TO THE EDITOR:
Heparin-induced thrombocytopenia (HIT) is a severe adverse reaction to heparin treatment characterized by antibodies to platelet factor 4 (PF4)-heparin complexes.1-3 Recent data suggest that therapies currently in use are associated with a significant likelihood of thrombosis, amputation, and death.4 Several off-label treatments such as IV immunoglobulin G (IVIg),5,6 direct oral anticoagulants,7 and therapeutic plasma exchange (TPE)8-21 are increasingly being used to treat severe or refractory cases. In performing TPE, a portion of the patient’s plasma is removed and replaced by an alternative fluid, typically 5% human albumin, and less often, normal plasma. In fact, several studies of TPE in HIT, including the largest report summarizing the experience in 28 patients21 and a recent article in Blood,13 used albumin as the sole replacement fluid. Other publications report using mostly albumin and some plasma,9,11,12,15,18,19 and in 1 study, plasma alone was used.8 Heterogeneity of patient presentation, differences in treatment schedule, and variability in replacement fluid used in these studies preclude firm conclusions from being drawn concerning the efficacy of specific TPE replacement solutions in treating HIT.22
Recent studies5,23 demonstrate that normal immunoglobulin G (IgG) is a potent inhibitor of HIT antibody–mediated platelet activation and that platelets from patients with the HH131, and to a lesser extent, RR/HR131 genotypes of the platelet IgG receptor FcγRIIa are particularly susceptible to this inhibition. Data suggest that although IgG1 binds both RR and HH131 isoforms of FcγRIIa, IgG2, which constitutes about one-third of IgG in plasma (and IVIg), does not recognize RR131.5,23 TPE impacts plasma Ig levels in 2 ways: it decreases the titer of pathogenic HIT antibodies and depletes nonpathogenic plasma IgGs. The latter effect could theoretically counteract the salutary impact of HIT antibody removal and potentially even worsen disease. This study was carried out to assess the impact of the diluent used for exchange on HIT antibody–mediated platelet activation by using an in vitro model that mimics TPE.
Samples from 3 patients with severe HIT (HIT-1, HIT-2, and HIT-3) who required TPE treatment were used for these studies. The PF4-dependent P-selectin expression assay (PEA) using platelets pretreated with low-dose PF4 (PEA, low PF4: 3.75 μg/mL) was performed as previously described5 (for full details, see supplemental Data available on the Blood Web site). A 1-volume plasma exchange removes about 60% of an intravascular substance assuming no recirculation or rapid intravascular-extravascular exchange. To mimic TPE in an in vitro system, HIT samples were diluted in 5% human albumin (Grifols, Los Angeles, CA), 3 different normal plasmas, IgG-depleted normal plasmas, and IgG-depleted normal plasmas to which IVIg had been added to reestablish baseline IgG levels (IgG-repleted plasma) and were then tested in the PEA. Studies were approved by the institutional review board of the Medical College of Wisconsin (Protocol PRO00023318).
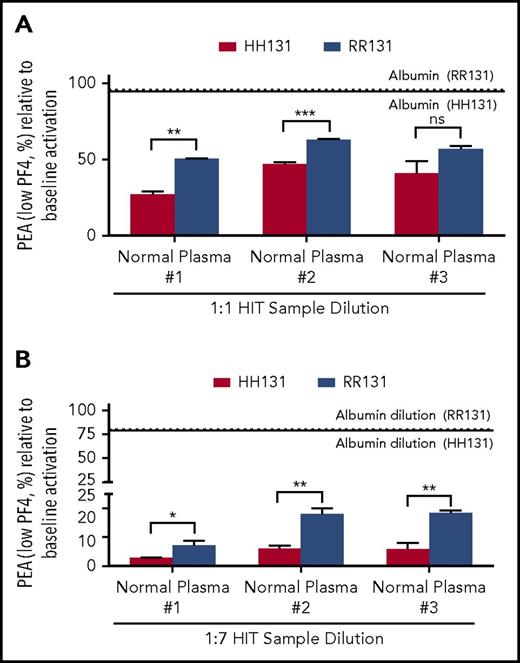
When a HIT sample (HIT-1) was diluted 1:1 in normal plasma to mimic a single exchange performed with this medium, P-selectin expression induced in both HH131 and RR131 platelets was significantly lower than when the HIT sample dilution was performed with 5% human serum albumin (Figure 1A). Similar results were obtained when 2 additional normal plasma samples were used as diluents (normal plasma #2 and #3; Figure 1A). Inhibition of P-selectin expression was greater when using HH131 than when using RR131 platelets with 2 of the 3 normal plasmas used (trending to significance in the third sample; P = .069; Figure 1A). The effects of serial plasma exchange, which is often used in treating HIT patients, were mimicked by reducing the HIT antibody to final concentrations of 12.5% (1:7) of its initial value (3 serial TPEs) using the same diluents and testing the ability of the diluted HIT sample to activate platelets (Figure 1B). As shown, dilution in normal plasma markedly decreased the ability of the HIT-1 antibody to activate platelets in contrast to results obtained with albumin dilution. Similar to results obtained with 1:1 dilution, P-selectin expression was more significantly inhibited in HH131 compared with RR131 platelets with dilution by all 3 normal plasmas (Figure 1B). Comparable results were obtained with 2 other HIT antibodies tested similarly (HIT-2 and HIT-3; supplemental Figures 1A-B and 2A-B).
Normal plasma inhibits HIT-1–mediated platelet activation more effectively than 5% albumin. (A-B) PEA test results are depicted as a percentage of the value obtained with undiluted HIT-1. Red and blue bars show results obtained with HH131 and RR131 FcγRIIa platelets, respectively. Results obtained with 5% albumin dilution are represented by the horizontal dotted (RR131 platelets) and solid (HH131 platelets) lines. Comparable results were obtained with plasma from 3 different normal individuals (normal plasma #1-#3). Means +1 standard deviation of triplicate determinations are presented and were compared by using the Student t test. P < .05 was considered significant. *P < .05; **P < .01; ***P < .001. ns, not significant.
Normal plasma inhibits HIT-1–mediated platelet activation more effectively than 5% albumin. (A-B) PEA test results are depicted as a percentage of the value obtained with undiluted HIT-1. Red and blue bars show results obtained with HH131 and RR131 FcγRIIa platelets, respectively. Results obtained with 5% albumin dilution are represented by the horizontal dotted (RR131 platelets) and solid (HH131 platelets) lines. Comparable results were obtained with plasma from 3 different normal individuals (normal plasma #1-#3). Means +1 standard deviation of triplicate determinations are presented and were compared by using the Student t test. P < .05 was considered significant. *P < .05; **P < .01; ***P < .001. ns, not significant.
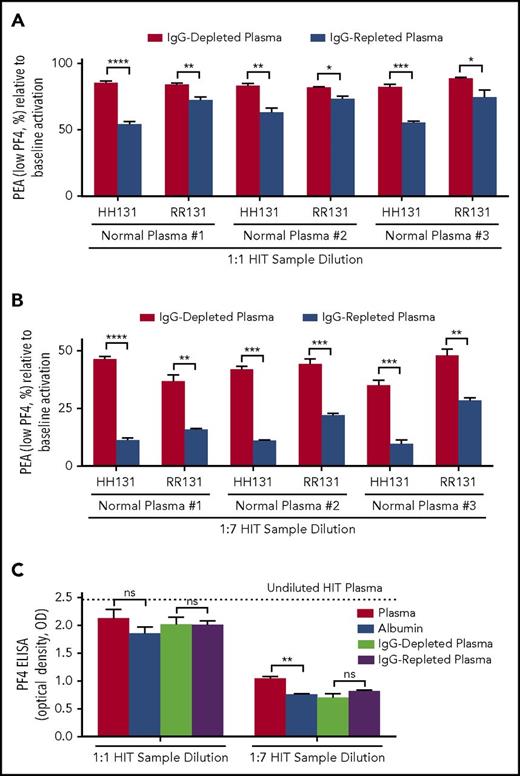
When IgG-depleted and -repleted plasmas were used for dilution at a 1:1 ratio, significantly greater inhibition of P-selectin expression was seen with IgG-repleted plasma using both HH131 and RR131 platelets (Figure 2A). Inhibition of platelet activation was higher, as expected, using HH131 than RR131 platelets, with all 3 normal plasma samples tested (Figure 2A). Similar results were obtained with 2 other HIT antibodies (HIT-2 and HIT-3; supplemental Figures 1C and 2C). These differences were maintained when higher dilutions (1:7) of the 3 HIT samples were used to mimic serial TPE (Figure 2B; supplemental Figures 1D and 2D). In contrast to findings made by using a functional assay (PEA), activity of the HIT antibodies in the solid-phase enzyme-linked immunosorbent assay (ELISA) was reduced in proportion to the degree of dilution (Figure 2C; supplemental Figures 1E and 2E). With some HIT samples, a slightly higher signal was obtained with IgG-replete solutions (plasma and IgG-repleted plasma) likely as a result of the higher background signal related to increased IgG content (Figure 2C; supplemental Figures 1E and 2E).
IgG-repleted normal plasma inhibits platelet activation induced by HIT-1 more effectively than IgG-depleted plasma. (A-B) The abscissa indicates dilution ratio of HIT-1 with IgG-depleted and -repleted samples obtained from 3 different normal plasmas (normal plasma #1-#3), and the ordinate depicts the PEA as a percentage of the value obtained with undiluted HIT-1. FcγRIIa genotype is indicated on the abscissa. Red and blue bars represent HIT-1 diluted with IgG-depleted and -repleted normal plasma, respectively. (C) IgG status of the diluent has little to no effect on results obtained with HIT-1 dilution in the PF4 enzyme-linked immunosorbent assay (ELISA). The abscissa indicates dilution ratio used, and the ordinate depicts the optical density (OD) of IgG-specific PF4-polyvinylsulfonate ELISA (PF4 ELISA). All PF4 ELISA reactions (ODs) were inhibited ≥50% with high-dose (100 U/mL) heparin (data not shown). Means +1 SD of triplicate determinations are presented and were compared by using the Student t test. P < .05 was considered significant. *P < .05; **P < .01; ***P < .001; ****P < .0001.
IgG-repleted normal plasma inhibits platelet activation induced by HIT-1 more effectively than IgG-depleted plasma. (A-B) The abscissa indicates dilution ratio of HIT-1 with IgG-depleted and -repleted samples obtained from 3 different normal plasmas (normal plasma #1-#3), and the ordinate depicts the PEA as a percentage of the value obtained with undiluted HIT-1. FcγRIIa genotype is indicated on the abscissa. Red and blue bars represent HIT-1 diluted with IgG-depleted and -repleted normal plasma, respectively. (C) IgG status of the diluent has little to no effect on results obtained with HIT-1 dilution in the PF4 enzyme-linked immunosorbent assay (ELISA). The abscissa indicates dilution ratio used, and the ordinate depicts the optical density (OD) of IgG-specific PF4-polyvinylsulfonate ELISA (PF4 ELISA). All PF4 ELISA reactions (ODs) were inhibited ≥50% with high-dose (100 U/mL) heparin (data not shown). Means +1 SD of triplicate determinations are presented and were compared by using the Student t test. P < .05 was considered significant. *P < .05; **P < .01; ***P < .001; ****P < .0001.
Plasma exchange is an important component of the arsenal of HIT treatment modalities, especially for severely affected patients. TPE is typically implemented in a series of treatments to attain significant depletion of HIT IgG.22 On occasion, single or only a few treatments are used in urgent situations to enable patients with acute or subacute HIT to be exposed to heparin. The replacement solution used in TPE is usually 5% human albumin, except in conditions such as thrombotic thrombocytopenic purpura, in which donor plasma is used to provide a source of the metalloprotease ADAMTS-13.22 Studies that demonstrate IVIg-mediated inhibition of HIT antibody-induced platelet activation23 suggested that normal plasma (IgG replete) should be highly effective in maintaining platelet quiescence and, in our hands, IVIg inhibited HIT antibody-mediated platelet activation in all patient samples (8) tested to date.5 Results obtained in this study show that IgG-replete, but not IgG-depleted plasma or 5% albumin, is highly effective in inhibiting HIT antibody-mediated platelet activation. These in vitro results are consistent with the possibility that a reduction in antibody activity with the use of plasma will translate into a beneficial effect for patients with severe HIT.
Of note, although IgG-repleted plasma was significantly more effective than IgG-depleted plasma in maintaining platelet quiescence, inhibition of P-selectin expression seen with the former sample type was still less than that observed with unmanipulated plasma (eg, Figure 1A vs Figure 2A). It is possible that additional (non-IgG) substances that have the ability to counteract HIT antibody-mediated platelet activation are present in normal plasma and may have been modified or diluted during the process of IgG depletion. Another limitation is that this study does not examine the impact of TPE replacement fluid on HIT antibody-mediated activation of monocytes, a cell type that may have an important role in HIT pathogenesis.24
These results suggest that plasma (or possibly IVIg infusion after albumin-based TPE) is a preferred replacement solution for TPE in HIT. Plasma, a blood product, has associated risks including transfusion reactions and transmission of infectious diseases, although the risk for serious reactions is low.25 IVIg has high cost and several risks, including thrombosis, hemolysis, renal failure, and aseptic meningitis.26 These factors should be taken into account, and a risk-benefit assessment should be performed before decisions regarding TPE replacement fluid are made.
Presented at the American Society of Hematology Annual Meeting and Exposition, 9-12 December 2017, Atlanta, GA.
The online version of this article contains a data supplement.
Acknowledgments
This study was supported in part by funds from the National Institutes of Health, National Heart, Lung, and Blood Institute grants HL013629 (R.H.A.) and HL133479 (A.P.) and the Clinical and Translational Sciences Institute of Southeastern Wisconsin (A.P.).
Authorship
Contribution: A.P. conceived the study; C.G.J. and S.M.P. performed the experiments; B.R.C., D.W.B., and R.H.A. provided advice on experimental design and interpretation; A.P. analyzed the data; C.G.J. wrote the first draft; and all authors edited the manuscript and approved its final version.
Conflict-of-interest disclosure: C.G.J., D.W.B., R.H.A., and A.P. state that a patent application has been filed related to diagnostic testing in HIT (Method of Detecting Platelet-Activating Antibodies That Cause Heparin-Induced Thrombocytopenia/Thrombosis; PCT/US14/62591). The remaining authors declare no competing financial interests.
Correspondence: Anand Padmanabhan, Blood Center of Wisconsin, 8733 Watertown Plank Rd, Milwaukee, WI 53233; e-mail: anand.padmanabhan@bcw.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal