as the percentage of mCherry/TOP-GFP double-positive live cells. The mean ± standard deviation of 3 independent experiments in triplicate is shown. Not significant (ns) P > .05 using 1-way analysis of variance with Bonferroni correction. (B) Western blot analysis of phospho-LRP6 (pLRP6) and total LRP6 (tLRP6) in control-transduced (EXT1 WT) or EXT1 KO HMCLs XG1 and LME1 treated with Wnt3a, R-spondin, or both. Tubulin served as a loading control. For quantification, the phosphorylation level in Wnt3a and R-spondin–treated WT cells was normalized to an arbitrary level of 100 units.
Loss of HS interferes with upstream, receptor-proximal Wnt pathway activation. (A) Flow cytometry analysis of Wnt reporter activity in control-transduced (EXT1 wild-type [WT]) or EXT1 KO HMCLs XG1 and LME1 treated with the GSK3 inhibitor CHIR99021 or DMSO. Wnt activity is plotted as the percentage of mCherry/TOP-GFP double-positive live cells. The mean ± standard deviation of 3 independent experiments in triplicate is shown. Not significant (ns) P > .05 using 1-way analysis of variance with Bonferroni correction. (B) Western blot analysis of phospho-LRP6 (pLRP6) and total LRP6 (tLRP6) in control-transduced (EXT1 WT) or EXT1 KO HMCLs XG1 and LME1 treated with Wnt3a, R-spondin, or both. Tubulin served as a loading control. For quantification, the phosphorylation level in Wnt3a and R-spondin–treated WT cells was normalized to an arbitrary level of 100 units.
Loss of HS attenuates cell-surface binding of Wnt3a and R-spondin
For several growth factors, binding to cell-surface HSPGs has been shown to potentiate or to be even essential for signaling.53,54 For Wnts, binding to HSPGs has been shown in several cell types, including Drosophila cells and mouse intestinal epithelial cells.55,56 To study whether HS on MM cells indeed binds Wnt proteins, we employed a flag-tagged,57 functional Wnt3a protein (supplemental Figure 8) and assessed its binding to the cell surface of EXT1 KO or empty vector control MM cells. As shown in Figure 7A-B, loss of HS resulted in reduced Wnt3a cell-surface binding to the HMCL LME1 and primary MM cells.
Loss of HS attenuates cell-surface binding of Wnt3a and R-spondin. (A) Flow cytometry analysis of cell-surface binding of Wnt3a-flag on control-transduced (EXT1 wild-type [WT]) or EXT1 KO LME1 cells. (B) Flow cytometry analysis of cell-surface binding of Wnt3a-flag to primary MM cells treated with heparitinase or buffer (control). (C) Flow cytometry analysis of cell-surface binding of R-spondin–his to control empty vector–transduced (control), EXT1 KO, and LGR4 KO LME1 cells. (D) Flow cytometry analysis of cell-surface binding of R-spondin–his on primary MM cells treated with heparitinase or buffer (control). (E) Flow cytometry analysis of Wnt reporter activity in control-transduced (EXT1 WT) or EXT1 KO HMCLs treated with Wnt3a, R-spondin–∆AA, or R-spondin–∆TSP condition medium or their combination. The mean ± standard deviation of 3 independent experiments in triplicate is shown. Not significant (ns) P > .05, *P ≤ .05 using 1-way analysis of variance with Bonferroni correction. (F) Model for syndecan-1 promotes Wnt signaling in MM. In human BM microenvironment, the stromal cells (niche cells) and MM cells secrete Wnt ligands, whereas (pre)osteoblasts (niche cells) produce R-spondins. HS chains decorating syndecan-1 promote autocrine and paracrine Wnt signaling and Wnt-mediated proliferation and survival in MM cells by presenting Wnt ligands and R-spondins.
Loss of HS attenuates cell-surface binding of Wnt3a and R-spondin. (A) Flow cytometry analysis of cell-surface binding of Wnt3a-flag on control-transduced (EXT1 wild-type [WT]) or EXT1 KO LME1 cells. (B) Flow cytometry analysis of cell-surface binding of Wnt3a-flag to primary MM cells treated with heparitinase or buffer (control). (C) Flow cytometry analysis of cell-surface binding of R-spondin–his to control empty vector–transduced (control), EXT1 KO, and LGR4 KO LME1 cells. (D) Flow cytometry analysis of cell-surface binding of R-spondin–his on primary MM cells treated with heparitinase or buffer (control). (E) Flow cytometry analysis of Wnt reporter activity in control-transduced (EXT1 WT) or EXT1 KO HMCLs treated with Wnt3a, R-spondin–∆AA, or R-spondin–∆TSP condition medium or their combination. The mean ± standard deviation of 3 independent experiments in triplicate is shown. Not significant (ns) P > .05, *P ≤ .05 using 1-way analysis of variance with Bonferroni correction. (F) Model for syndecan-1 promotes Wnt signaling in MM. In human BM microenvironment, the stromal cells (niche cells) and MM cells secrete Wnt ligands, whereas (pre)osteoblasts (niche cells) produce R-spondins. HS chains decorating syndecan-1 promote autocrine and paracrine Wnt signaling and Wnt-mediated proliferation and survival in MM cells by presenting Wnt ligands and R-spondins.
Like Wnts, R-spondins can also interact with HSPGs. In Xenopus embryos, R-spondin3 binding to syndecan-4 has been shown to mediate Wnt/planar cell polarity signaling.58 To study whether HS moieties on MM cells also bind R-spondin, we employed an His-tagged R-spondin and assessed its binding to the cell surface by flow cytometry. As shown in Figure 7C-D, loss of HS completely disrupted the cell-surface binding of R-spondin to the HMCL LME1 and primary MM cells. By contrast, KO of LGR4 (supplemental Figure 9), the cognate R-spondin receptor on MM cells,34 had no measurable effect on R-spondin cell-surface binding (Figure 7C).
All 4 R-spondins have a common TSP domain, which mediates glycosaminoglycan binding.59-61 To further study the functional role of R-spondin interaction with HS, we employed a mutant R-spondin (R-spondin–∆TSP)lacking the TSP domain and C-terminal basic amino acid–rich domain. As a control, we used R-spondin lacking the basic amino acid–rich domain only (R-spondin–∆AA). Deletion of the TSP domain strongly diminished the Wnt signaling–potentiating effect of R-spondin compared with the control R-spondin–∆AA. Whereas loss of cell-surface HS significantly attenuated the potentiating effect of R-spondin–∆AA, it did not affect the (minor) potentiating effect of R-spondin–∆TSP on Wnt signaling (Figure 7E). This indicates that the Wnt signaling–promoting effect of R-spondin is largely HS dependent.
Taken together, our data suggest that loss of HS inhibits Wnt signaling by impairing the binding of Wnt ligands and R-spondins to the cell surface.
Discussion
Aberrant Wnt/β-catenin pathway activity mediates cell proliferation in MM.18-22,34 In the current study, we reveal that the HS chains decorating syndecan-1 promote autocrine and paracrine Wnt signaling and Wnt-mediated proliferation in MM cells by binding Wnt ligands as well as R-spondins (Figure 7F). These results establish an important role for syndecan-1 in aberrant Wnt/β-catenin signaling and Wnt ligand–mediated cell growth in MM.
We confirm that HMCLs display constitutive Wnt signaling and demonstrate that inhibition of Wnt signaling, either downstream by dnTCF4 or upstream by the Wnt secretion inhibitors IWP-2 and LGK974, attenuates MM cell growth. These results indicate a role for autocrine Wnt ligands in mediating MM growth. Interestingly, HS-deficient EXT1 KO HMCLs exhibited decreased levels of cytoplasmic and nuclear β-catenin and strongly reduced Wnt reporter activity. Furthermore, in line with a previous study from our laboratory,6 targeting of HS expression by inducible short hairpin RNA–mediated EXT1 KD resulted in reduced MM cell growth. Interestingly, this growth reduction could largely be reversed by downstream activation of Wnt signaling by means of an active mutant β-catenin (S33Y) or by overexpression of the Wnt target gene c-Myc, suggesting that the attenuated growth may largely be caused by inhibition of Wnt signaling, involving c-Myc downregulation.
Although the Wnt pathway is constitutively active in many MMs and HMCLs, these cells still respond to exogenous Wnt ligands.19,34 In the BM microenvironment, these ligands, including Wnt3a, Wnt5a, Wnt10b, and/or Wnt16, are secreted by stromal cells.19,22,44,45 We employed recombinant Wnt3a, either alone or in combination with R-spondin, to mimic this paracrine stimulation. Importantly, loss of HS significantly reduced the response of MM cells to Wnt3a stimulation and impaired binding of Wnt3a to the MM cell surface. At limited concentrations of Wnt3a ligand, which presumably represent physiological conditions, the inhibition of Wnt signaling activation caused by EXT1 KO was much more prominent than at saturating ligand concentrations (supplemental Figure 10). Consistent with these findings, studies in model organisms including Drosophila and Xenopus have demonstrated that HSPGs are required to increase the local concentration of Wg (Wnt) ligand to activate its receptors.62,63 Wnt proteins are relatively unstable and insoluble because of their hydrophobic nature, which constrains long-range signaling.23 Binding to HSPGs may stabilize Wnt proteins and facilitate their interaction with Fzd receptor. In Drosophila, abrogation of HS synthesis by mutation of EXT family genes leads to reduced extracellular Wingless levels.64 Furthermore, in mouse intestinal epithelium, HSPGs were shown to increase the cell-surface binding affinity of Wnt ligands, enhance Wnt/β-catenin signaling, and facilitate crypt regeneration after intestinal epithelial injury,55 and Alexander et al17 reported that syndecan-1 is required for Wnt-mediated mammary tumorigenesis.
R-spondin family proteins have recently been identified as important positive regulators of Wnt signaling by interacting with LGR4-6 receptors.29,30 In Xenopus embryos, R-spondin3 binds syndecan-4 and mediates Wnt/planar cell polarity signaling.58 Interestingly, we recently found that LGR4 is aberrantly expressed by most MMs, resulting in paracrine Wnt pathway (hyper)activation by osteoblast-derived R-spondins.34 Our current results confirm that R-spondin indeed strongly amplifies the response of MM cells to Wnt3a stimulation, and we show that KO of EXT1, with consequent loss of HS, disrupts R-spondin binding to the surface of MM cells. Functionally, EXT1 KO attenuated the capacity of R-spondins to promote Wnt signaling. All 4 R-spondins contain a TSP domain that can bind to HSPGs/heparin.60,61,65 Deletion of the TSP domain of R-spondin resulted in a reduced capacity to promote Wnt signaling in MM. Activation of Wnt signaling by this TSP domain–deficient R-spondin mutant was markedly reduced and was no longer dependent on cell-surface HS expression. Together, these data indicate that R-spondins interact with MM cell-surface HS via their TSP domain. A crystallographic study of the structure of R-spondin1–LGR5-RNF43 showed that the Furin1 domain of R-spondin binds to RNF43, whereas the Furin2 domain of R-spondin interacts with LGR5. Hence, the TSP domain would indeed be accessible for binding other molecules, including HSPGs.66 Similarly, Glinka et al67 also showed that the TSP domain of the R-spondin is not involved in binding to the LGR receptors. Taken together, these findings suggest that R-spondins potentiate Wnt signaling by interacting with both LGR receptors and HSPGs, interactions that involve distinct R-spondin domains.
Although we found that downstream activation of Wnt signaling by S33Y β-catenin can largely overcome the growth reduction as a consequence of HS deletion, this by no means excludes a role for HS in mediating other growth factor pathways. For several growth factors, including HGF, EGF family ligands, and APRIL, interaction with HS has been shown to promote signaling.5,8 In fact, we previously found that syndecan-1 HS is even more critical for MM growth in vivo than in vitro.6 In primary MM, distinct MM (sub)clones may differ in their dependence on various HS-binding growth factors for their growth and survival. By targeting HS or its biosynthesis machinery in vivo, these will in principle be simultaneously inhibited, thus rendering HS-directed therapy an attractive option. Interestingly, it has recently been shown that a monoclonal antibody primarily targeting the HS chains of glypican-3 inhibited Wnt/β-catenin signaling in hepatocellular carcinoma cells and had potent antitumor activity in vivo.68 However, because HSPGs are widely expressed in normal tissues, generic targeting of HSPGs might cause adverse effects. These may potentially be circumvented by selective targeting of specific HSPG-modifying enzymes with small molecules or of specific HS modifications with antibodies or glycomimetics. Indeed, several studies have demonstrated that postpolymerization modifications of HS, such as O-sulfation and epimerization, can specifically modulate the interaction of HSPGs with growth factors, including Wnts.56,69 Furthermore, as recently shown by Kumagai et al,70 the HS-specific endosulfatase-2 can also modulate Wnt signaling in renal cell carcinomas.
Taken together, our results indicate an important role for syndecan-1 in mediating Wnt/β-catenin signaling and cell growth in MM. Therapeutic targeting of the HS chains decorating syndecan-1 or of the HS biosynthesis and modification machinery may significantly diminish the Wnt/β-catenin signaling–mediated growth of MMs and may also interfere with other growth factor signals emanating from the BM microenvironment.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work is supported by grant UVA 2011-5205 from the Dutch Cancer Society to M.S. and S.T.P and a CSC Chinese Government Scholarship to Z.R.
Authorship
Contribution: Z.R. designed the research, performed experiments, analyzed the data, designed the figures, and wrote the paper; H.v.A, W.d.L., and R.B.H. performed experiments; W.d.L., M.M.M., H.C., and M.J.K. provided materials; M.S. and S.T.P. supervised the study, designed the research, and analyzed the data; and S.T.P. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Steven T. Pals, Department of Pathology, Academic Medical Center, University of Amsterdam, Meibergdreef 9, 1105 AZ Amsterdam, The Netherlands; e-mail: s.t.pals@amc.uva.nl.
References
Author notes
M.S. and S.T.P. contributed equally to this study.

![Figure 2. Deletion of heparan sulfate by EXT1 KO reduces Wnt signaling in HMCLs. (Ai) Flow cytometry analysis of cell-surface expression of HS in control-transduced cells (Cas9-empty control) or upon CRISPR/Cas9-mediated EXT1 KO (Cas9-sgEXT1) in HMCLs. (Aii) Confocal microscopy analysis of HS expression in control-transduced cells (EXT1 wild type [WT]) or upon CRISPR/Cas9-mediated EXT1 KO in HMCL LME1. Scale bars represent 2.5 μm. (B) Analysis of the nuclear and cytoplasmic distribution of β-catenin in HMCLs after EXT1 KO by western blot. Tubulin (cytoplasm) and TBP (nucleus) served as a fractionation and loading controls. For quantification, the expression level in WT cells was normalized to an arbitrary level of 100 units. (C) Flow cytometry analysis of Wnt reporter activity in LP1 cells transduced with Wnt signaling reporter TOP-GFP or control FOP-GFP. (D) Flow cytometry analysis of Wnt reporter activity in EXT1 WT or EXT1 KO LP1 cells. A representative plot is shown at left. Quantification of Wnt reporter activity is plotted as the percentage of mCherry/GFP double-positive live cells (right). The mean ± standard deviation of 3 independent experiments in triplicate is shown. **P ≤ .01 using 1-sample t test. (E) Analysis of c-Myc and cyclin D1 expression after EXT1 KO by western blot. Actin served as loading control. For quantification, the expression level in WT cells was normalized to an arbitrary level of 100 units.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/131/9/10.1182_blood-2017-07-797050/4/m_blood797050f2.jpeg?Expires=1770140368&Signature=eRYVBiQhAD9UTetpyGy8oC~F3ue2dvdAGagb--D6ePs0WfE-w2WOVUDTlhvdgP2tYuyfpBiXx2ohbPmd61kmgk74G1EwrzUgpAfm7hgXTyCFBvTRlByRVGVLrC3RBQuY1kX3E~8To3GxpOMFSfXDvCC82iCRBh0KtfS36IejSQnbYWjCgBTgl7C2fFwCh0-lKbrGbdHFRm4zrDXQSJhV63Mly8oopWXpo4PyIJISpZmK33KKdHsZwfD3zzvwVFqL4eNZBpwJDi-htPTkh-pPsNJTAagohkxXxuPNqmTCvAbp7lhK5FyWL-q1WpAWxDSuNUV4W5-5D1eXccrC~KnLwQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 4. Loss of HS impairs Wnt pathway activation by paracrine Wnts. (A) Flow cytometry analysis of Wnt reporter activity in 6 TOP-GFP–transduced HMCLs treated with Wnt3a, R-spondin, or both. Wnt reporter activity is plotted as the percentage of mCherry/TOP-GFP double-positive live cells. The mean ± standard deviation (SD) of 3 independent experiments in triplicate is shown. (B) Western blot analysis of the nuclear and cytoplasmic distribution of β-catenin in control-transduced (wild-type [WT]) or EXT1 KO HMCLs after treatment with Wnt3a. Tubulin (cytoplasm) and TBP (nucleus) served as fractionation and loading controls. For quantification, the expression level in Wnt3a-treated WT cells was normalized to an arbitrary level of 100 units. (C) Flow cytometry analysis of Wnt reporter activity in control-transduced (EXT1 WT) or EXT1 KO HMCLs after treatment with Wnt3a. Left panel: representative picture of TOP-GFP reporter assay in HMCL OPM2 is shown. Right panel: Wnt reporter activity is plotted as the percentage of mCherry/TOP-GFP double-positive cells. The mean ± SD of 3 independent experiments in triplicate is shown. *P ≤ .05, **P ≤ .01 using 1-way analysis of variance with Bonferroni correction.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/131/9/10.1182_blood-2017-07-797050/4/m_blood797050f4.jpeg?Expires=1770140368&Signature=g4jRwdoBE0gsj9q0OBqgfTjQWf0Kj8uqlt4NBN8nvahVEvqTnMOXzHL-L1CJT1SiXwxpndG57GgLxUQDPYFSjVnKqjJcc7m5cvu29myiu0i16vpf5X0dF8uUj74zfDALbPFxLvCknEs5T9~5av~ikTOVtvnVsktuP91Ft4gRxIz4NS6ymKOvVNknb7IOdlDhlsoU5xoRE4yYtrzRIZTx8qnT2tMxwndyeWnEgyOpqSal-ouAjLRbG6Xe6bIp09KvJPb~RwF5RVPf4Vu-SM-2q0D7h02ygWXPBva9GYM0R5ilBJnS32Y7AGtUJ0TVuqQg77PB2ZSfMG-E3qGZNx13rQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 5. Loss of HS mitigates the potentiating effect of R-spondin on Wnt signaling. (A) Western blot analysis of nuclear and cytoplasmic distribution of β-catenin in control-transduced (EXT1 wild-type [WT]) or EXT1 KO HMCLs after treatment with recombinant Wnt3a, R-spondin, or both. Tubulin (cytoplasm) and TBP (nucleus) served as fractionation and loading controls. For quantification, the expression level in Wnt3a and R-spondin–treated WT cells was normalized to an arbitrary level of 100 units. (B) Western blot analysis of β-catenin accumulation in control (buffer)– or heparitinase-treated primary MM cells stimulated with recombinant Wnt3a, R-spondin, or both. Actin served as a loading control. For quantification, the signal in Wnt3a and R-spondin–treated control cells was normalized to an arbitrary level of 100 units. (C) Flow cytometry analysis of Wnt reporter activity in control-transduced (EXT1 WT) or EXT1 KO HMCLs treated with Wnt3a, R-spondin, or both. Wnt reporter activity is plotted as the percentage of mCherry/TOP-GFP double-positive live cells. The mean ± standard deviation of 3 independent experiments in triplicate is shown. **P ≤ .01 using 1-way analysis of variance with Bonferroni correction.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/131/9/10.1182_blood-2017-07-797050/4/m_blood797050f5.jpeg?Expires=1770140368&Signature=zN6i0g56QxS7rRQn82f31AEeTzLHspJmI~JwEvgqYEGRCGEQH6Txzbyuc-7yjpEu3xGMVXyukuwFCbCFmlkZL1uAb7~BfF6o5lEa2jn4Z5HvPe82sYZaercPHhOsVqgi7Vd7dfOLK-0xn3DOSx0HzTv2w-soXg~477Tr2DHdiUuHfKU1~x8mbYFx8qX1le2wQYZamSUQa11E1KpAPRsG-UFgFZgdeGqzb3YDBASz2Am7hyUSi7U5jHApDjSM2lWJKnaqzWo9rJjTdAt5-Hb75mH~679MhQWGbCasP-TQJvzgPg~eTY-~J-wq61IYDUankWVK~pykK3y~N8wGfytvXg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 6. Loss of HS interferes with upstream, receptor-proximal Wnt pathway activation. (A) Flow cytometry analysis of Wnt reporter activity in control-transduced (EXT1 wild-type [WT]) or EXT1 KO HMCLs XG1 and LME1 treated with the GSK3 inhibitor CHIR99021 or DMSO. Wnt activity is plotted as the percentage of mCherry/TOP-GFP double-positive live cells. The mean ± standard deviation of 3 independent experiments in triplicate is shown. Not significant (ns) P > .05 using 1-way analysis of variance with Bonferroni correction. (B) Western blot analysis of phospho-LRP6 (pLRP6) and total LRP6 (tLRP6) in control-transduced (EXT1 WT) or EXT1 KO HMCLs XG1 and LME1 treated with Wnt3a, R-spondin, or both. Tubulin served as a loading control. For quantification, the phosphorylation level in Wnt3a and R-spondin–treated WT cells was normalized to an arbitrary level of 100 units.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/131/9/10.1182_blood-2017-07-797050/4/m_blood797050f6.jpeg?Expires=1770140368&Signature=x4gZ55fE2j1hyvhxD-lx12QZ92r-DjnBxV1ckZsTnLZxqXkNOIZKPo3WkcS6Jxt-yr3WgXA3O4N6eE-hvNvJJlooYSzTQf7MveT84x4Y6fw7M71iJyXqFFRMWtMOt4fxOvnlvVgcs168BfS5WtUWDmfHQQJgzupi~GrOfzgs72patOfBvzHrlV0ZkwdmiLyWICwue3hRGiDUc7dqOwII7BAAsQ8Z3iV976Xwzxu1~aUp9lgdxy-zNGyiArY9LnQlh4UJFsf~4jXmITVdjg~N~3D-mLp0vIppl2rduTq6er~3WX6KSJFUXcssSRKzwc3c~5f8wccswagyTWc1eU4YDw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 7. Loss of HS attenuates cell-surface binding of Wnt3a and R-spondin. (A) Flow cytometry analysis of cell-surface binding of Wnt3a-flag on control-transduced (EXT1 wild-type [WT]) or EXT1 KO LME1 cells. (B) Flow cytometry analysis of cell-surface binding of Wnt3a-flag to primary MM cells treated with heparitinase or buffer (control). (C) Flow cytometry analysis of cell-surface binding of R-spondin–his to control empty vector–transduced (control), EXT1 KO, and LGR4 KO LME1 cells. (D) Flow cytometry analysis of cell-surface binding of R-spondin–his on primary MM cells treated with heparitinase or buffer (control). (E) Flow cytometry analysis of Wnt reporter activity in control-transduced (EXT1 WT) or EXT1 KO HMCLs treated with Wnt3a, R-spondin–∆AA, or R-spondin–∆TSP condition medium or their combination. The mean ± standard deviation of 3 independent experiments in triplicate is shown. Not significant (ns) P > .05, *P ≤ .05 using 1-way analysis of variance with Bonferroni correction. (F) Model for syndecan-1 promotes Wnt signaling in MM. In human BM microenvironment, the stromal cells (niche cells) and MM cells secrete Wnt ligands, whereas (pre)osteoblasts (niche cells) produce R-spondins. HS chains decorating syndecan-1 promote autocrine and paracrine Wnt signaling and Wnt-mediated proliferation and survival in MM cells by presenting Wnt ligands and R-spondins.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/131/9/10.1182_blood-2017-07-797050/4/m_blood797050f7.jpeg?Expires=1770140368&Signature=yqS8DjQ~m-M5LxqzpRx7Kb8q1LQmFPMX1RhXiPGt5luH4VFA~fH0uHHvdaL3mumvQycPVfYVxv9mSyAxuigdxr2rz4LAUzA62xE9ZN9dIqwYVZHGkVIeIHjDlkTI2mqYqTDiMKCntK0jjQheLLeTpuE3riHAFvMbPO6bM4eJY9O3MsZkaQGEzYjK9hF5ehxULjPq2JcAqG8gFfmUJxiMEBMptXsD04Y2SSH4GV64-DXxNNTkxo-Ms8u9qLre5c20rvNyOzSGvuG5-VN5yAb3zAkuJ0ajZjbaalIgzRKrui9MAX1zYK4CukPJDY-OBnO6TpzdEciMSNz34Ahh6iobvQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal