TO THE EDITOR:
X-linked lymphoproliferative syndrome (XLP) is a primary immunodeficiency caused by mutations in either SH2D1A (XLP1) or XIAP (XLP2) genes.1-3 XLP patients are highly susceptible to Epstein-Barr virus (EBV), which can trigger potentially fatal hemophagocytic lymphohistiocytosis (HLH). Although some XLP1 patients develop lymphoma, those with XLP2 commonly present with chronic inflammatory bowel disease.4
One surprising feature of XLP is its great variability in clinical presentation, such that even affected siblings may develop HLH at different ages and with variable severity or not at all.4 This is reminiscent of atypical familial HLH (FHL; FHL2-5) caused by hypomorphic mutations in FHL-related genes.5 However, a clear point of distinction from atypical FHL2-5, is XLP2’s variable effect on natural killer (NK) cell function, ranging from severe impairment to normal cytotoxicity. Intriguingly, no clear mechanistic link has ever been established between X-linked inhibitor of apoptosis (XIAP) deficiency and NK cytotoxicity. By investigating monozygotic twins from a nonconsanguineous family, we have here uncovered a surprising mechanism that accounts for functional NK deficiency in XLP2 patients, while also explaining the frequent responses of these patients to the anti-CD20 agent, rituximab.6
Isolated peripheral blood mononuclear cells were cultured at 106 cells per mL for 18 hours in RPMI 1640 medium with or without 100 U/mL interleukin 2 (IL2). NK- or T-cell function was assessed by 51Cr-release cytotoxicity assay and by CD107a externalization assay using K562 target cells.7 Detailed protocols, reagents used in this study, and gating strategy for NK-cell phenotype are described in supplemental Methods (available on the Blood Web site).
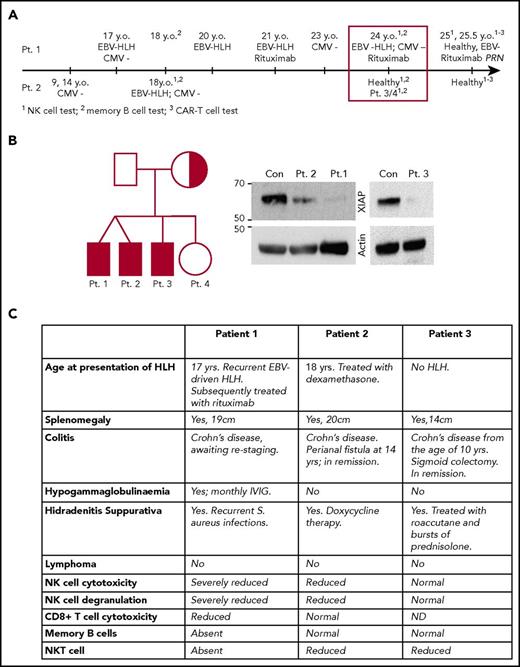
Family tree, timeline, and clinical presentation of patients 1 to 3 are shown in Figure 1. A 17-year-old boy (patient 1) was admitted to the hospital with suspected diagnosis of EBV-related FHL. He made a full recovery following a short course of corticosteroids. One year later, his monozygotic twin brother (patient 2) also presented with suspected EBV-related HLH-like symptoms that rapidly responded to dexamethasone. At the time, patient 2 had severely reduced NK-cell numbers (0.1% of total lymphocytes) that showed marginal cytotoxic activity (not shown). He has had no further recurrence of HLH. Their healthy nonconsanguineous parents had no family history of primary immunodeficiency.
Timeline and clinical manifestations of the disease in the patients. (A) Timeline of clinical presentation of the disease in patients 1 and 2. (B) Family tree shows the carrier of the XIAP mutation (half-shaded) and the patients (shaded). The western immunoblot demonstrates a dramatic reduction in mutant XIAP expression in patients 1 to 3. (C) Clinical manifestation of XLP2 in patients 1 to 3. CAR-T, chimeric antigen receptor T cell; Con, control; IVIG, IV immunoglobulin; Pt., patient; y.o., years old.
Timeline and clinical manifestations of the disease in the patients. (A) Timeline of clinical presentation of the disease in patients 1 and 2. (B) Family tree shows the carrier of the XIAP mutation (half-shaded) and the patients (shaded). The western immunoblot demonstrates a dramatic reduction in mutant XIAP expression in patients 1 to 3. (C) Clinical manifestation of XLP2 in patients 1 to 3. CAR-T, chimeric antigen receptor T cell; Con, control; IVIG, IV immunoglobulin; Pt., patient; y.o., years old.
Unlike patient 2, patient 1 suffered several EBV-related HLH episodes over the next 5 years. At the age of 24 years, he presented once again with a high EBV viral load (30 × 106 copies per mL), drenching night sweats, fatigue, and 9 kg weight loss over several weeks. His NK cells exhibited significantly reduced cytotoxicity in vitro, which partially recovered in response to overnight incubation with 100 U/mL IL2 (Figure 2A). Although asymptomatic at that time, patient 2 was also found to have poor NK cytotoxicity (Figure 2A). We also found on routine testing that patient 1, but not patient 2, lacked circulating memory B cells (Figure 2B). Retrospective analysis revealed that this phenotype was persistent over time, as patient 1 also had severely reduced memory B cells at 18 years of age, when he remained asymptomatic while his brother developed HLH (Figures 1 and 2B). The older brother (patient 3) and the eldest sister of patients 1 and 2 had never developed clinical HLH, and their NK activity appeared normal/slightly reduced (supplemental Figure 1A-B). Importantly, the 3 brothers (patients 1-3) all suffered from Crohn disease, while their sister remained healthy.
NK cells of XLP2 patients had impaired natural cytotoxicity and developed adaptive-like phenotype. (A) Cytotoxic activity of NK cells in patients 1 and 2 at 24 years of age, when they were diagnosed with XLP2 (see timeline in Figure 1). Shown is the result of the 51Cr-release assay (for clarity, error bars are not shown). (B) Memory B-cell phenotype (CD3−CD19+C20+CD27+) at the first onset of HLH, 6 years later (at the time of XLP2 diagnosis, aged 24 years), and 1.5 years after the diagnosis (aged 25.5 years). (C) Cytotoxic activity of the NK cells of patients 1 and 2, 1 year after XLP2 diagnosis. Shown are the 51Cr-release assay (for clarity, error bars are not shown) and the CD107a degranulation assay. (D) Cytotoxic activity of CD8+ T cells (please see supplemental Methods for details) as measured by 51Cr-release assay (mean ± standard error of the mean [SEM], n = 3) and CD107a degranulation assay. Tests were done at the same time as in panel C. (E) NK cells of patient 1 and 2, but not patient 3, developed adaptive-like phenotype (for gating strategy, please see supplemental Figure 4). NK cells in patients 1/2 were assessed 1 year (as in panel C) and 1.5 years after the diagnosis (depicted by gray and blue bars, respectively), and in patient 3 – 1.75 years after the diagnosis. Shown is mean ± standard deviation (SD) of 10 (FcεRγ−) or 11 (NKG2C+ and NKG2A+) controls. There was a similar trend for NKG2A+ and NKG2C+ cells, when gated on both CD16+CD56high and CD16+CD56dim NK cells (data not shown). (F) NK cells from patients 1 and 2 show enhanced response to rituximab (RTX)-treated Raji cells through ADCC. E/T, effector/target ratio.
NK cells of XLP2 patients had impaired natural cytotoxicity and developed adaptive-like phenotype. (A) Cytotoxic activity of NK cells in patients 1 and 2 at 24 years of age, when they were diagnosed with XLP2 (see timeline in Figure 1). Shown is the result of the 51Cr-release assay (for clarity, error bars are not shown). (B) Memory B-cell phenotype (CD3−CD19+C20+CD27+) at the first onset of HLH, 6 years later (at the time of XLP2 diagnosis, aged 24 years), and 1.5 years after the diagnosis (aged 25.5 years). (C) Cytotoxic activity of the NK cells of patients 1 and 2, 1 year after XLP2 diagnosis. Shown are the 51Cr-release assay (for clarity, error bars are not shown) and the CD107a degranulation assay. (D) Cytotoxic activity of CD8+ T cells (please see supplemental Methods for details) as measured by 51Cr-release assay (mean ± standard error of the mean [SEM], n = 3) and CD107a degranulation assay. Tests were done at the same time as in panel C. (E) NK cells of patient 1 and 2, but not patient 3, developed adaptive-like phenotype (for gating strategy, please see supplemental Figure 4). NK cells in patients 1/2 were assessed 1 year (as in panel C) and 1.5 years after the diagnosis (depicted by gray and blue bars, respectively), and in patient 3 – 1.75 years after the diagnosis. Shown is mean ± standard deviation (SD) of 10 (FcεRγ−) or 11 (NKG2C+ and NKG2A+) controls. There was a similar trend for NKG2A+ and NKG2C+ cells, when gated on both CD16+CD56high and CD16+CD56dim NK cells (data not shown). (F) NK cells from patients 1 and 2 show enhanced response to rituximab (RTX)-treated Raji cells through ADCC. E/T, effector/target ratio.
On the basis of these observations, we suspected that patients 1 to 3 might suffer from XLP2, and this was indeed confirmed when a novel missense mutation c.644G>C (p.Arg215Pro; Arg215 is highly conserved in mammals) in XIAP was found to have been inherited by patients 1 to 3 (but not by their sister) from their mother. This mutation resulted in a significant reduction of protein expression in patients 1 to 3 (Figure 1B). No mutations were identified in FHL-causing genes PRF1, UNC13D, STX11, STXBP2, LYST, SH2D1A, or RAB27A.
Following the initial diagnosis, patient 1 received 4 infusions of rituximab (375 mg/m2 per week) administered every 3 weeks to eliminate EBV-infected B cells, to which he responded strongly. His high EBV titres returned just over a year later, and after 2 infusions of rituximab (375 mg/m2) per week for 2 weeks, the patient was placed on maintenance rituximab (375 mg/m2) administered once every 4 months. He remains asymptomatic, with very low plasma EBV DNA titres (<400 copies/mL) detected on regular surveillance. Due to the lack of circulating memory B cells (Figure 2B), patient 1 requires regular IV immunoglobulin infusions. Consistent with XLP2, patients 1 to 3 had reduced or absent NKT cells (CD3+CD1d/αGalCer+ [supplemental Figure 2A]).8 Despite maintaining excellent health, patient 1’s NK-cell function remained reduced, with some recovery of function observed in the presence of IL2 (Figure 2C; supplemental Figure 2B); in contrast, his CD8+ T-cell function was only slightly lower (by 50%) than in control and patient 2 (Figure 2D). Such a variable phenotype of memory B, NK, and CD8+ T cells in patients 1 to 3 suggested that the XIAP deficiency had no direct effect on the function of these cells. Nevertheless, it seemed surprising that EBV-infected B cells could be efficiently cleared by antibody-dependent cell-mediated cytotoxicity (ADCC) given the abundance of functionally impaired NK cells in patient 1 and normal levels of expression of perforin and granzyme B (supplemental Figure 3).
Several recent studies have shown that chronic viral infections such as human or murine cytomegalovirus (CMV) and HIV9-14 can result in persistent epigenetic reprogramming of NK cells leading to their adaptive differentiation, commonly characterized as the NKG2C+ and/or FcεRγ− phenotype; this typically leads to impaired natural cytotoxicity, but normal or enhanced ADCC.11,12 Whether other chronic viral infections may cause a similar response in patients is currently unknown, but EBV was ruled out in 1 recent study.15 EBV infects a significant proportion of the general population, but many individuals who are unable to control EBV are also coinfected with CMV or HIV, thus making it difficult to determine the impact of EBV alone. In that respect, XLP is unusual in that the patients may be highly susceptible, specifically to EBV, and are unable to clear the infection.
On this basis, we explored whether patients 1 and 2 (who significantly both remained CMV−; Figure 1A) had developed the adaptive-like NK phenotype. Indeed, we found that patient 1, who had several bouts of EBV-triggered HLH, had a significant and persistent increase of the FcεRγ− population of NK cells, and patient 2, who only had a single episode of EBV-triggered HLH, had a more modest, but persistent, increase (Figure 2E). In contrast, patient 3, who never experienced EBV-induced HLH, had a level of the FcεRγ− population of NK cells similar to controls (Figure 2E). Similarly, the proportion of NKG2C+ NK cells was increased whereas NKG2A+ NK cells were decreased, consistent with the phenotype of the FcεRγ− NK cells being predominately NKG2C+NKG2A− (Figure 2E).13,14 On the basis of these findings, we predicted that in contrast to their severely diminished natural cytotoxicity, patients 1 and 2 might have significant NK-mediated ADCC.11,12 Indeed, we found that NK-driven ADCC considerably improved in the presence of rituximab (Figure 2F), potentially explaining the strong clinical response to the drug.
The current study explains a commonly observed dichotomy of NK function in XLP2 patients, where individuals with the same genotype may present with a broad range of NK cytotoxicity, ranging from normal to severely diminished. Our discovery of EBV-induced adaptive-like NK differentiation expands the repertoire of viral infections that may cause this transformation, and also provides a rational explanation for how XLP patients can overcome chronic infection through NK-dependent ADCC of infected target cells.6 Finally, it is theoretically possible that early intervention may prevent/reduce the frequency/severity of HLH episodes by reducing the chance of persistent/permanent adaptive NK transformation. It remains to be seen whether these observations remain consistent across a larger cohort of XLP patients.
The online version of this article contains a data supplement.
Acknowledgments
This work was supported by National Health and Medical Research Council of Australia project grant 1062990 and fellowship 1059126 (both I.V.), and project grant 1048536 (A.J.).
Authorship
Contribution: S.O., A.C.H., A.J., and I.V. designed and conducted experiments and cowrote the manuscript; K.T., S.O., A.Y., B.R., G.M., J.S., and A.P.S. observed and treated the patients; M.C., G.R., P.B., and T.N. conducted experiments; and J.A.T. designed the study and cowrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ilia Voskoboinik, Peter MacCallum Cancer Centre, St. Andrews Place, Melbourne, VIC 3002, Australia; e-mail: ilia.voskoboinik@petermac.org; or Anthony Jaworowski, Burnet Institute, Melbourne, VIC 3004, Australia; e-mail: anthony.jaworowski@burnet.edu.au.
REFERENCES
Author notes
S.O., A.C.H., and K.T. contributed equally to this work.
A.J. and I.V. are joint senior authors.

![Figure 2. NK cells of XLP2 patients had impaired natural cytotoxicity and developed adaptive-like phenotype. (A) Cytotoxic activity of NK cells in patients 1 and 2 at 24 years of age, when they were diagnosed with XLP2 (see timeline in Figure 1). Shown is the result of the 51Cr-release assay (for clarity, error bars are not shown). (B) Memory B-cell phenotype (CD3−CD19+C20+CD27+) at the first onset of HLH, 6 years later (at the time of XLP2 diagnosis, aged 24 years), and 1.5 years after the diagnosis (aged 25.5 years). (C) Cytotoxic activity of the NK cells of patients 1 and 2, 1 year after XLP2 diagnosis. Shown are the 51Cr-release assay (for clarity, error bars are not shown) and the CD107a degranulation assay. (D) Cytotoxic activity of CD8+ T cells (please see supplemental Methods for details) as measured by 51Cr-release assay (mean ± standard error of the mean [SEM], n = 3) and CD107a degranulation assay. Tests were done at the same time as in panel C. (E) NK cells of patient 1 and 2, but not patient 3, developed adaptive-like phenotype (for gating strategy, please see supplemental Figure 4). NK cells in patients 1/2 were assessed 1 year (as in panel C) and 1.5 years after the diagnosis (depicted by gray and blue bars, respectively), and in patient 3 – 1.75 years after the diagnosis. Shown is mean ± standard deviation (SD) of 10 (FcεRγ−) or 11 (NKG2C+ and NKG2A+) controls. There was a similar trend for NKG2A+ and NKG2C+ cells, when gated on both CD16+CD56high and CD16+CD56dim NK cells (data not shown). (F) NK cells from patients 1 and 2 show enhanced response to rituximab (RTX)-treated Raji cells through ADCC. E/T, effector/target ratio.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/131/6/10.1182_blood-2017-08-803668/4/m_blood803668f2.jpeg?Expires=1764953969&Signature=l47u54ZIlvBGxI2qmSxNSVcNf9-MBrQ7q4~B6E2J2ZoFZeqX6MAiSA74P7B8o7yqhlVOzSfWVcEBreKKFTBW10Y37Naj6NH0E2UM3cbgLT0V1TvkXdisHv8q62BYsyiJUOzZJqinR7j9xOI9F1YaPDMSTVF4ss3pS5LAgUG028WqyXssmNwMwLPtyJ1Thx6Ok7AcE9i6WiWzXGcMaeRBZsXg7lip2xIwD9dfbnDAUfo41Hel8L9omwo15AcYgCTqgdwVMiIHtyHvDv1qQStMZOUsFkgo68D0XDsW1~XfG5CLxsXegg2ofIc2MY3klqvU-~supn~4yKxE9VJ62O8FUQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal