Key Points
Myosin IIa is required for cAMP-mediated endothelial VWF secretion critical for hemostasis and thrombosis.
Myosin IIa regulates mature WPB positioning and facilitates WPB exocytosis via zyxin-mediated actin framework formation before fusion.
Abstract
Nonmuscle myosin II has been implicated in regulation of von Willebrand factor (VWF) release from endothelial Weibel-Palade bodies (WPBs), but the specific role of myosin IIa isoform is poorly defined. Here, we report that myosin IIa is expressed both in primary human endothelial cells and intact mouse vessels, essential for cyclic adenosine monophosphate (cAMP)-mediated endothelial VWF secretion. Downregulation of myosin IIa by shRNAs significantly suppressed both forskolin- and epinephrine-induced VWF secretion. Endothelium-specific myosin IIa knockout mice exhibited impaired epinephrine-stimulated VWF release, prolonged bleeding time, and thrombosis. Further study showed that in resting cells, myosin IIa deficiency disrupted the peripheral localization of Rab27-positive WPBs along stress fibers; on stimulation by cAMP agonists, myosin IIa in synergy with zyxin promotes the formation of a functional actin framework, which is derived from preexisting cortical actin filaments, around WPBs, facilitating fusion and subsequent exocytosis. In summary, our findings not only identify new functions of myosin IIa in regulation of WPB positioning and the interaction between preexisting cortical actin filaments and exocytosing vesicles before fusion but also reveal myosin IIa as a physiological regulator of endothelial VWF secretion in stress-induced hemostasis and thrombosis.
Introduction
Endothelial exocytosis is one of the first lines of protection against vascular injury. Weibel-Palade bodies (WPBs),1 endothelium-specific secretory granules, deliver hemostatic and inflammatory mediators in response to a variety of agonists.2 The most abundant cargo in WPBs is von Willebrand factor (VWF), a multimeric glycoprotein that mediates platelet adhesion to the vascular wall and platelet aggregation, as well as serving as a plasma carrier for factor VIII.3 Impaired production and secretion of VWF cause von Willebrand disease.4 The cyclic adenosine monophosphate (cAMP)-mediated endothelial VWF secretion is a fundamental process in regulating plasma VWF level in response to epinephrine under physiological conditions.5-7 This mechanism has been exploited therapeutically using VWF-raising drugs, such as desmopressin, a synthetic analog of vasopressin, to treat patients with VWD. Despite the importance of this pathway, how cAMP signaling controls the behaviors of secretory WPBs, such as peripheral distribution, docking, priming, and fusion, remains largely unclear.
WPBs are the large secretory organelles, which have a diameter of 0.1 to 0.3 μm and length of 1 to 5 μm.1,3 Recent studies using live-cell imaging in culture cells demonstrate that the exocytosis of large vesicles including WPBs requires the contraction of the actomyosin coat to expel cargo content.8,9 Further studies using time-resolved 3-dimensional imaging visualized actomyosin-dependent vesicle compression and dissected the molecular mechanism of the actin coat formation in an animal model.10,11 All these findings represent a new function for actin: after granule fusion with the plasma membrane, a dynamic actin coat is de novo generated via actin polymerization and myosin II is rapidly recruited or assembled on the surface of the fused organelle.12 However, the role of myosin II in regulation of WPB behavior before fusion remains elusive, likely because of the limitation of conventional microscopy employed in these studies. Very recently, using live-cell superresolution microscopy,13 we visualized the dynamic interaction between fine actin filaments and WPBs and found previously unappreciated reorganization of preexisting actin filaments around WPBs before fusion. In addition, we show that focal adhesion protein zyxin mediates the formation of actin frameworks on exocytic WPBs, facilitating fusion.14
Despite this progress, the specific role of myosin IIa isoform in actomyosin II-mediated VWF secretion remains to be defined. In an elegant study, myosin II activity was convincingly shown to be required for VWF release from fused WPBs, but myosin IIa isoform was hardly detected in the endothelial cells (ECs) used.9 Here, we report that myosin IIa plays a fundamental role in the in vitro and in vivo release of VWF from both human and mouse endothelial cells, which is indispensable for stress-induced hemostasis and thrombosis. Under quiescent condition, myosin IIa is critically required for proper peripheral distribution of mature WPBs, whereas on stimulation, it contributes to the formation of a zyxin-mediated framework around exocytosing granules before fusion, facilitating cAMP-mediated WPB exocytosis.
Methods
Cell cultures
Human primary umbilical vascular ECs (HUVECs) were isolated, cultured, and maintained for VWF secretion assay, live-cell imaging with GFP-Lifeact and mCherry-PSL-lum, or immunofluorescence, as previously described.14 For acute blebbistatin inhibition, medium containing both blebbistatin (25 μmol/L) and forskolin (10 μmol/L) was added after forskolin stimulation.
DNA constructs, virus expression system, VWF enzyme-linked immunosorbent assay, and protein detection
The construct of wild-type (WT) myosin IIa was generated by amplifying gene-specific cDNA from HUVECs and ligating it into the pRetroQ-AcGFP vector (Addgene) with GFP deletion in the C terminal. The myosin IIa mutants at serines 1916 (S1916) and 1943 (S1943) were generated by overlap extension polymerase chain reaction, using WT myosin IIa as a template. The constructs of zyxin mutants were previously described.14 The Rab27A-GFP was amplified from HUVEC cDNAs and ligated into the pRetroQ-AcGFP vector. The overexpression and shRNA-expressing viruses infecting HUVECs were prepared as before for subsequent protein expression analysis and VWF enzyme-linked immunosorbent assay.14 The lysates of 3 parallel control wells of HUVECs were harvested for total VWF level measurement. Basal and stimulated release in supernatants was presented as a percentage of the total VWF present in the cells. For the measurement of plasma VWF, pooled plasma from 5 C57BL/6 WT mice was used as a reference (100%), and results were expressed as percentage of WT values.15
Antibodies, immunofluorescence staining, and imaging
The following antibodies were used for Western blotting and immunoprecipitation: rabbit polyclonal antibody to ERK2 (Santa Cruz, sc-292838), rabbit antiserum against P85 was obtained as preciously described,16 rabbit polyclonal antibody to myosin IIa (Sigma, M8064), rabbit polyclonal antibody to phospho-myosin IIa S1916 (CST, MP5191), rabbit polyclonal antibody to phospho-myosin IIa S1943 (CST, MP5026), rabbit polyclonal antibody to myosin IIb (Covance, PRB-445P-100), rabbit monoclonal antibodies to zyxin (Abcam, ab109316), mouse monoclonal antibody to flag (Sigma, F3165), mouse monoclonal antibody to CK2α (Santa Cruz, SC-12738) and CK2β (Santa Cruz, SC-12739), rabbit polyclonal antibody to phospho-CK2 substrate (CST, 8738), rabbit polyclonal antibody to myosin IIc (Biolegend, PRB-444P), rabbit monoclonal antibody to myosin IIc (CST, 8189), and HRP-conjugated secondary antibodies (anti-rabbit NA9340 and anti-mouse NA9310, GE). Confluent ECs with or without forskolin stimulation (10 mmol/L) were washed, fixed, and immunostained as previously described.14 Actin filaments were labeled with Alexa Fluor 488-conjugated phalloidin (A12379), along with secondary antibodies for 1 hour at room temperature.
EC-specific myosin IIa-knockout mice (VEcreERT2/myh9loxp/loxp)
A mouse line expressing tamoxifen-inducible Cre-recombinase (VEcreERT2) regulated by the vascular endothelial cadherin promoter on a C57BL/6J background was used.17 Inducible endothelial myosin IIa-specific knockout mice (iEMKO; VEcreERT2/myh9loxp/loxp) were generated by mating the VEcreERT2 mice with myosin IIa loxp mice (myh9loxp/loxp, Jackson Laboratories). iEMKO mice were given tamoxifen (1 mg per mouse per day) for 5 days by intraperitoneal injection. The experiments were performed from days 10 to 14 after induction. Whole-mount staining of mouse ears was used to check the knockout efficiency of myosin IIa in ECs, using CD31 antibody (BD, 1:500) and myosin IIa antibody (Sigma, 1:500) for staining ECs and myosin. Ears were placed on coverslips for confocal imaging.
Bleeding time and thrombus formation
Ten-week-old mice were stimulated with epinephrine (0.5 mg/kg), and the measurement of bleeding time and the thrombus formation happened according to the methods described earlier.14 All animal procedures were carried out according to the rules of the Association for Assessment and Accreditation of Laboratory Animal Care International and were approved by the Animal Care and Use Committee of Peking University.
Statistics
All experiments were performed in at least 3 independent experiments. Data were expressed as mean ± standard error of the mean or mean ± standard deviation. For statistical analysis, the Prism Version 5.0 software (GraphPad Software) was used. Statistical differences were assessed with an unpaired 2-tailed Student t test when only 2 groups were compared. Values of P < .05 were considered statistically significant.
Results
Myosin IIa is required for cAMP-mediated VWF secretion from endothelial cells
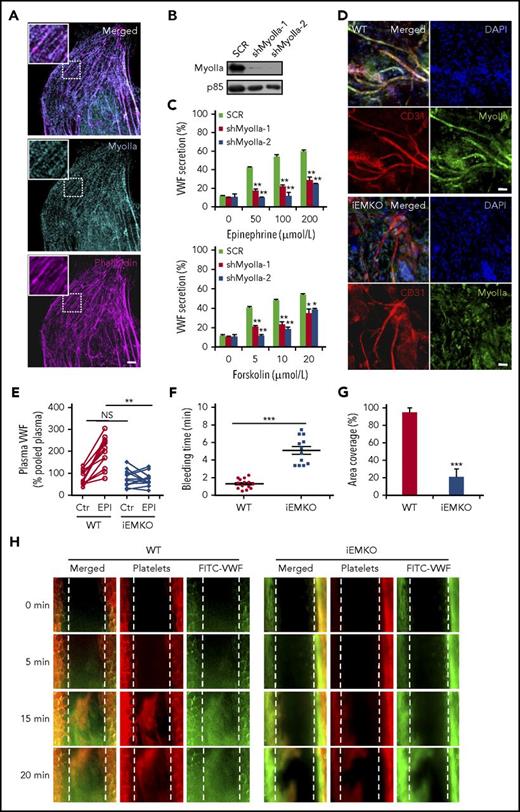
In mammals, 3 genes encode 3 nonmuscle myosin II isoforms: myosin IIa (Myh9), IIb (Myh10), and IIc (Myh14).18 There is a controversy in the expression level of myosin IIa in human primary umbilical vascular endothelial cells (HUVECs).9,18,19 We thus examined the expression of myosin IIa using immunostaining and western blot. As shown in Figure 1A, immunostaining showed a typical myosin II pattern predominantly colocalized with actin cytoskeleton. In addition, myosin IIa protein was easily detected in the lysates of HUVECs (Figure 1B). We then studied the role of myosin IIa in cAMP-mediated VWF secretion from HUVECs, using cAMP-elevating agonists (epinephrine, a hormone released under physiological conditions,20 and forskolin). The short-hairpin RNAs (shRNAs) of myosin IIa (shMyoIIa), which did not affect the expression of either myosin IIb or myosin IIc that was not detectable in HUVECs (supplemental Figure 1, available on the Blood Web site), significantly reduced both forskolin- and epinephrine-induced VWF secretion (Figure 1C).
Myosin IIa is required for cAMP-mediated VWF secretion from endothelial cells both in vitro and in vivo. (A) Immunostaining of myosin IIa (cyan) and f-actin (magenta) in HUVECs (scale bar, 5 μm). (B) The western blot of the knockdown efficiency of the shRNAs against myosin IIa (shMyoIIa-1 and shMyoIIa-2). The p85 protein was used as a loading control. (C) VWF secretion from HUVECs expressing scrambled (SCR) or myosin IIa shRNAs (shMyoIIa-1 and shMyoIIa-2) with stimulation of forskolin or epinephrine at the indicated concentrations (n = 12; *P < .05, **P < .01). (D) Whole-mount staining to check the knockout (KO) efficiency of myosin IIa in the iEMKO mouse. Mouse ears were fixed and stained with anti-myosin IIa (green) and anti-CD31 (red) to label vascular endothelial cells (scale bars, 60 μm). (E) Normalized plasma levels of VWF in WT (n = 12) and iEMKO (n = 12) mice before (NS > 0.05) and after epinephrine (EPI) stimulation (**P < .01). Results were expressed as percentage of the value of pooled plasma from 5 WT mice. (F) Bleeding times in WT (n = 14) and iEMKO (n = 12) mice after epinephrine stimulation (***P < .001). (G) The graph showed the coverage of the area by thrombus in (H) at 20 min. (***P < .001; mean ± standard error of the mean). (H) FeCl3-induced thrombus formation in mesenteric vessels of WT (n = 10) and iEMKO (n = 8) mice at different points. Thrombus indicated by rhodamine-labeled platelets and fluorescein isothiocyanate (FITC)-conjugated anti-VWF antibody. All error bars represent standard deviation.
Myosin IIa is required for cAMP-mediated VWF secretion from endothelial cells both in vitro and in vivo. (A) Immunostaining of myosin IIa (cyan) and f-actin (magenta) in HUVECs (scale bar, 5 μm). (B) The western blot of the knockdown efficiency of the shRNAs against myosin IIa (shMyoIIa-1 and shMyoIIa-2). The p85 protein was used as a loading control. (C) VWF secretion from HUVECs expressing scrambled (SCR) or myosin IIa shRNAs (shMyoIIa-1 and shMyoIIa-2) with stimulation of forskolin or epinephrine at the indicated concentrations (n = 12; *P < .05, **P < .01). (D) Whole-mount staining to check the knockout (KO) efficiency of myosin IIa in the iEMKO mouse. Mouse ears were fixed and stained with anti-myosin IIa (green) and anti-CD31 (red) to label vascular endothelial cells (scale bars, 60 μm). (E) Normalized plasma levels of VWF in WT (n = 12) and iEMKO (n = 12) mice before (NS > 0.05) and after epinephrine (EPI) stimulation (**P < .01). Results were expressed as percentage of the value of pooled plasma from 5 WT mice. (F) Bleeding times in WT (n = 14) and iEMKO (n = 12) mice after epinephrine stimulation (***P < .001). (G) The graph showed the coverage of the area by thrombus in (H) at 20 min. (***P < .001; mean ± standard error of the mean). (H) FeCl3-induced thrombus formation in mesenteric vessels of WT (n = 10) and iEMKO (n = 8) mice at different points. Thrombus indicated by rhodamine-labeled platelets and fluorescein isothiocyanate (FITC)-conjugated anti-VWF antibody. All error bars represent standard deviation.
Endothelium-specific deficiency of myosin IIa leads to impaired cAMP-induced VWF release into plasma and prolonged bleeding time in mice
We set out to study the role of myosin IIa-mediated endothelial VWF secretion in vascular homeostasis.15,21 Because myosin IIa-deficient mice die at embryonic days 6.5 to 7, accompanied by a failure to organize normal germ layers,22 we used tamoxifen-iEMKO mice (VEcreERT2/myh9loxp/loxp).17,23 When ECs from the ears of WT and iEMKO mice were identified using CD31, a pan-vascular endothelial marker, whole-mount staining showed a high expression level of myosin IIa in vascular ECs of the WT mouse ear, which was consistent with the results in HUVECs. As shown in Figure 1D and supplemental Figure 2, myosin IIa was efficiently depleted in ECs of iEMKO mice. The VWF enzyme-linked immunosorbent assay results showed that depletion of myosin IIa significantly suppressed epinephrine-induced VWF release (Figure 1E). Consistently, the bleeding times of the mouse tail vein after epinephrine stimulation were markedly longer in iEMKO mice than the controls (Figure 1F). Similarly, after epinephrine stimulation, compared with control mice, the FeCl3-induced thrombus formation of mesenteric vessels in iEMKO mice was significantly impaired, as shown by rhodamine-labeled platelets (Figure 1G-H).
In summary, these data demonstrate that myosin IIa-mediated endothelial VWF secretion is essential for homeostasis and thrombosis in response to injury.
Myosin IIa, but not myosin IIb, is required for the peripheral distribution of WPBs
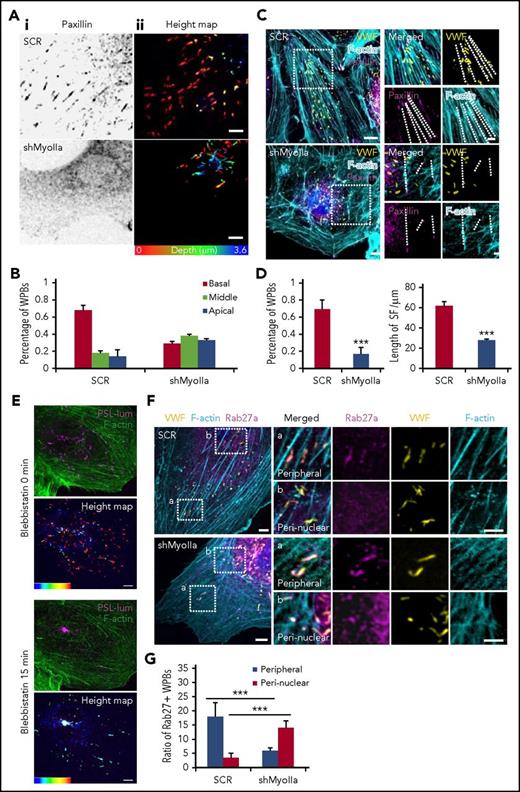
We then continued to study the mechanism underlying myosin IIa-mediated endothelial VWF secretion. Because only the peripheral located WPBs are secreted from ECs in response to cAMP agonists,24 we first examined the effect of myosin IIa deficiency on the distribution of WPBs. Interestingly, the peripheral distribution of WPBs was disrupted in the ECs deficient in myosin IIa (Figure 2A-B), but not myosin IIb (supplemental Figure 3). In normal HUVECs, 70% of WPBs were localized in the peribasal membrane region, whereas in myosin IIa-depleted cells, most WPBs were distributed in the perinuclear region. WPBs have been reported to be localized along focal adhesions (FAs) anchored to stress fibers (SFs) in normal HUVECs. As shown in Figure 2C-D, in contrast to the high ratio of WPBs (68%) localized along FA-anchored SFs in scrambled cells, less than 20% of WPBs were localized along SFs, which was relatively shorter than normal cells after the downregulation of myosin IIa. Furthermore, inhibition of myosin II activity by blebbistatin led to the decreased number of WPBs along SFs, accompanied with reduced number and length of FAs and shorter SFs (supplemental Figure 4). To visualize WPB movement within the cortical actin network, serum-starved HUVECs coexpressing GFP-Lifeact (an actin-binding peptide to monitor the behavior of actin filaments25 ) and mCherry-PSL-lum (a fusion protein of the luminal part of P-selectin with mCherry at the C-terminus14 ), a well-characterized combination for imaging actin structures and WPBs simultaneously,26 were imaged in real time by spinning disk confocal microscopy. By real-time imaging, we found that the inhibition of myosin II activity resulted in the translocation of WPBs from peripheral regions to the perinuclear regions (Figure 2E; supplemental Movie 1).
Myosin IIa, but not myosin IIb, is required for the peripheral distribution of Rab27a-positive WPBs. (Ai) Immunostaining of paxillin (black) in HUVECs expressing scrambled (SCR) and shRNA targeting myosin IIa (shMyoIIa). (Aii) Spinning disk confocal microscopy of the height map (depth was defined as the distance of WPBs to the basal membrane) of WPBs relative to the basal membrane in these HUVECs. Height of the basal membrane was defined as 0 μm (red), and the apical membrane was indicated in blue. (B) Statistics for the height of WPBs as in A. The height of WPBs was evenly divided into 3 layers: basal (red), middle (green), and apical (blue). (C) Representative images of immunostaining of VWF (yellow), F-actin (cyan), and paxillin (magenta) in cells expressing SCR and shMyoIIa. Dashed lines indicated FA-anchored SFs. (D) Percentage of WPBs along stress fibers and length of FA-anchored SFs, as in D. **P < .01, ***P < .001. (E) Representative real-time images and height maps with blebbistatin treatment in cells expressing P-selectin-lum-mCherry (PSL-lum-mCherry) and Lifeact-GFP. (F) Representative images of immunostaining of VWF (yellow), f-actin (cyan), and Rab27a (magenta) in cells expressing SCR and shMyoIIa. The magnified panels showed the peripheral region (a) and the peri-nuclear region (b). The peripheral region was defined as the region whose distance to nuclear was more than 10 μm. Arrows indicate the Rab27a-positive WPBs and arrowheads indicate the Rab27a-negative WPBs. (G) Quantitative analysis of the number of Rab27a-positive WPBs in perinuclear and peripheral regions (mean ± standard deviation. ***P < .001, Student’s t-test (scale bars, 5 μm; mean ± standard deviation).
Myosin IIa, but not myosin IIb, is required for the peripheral distribution of Rab27a-positive WPBs. (Ai) Immunostaining of paxillin (black) in HUVECs expressing scrambled (SCR) and shRNA targeting myosin IIa (shMyoIIa). (Aii) Spinning disk confocal microscopy of the height map (depth was defined as the distance of WPBs to the basal membrane) of WPBs relative to the basal membrane in these HUVECs. Height of the basal membrane was defined as 0 μm (red), and the apical membrane was indicated in blue. (B) Statistics for the height of WPBs as in A. The height of WPBs was evenly divided into 3 layers: basal (red), middle (green), and apical (blue). (C) Representative images of immunostaining of VWF (yellow), F-actin (cyan), and paxillin (magenta) in cells expressing SCR and shMyoIIa. Dashed lines indicated FA-anchored SFs. (D) Percentage of WPBs along stress fibers and length of FA-anchored SFs, as in D. **P < .01, ***P < .001. (E) Representative real-time images and height maps with blebbistatin treatment in cells expressing P-selectin-lum-mCherry (PSL-lum-mCherry) and Lifeact-GFP. (F) Representative images of immunostaining of VWF (yellow), f-actin (cyan), and Rab27a (magenta) in cells expressing SCR and shMyoIIa. The magnified panels showed the peripheral region (a) and the peri-nuclear region (b). The peripheral region was defined as the region whose distance to nuclear was more than 10 μm. Arrows indicate the Rab27a-positive WPBs and arrowheads indicate the Rab27a-negative WPBs. (G) Quantitative analysis of the number of Rab27a-positive WPBs in perinuclear and peripheral regions (mean ± standard deviation. ***P < .001, Student’s t-test (scale bars, 5 μm; mean ± standard deviation).
Together, these results indicate that myosin IIa and its activity are necessary for maintaining the peripheral location of WPBs.
Myosin IIa-dependent peripherally located WPBs are Rab27a-positive
It is known that mature WPBs are located in the periphery of cells, whereas immature WPBs otherwise accumulate around the nucleus.19,20,27 The small GTPase Rab27a is a marker of mature WPBs.28,29 We then examined the expression of Rab27a in myosin IIa-dependent peripherally distributed WPBs. Indeed, immunostaining showed that Rab27a-positive WPBs were mostly localized in the periphery of cells expressing the scrambled shRNA, whereas they translocated to the perinuclear region in myosin IIa-deficient cells. Notably, the total number of Rab27a-positive WPBs was not significantly altered in scrambled and myosin IIa-depleted cells, suggesting that myosin IIa does not influence the maturation of WPBs (Figure 2F-G). These data suggested that myosin IIa is crucial for the distribution of mature WPBs. Consistently, real-time imaging showed that blebbistatin treatment led to the translocation of Rab27a-positive granules from the periphery to the perinuclear region (supplemental Figure 5A). In addition, the main pool of peripheral Rab27a-positive WPBs underwent successful exocytosis on activation (supplemental Figure 5B-C). These results showed that myosin IIa is required for maintaining the peripheral distribution of Rab27a-positive mature WPBs, which is essential for the subsequent exocytosis.
Myosin IIa-deleted ECs exhibit impaired mature WPB distribution and decreased cAMP-induced VWF secretion
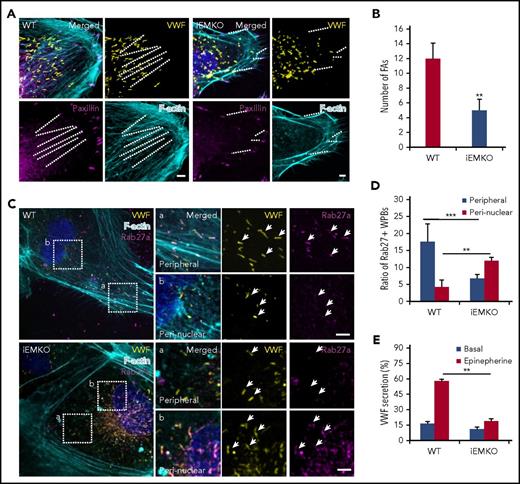
To directly assess the role of myosin IIa in regulation of the distribution and secretion of endothelial WPBs in iEMKO mice, we isolated cardiac ECs from WT and iEMKO mice. Immunostaining showed that the FAs and FA-anchored SFs were impaired in the ECs from iEMKO mice (Figure 3A-B). Myosin IIa-deficient mouse ECs displayed perinuclear distribution of Rab27a-positive WPBs (Figure 3C-D), consistent with earlier findings in HUVECs. We further examined the effect of myosin IIa on VWF secretion from cardiac ECs induced by epinephrine. The cAMP-induced VWF release was abolished in cardiac ECs isolated from iEMKO mice (Figure 3E).
Myosin IIa-deleted ECs exhibit impaired mature WPB distribution and decreased cAMP-induced VWF secretion. (A) Cardiac endothelial cells isolated from WT and iEMKO mice and immunostained with antibodies against VWF (yellow) and paxillin (magenta). F-actin was labeled with phalloidin (cyan). (Right) Magnified insets. (B) Quantitative analysis of the number of FAs per cell as in A (n = 15; **P < .01, 3 independent experiments). (C) Confocal images of WT and iEMKO CECs immunostained with antibodies against VWF (yellow) and Rab27a (magenta). F-actin was labeled with phalloidin (cyan). Arrows indicate WPBs. (Right) Magnified insets. (D) Number of Rab27a-positive WPBs along stress fibers as in C (**P < .01; ***P < .001). (E) VWF secretion in CECs isolated from WT and iEMKO mice with epinephrine stimulation (n = 12; **P < .01, Student’s t-test; mean ± standard deviation; scale bars, 5 μm).
Myosin IIa-deleted ECs exhibit impaired mature WPB distribution and decreased cAMP-induced VWF secretion. (A) Cardiac endothelial cells isolated from WT and iEMKO mice and immunostained with antibodies against VWF (yellow) and paxillin (magenta). F-actin was labeled with phalloidin (cyan). (Right) Magnified insets. (B) Quantitative analysis of the number of FAs per cell as in A (n = 15; **P < .01, 3 independent experiments). (C) Confocal images of WT and iEMKO CECs immunostained with antibodies against VWF (yellow) and Rab27a (magenta). F-actin was labeled with phalloidin (cyan). Arrows indicate WPBs. (Right) Magnified insets. (D) Number of Rab27a-positive WPBs along stress fibers as in C (**P < .01; ***P < .001). (E) VWF secretion in CECs isolated from WT and iEMKO mice with epinephrine stimulation (n = 12; **P < .01, Student’s t-test; mean ± standard deviation; scale bars, 5 μm).
Myosin II activity is required for cAMP-mediated formation of functional actin framework around WPBs before fusion and subsequent exocytosis
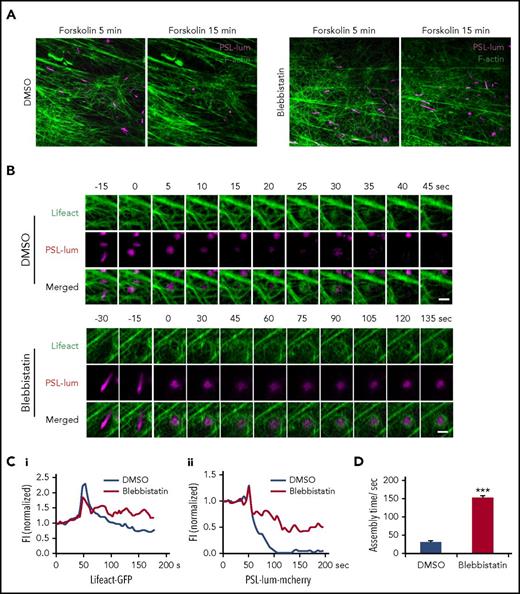
To capture the role of myosin II in regulation of spatiotemporal interaction of exocytosing WPBs with cortical actin filaments, we used superresolution live-cell microscopy13 to image exocytotic behaviors in cells expressing mCherry-PSL-lum and GFP-Lifeact. To avoid the interference of the function of myosin on the early stage of exocytosis, we added blebbistatin after forskolin stimulation. Strikingly, peripheral WPBs were mostly retained in HUVECs treated with blebbistatin in the presence of forskolin, whereas most of the peripheral WPBs were exocytosed in HUVECs treated with dimethyl sulfoxide (DMSO; Figure 4A). These data suggested that the activity of myosin II is critical to the successful exocytosis for the peripheral distributed WPBs.
Myosin II activity is required for cAMP-mediated formation of functional actin framework around WPBs before fusion and subsequent exocytosis. (A) Representative images showing VWF secretion with acute DMSO or blebbistatin treatment in the presence of forskolin in HUVECs coexpressing PSL-lum-mCherry (PSL-lum) and Lifeact-GFP (Lifeact). (B) Representative time-courses of actin framework formation in cells with acute DMSO or blebbistatin treatment. (C) Normalized fluorescence intensity (FI) of Lifeact-GFP around exocytosing WPBs (Ci) and of PSL-lum-mCherry (Cii) in DMSO- and blebbistatin-treated cells. (D) Assembly times for the actin framework as in B. ***P < .001; scale bars, 5 μm in A, 1 μm in E (mean ± standard deviation; Student’s t-test).
Myosin II activity is required for cAMP-mediated formation of functional actin framework around WPBs before fusion and subsequent exocytosis. (A) Representative images showing VWF secretion with acute DMSO or blebbistatin treatment in the presence of forskolin in HUVECs coexpressing PSL-lum-mCherry (PSL-lum) and Lifeact-GFP (Lifeact). (B) Representative time-courses of actin framework formation in cells with acute DMSO or blebbistatin treatment. (C) Normalized fluorescence intensity (FI) of Lifeact-GFP around exocytosing WPBs (Ci) and of PSL-lum-mCherry (Cii) in DMSO- and blebbistatin-treated cells. (D) Assembly times for the actin framework as in B. ***P < .001; scale bars, 5 μm in A, 1 μm in E (mean ± standard deviation; Student’s t-test).
Previously, we found that cAMP-mediated WPB exocytosis requires zyxin-mediated formation of an actin framework around exocytosing granules, which facilitates fusion and secretion (supplemental Movie 2).14 Interestingly, in forskolin-stimulated HUVECs under acute exposure to blebbistatin, the actin framework around exocytosing WPBs was so weak and unstable that it was unable to complete exocytosis over a prolonged assembly process (Figure 4B-D; supplemental Movie 3). This result suggests that myosin II is critical to the formation of a functional actin framework in promotion of WPB exocytosis. Interestingly, the formation of the actin framework before fusion was closely associated with the translocation of WPBs to the plasma membrane, as indicated by a sudden increment of the fluorescence intensity of PSL-lum-mCherry (Figure 4C). The inhibition of myosin II activity resulted in an oscillation in the fluorescence intensity of PSL-lum-mCherry (Figure 4C), suggesting that this inhibition reduces the translocation of WPBs close to the plasma membrane.
Collectively, these results showed that myosin II is critical to the formation of functional actin framework around cAMP-mediated exocytotic WPB, facilitating fusion and subsequent cargo release.
Interaction between myosin IIa and zyxin is required for cAMP-mediated actin framework formation and WPB exocytosis
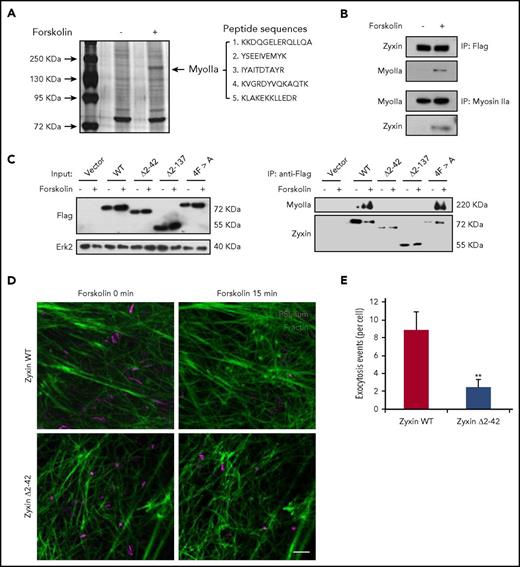
Because zyxin is critical for the formation of an actin framework around granules before fusion,14 we speculated that myosin IIa may interact with zyxin in regulating the formation of an actin framework associated with exocytosing WPBs. Strikingly, mass spectrometry identified myosin IIa as the most abundant interacting protein of zyxin in HUVECs overexpressing zyxin-flag in response to forskolin (Figure 5A). Immunoprecipitation confirmed the interaction between zyxin and myosin IIa (Figure 5B), but did not detect any interaction with myosin IIb (supplemental Figure 6).
Interaction between myosin IIa and zyxin is required for cAMP-mediated actin framework formation and WPB exocytosis. (A) Silver-stained gels showing immunoprecipitates from HUVECs with or without forskolin stimulation. Peptide sequences derived from the myosin IIa band (MyoIIa) were obtained by mass spectrometry. (B) Immunoprecipitation between myosin IIa and zyxin using HUVECs (bottom) or zyxin-flag-overexpressing HUVECs (upper). The immunoprecipitates were immunoblotted with either zyxin antibody (zyxin) or myosin IIa antibody (MyoIIa). (C) Immunoprecipitation of myosin IIa with the indicated antibodies using lysates of HUVECs expressing WT or the zyxin, zyxin Δ2-42, zyxin Δ2-137, or 4FA mutant with forskolin stimulation. (D) HUVECs coexpressing mCherry-PSL-lum (PSL-lum, magenta) and Lifeact-GFP (F-actin, green) were infected with shRNAs against zyxin (shZyxin), followed by introduction of WT or the zyxin Δ2-42 mutant. Images show the basal state (0 min) and the last imaging points (15 min; n = 4; 2 independent experiments). (E) Average numbers of exocytotic events in cells used in D (n = 4; **P < .01; 2 independent experiments; scale bars, 5 μm in A, B, and E, 1 μm in insets; mean ± standard deviation; Student’s t-test).
Interaction between myosin IIa and zyxin is required for cAMP-mediated actin framework formation and WPB exocytosis. (A) Silver-stained gels showing immunoprecipitates from HUVECs with or without forskolin stimulation. Peptide sequences derived from the myosin IIa band (MyoIIa) were obtained by mass spectrometry. (B) Immunoprecipitation between myosin IIa and zyxin using HUVECs (bottom) or zyxin-flag-overexpressing HUVECs (upper). The immunoprecipitates were immunoblotted with either zyxin antibody (zyxin) or myosin IIa antibody (MyoIIa). (C) Immunoprecipitation of myosin IIa with the indicated antibodies using lysates of HUVECs expressing WT or the zyxin, zyxin Δ2-42, zyxin Δ2-137, or 4FA mutant with forskolin stimulation. (D) HUVECs coexpressing mCherry-PSL-lum (PSL-lum, magenta) and Lifeact-GFP (F-actin, green) were infected with shRNAs against zyxin (shZyxin), followed by introduction of WT or the zyxin Δ2-42 mutant. Images show the basal state (0 min) and the last imaging points (15 min; n = 4; 2 independent experiments). (E) Average numbers of exocytotic events in cells used in D (n = 4; **P < .01; 2 independent experiments; scale bars, 5 μm in A, B, and E, 1 μm in insets; mean ± standard deviation; Student’s t-test).
Furthermore, to find the specific binding domain for myosin IIa in zyxin, several zyxin constructs (WT zyxin), the mutants Δ2-42 (deletion of amino-acids 2-42), Δ2-137 (deletion of amino-acids 2-137), and 4F > A (replacement of 4 phenylalanines by alanines)30 were introduced into HUVECs expressing shRNA against zyxin (shZyxin). Immunoprecipitation showed that the Δ2-42 mutant failed to interact with myosin IIa, suggesting that the domain from amino acids 2 to 42 is critical for the interaction between myosin IIa and zyxin (Figure 5C). Consistently, the Δ2-42 mutant also failed to rescue the WPB exocytosis in shZyxin-expressing cells, indicating a requirement of the interaction between myosin IIa and zyxin for WPB exocytosis (Figure 5D-E).
Taken together, the cAMP-induced interaction between myosin IIa and zyxin through the 2-42-amino-acid domain is necessary for the formation of an actin framework around WPBs, and the subsequent exocytosis.
Phosphorylation of different myosin IIa sites plays distinct roles in regulation of VWF secretion
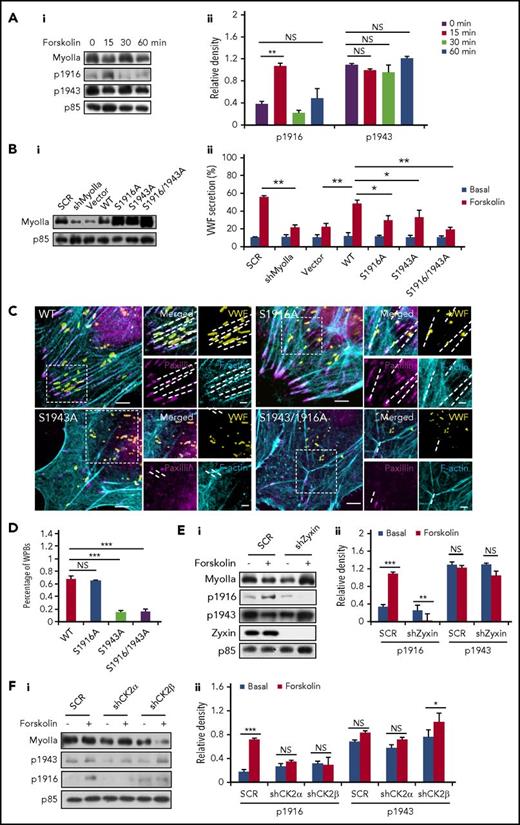
Phosphorylation sites at the tail of the myosin II heavy chain are important for regulating its function and cellular physiology.31,32 The phosphorylation of S1916 and S1943 is best studied for myosin IIa,31,32 and they have been reported to be involved in the translocation and secretion of granules.33,34 Western blot showed a significant increase in the phosphorylation of the S1916 on forskolin stimulation. Interestingly, the phosphorylation level of the S1943 remained unchanged after forskolin stimulation (Figure 6A; supplemental Figure 7).
Phosphorylation of different myosin IIa sites plays distinct roles in regulation of VWF secretion. (Ai) Western blot of phospho-myoIIa at S1916 (ppS1916) and S1943 (ppS1943) in HUVECs stimulated with forskolin for different points. (Aii) Ratio of phosphorylated myoIIa at S1916 (p1916) and S943 (p1943) to total myoIIa of left. (Bi) Western-blot of myosin mutants expressed in HUVECs. (Bii) Enzyme-linked immunosorbent assay of VWF secretion from HUVECs expressing SCR and shMyoIIa in the presence of forskolin, rescued by WT myosin IIa (WT) and the S1916A, S1943A, or S1916/1943A mutants of myosin IIa (n = 4; *P < .05, **P < .01, 3 independent experiments). (C) Immunostaining of VWF (yellow), F-actin (cyan), and paxillin (magenta) in HUVECs used in B. (D) Percentage of WPBs along the FA-anchored stress fibers in C. (Ei) Western blot of phospho-myoIIa at S1916 (p1916) and S1943 (p1943) in SCR and shZyxin HUVECs stimulated with forskolin for 15 minutes. (Eii) Ratio of p1916 and p1943 to total myoIIa of left. (Fi) Western blot of p1916 and p1943 in SCR HUVECs and shCK2 HUVECs stimulated with forskolin for 15 minutes. (Fii) Ratio of p1916 and p1943 to total myoIIa of left. (Scale bars, 5 μm in C; mean ± standard deviation; NS > 0.05, *P < .05, **P < .01, ***P < .001, Student’s t-test.)
Phosphorylation of different myosin IIa sites plays distinct roles in regulation of VWF secretion. (Ai) Western blot of phospho-myoIIa at S1916 (ppS1916) and S1943 (ppS1943) in HUVECs stimulated with forskolin for different points. (Aii) Ratio of phosphorylated myoIIa at S1916 (p1916) and S943 (p1943) to total myoIIa of left. (Bi) Western-blot of myosin mutants expressed in HUVECs. (Bii) Enzyme-linked immunosorbent assay of VWF secretion from HUVECs expressing SCR and shMyoIIa in the presence of forskolin, rescued by WT myosin IIa (WT) and the S1916A, S1943A, or S1916/1943A mutants of myosin IIa (n = 4; *P < .05, **P < .01, 3 independent experiments). (C) Immunostaining of VWF (yellow), F-actin (cyan), and paxillin (magenta) in HUVECs used in B. (D) Percentage of WPBs along the FA-anchored stress fibers in C. (Ei) Western blot of phospho-myoIIa at S1916 (p1916) and S1943 (p1943) in SCR and shZyxin HUVECs stimulated with forskolin for 15 minutes. (Eii) Ratio of p1916 and p1943 to total myoIIa of left. (Fi) Western blot of p1916 and p1943 in SCR HUVECs and shCK2 HUVECs stimulated with forskolin for 15 minutes. (Fii) Ratio of p1916 and p1943 to total myoIIa of left. (Scale bars, 5 μm in C; mean ± standard deviation; NS > 0.05, *P < .05, **P < .01, ***P < .001, Student’s t-test.)
To further examine their role in VWF secretion, 3 dominant-negative myosin IIa mutants (S1916A, S1943A, and S1916/S1943A, in which serines were changed to alanines to abolish phosphorylation) and 2 constitute activated myosin IIa mutants (S1916D and S1943D, in which 2 serines were changed to aspartic acid to mimic the phosphorylation) were introduced into shMyoIIa-expressing HUVECs, with the WT myosin IIa as a positive control. Remarkably, we found that, unlike WT myosin IIa, the 3 dominant-negative constructs did not significantly rescue the defect of VWF secretion in shMyoIIa-expressing HUVECs (Figure 6B). Triple staining showed that ∼85% of the WPBs in HUVECs expressing the S1943A or S1916/1943A mutant failed to localize along the FA-anchored stress fibers, whereas only 30% of WPBs in HUVECs expressing the S1916A mutant or WT myosin IIa were not distributed in the periphery along stress fibers (Figure 6C-D; supplemental Figure 8). These results indicate a critical role of S1943 in maintaining periphery distribution of WPBs, which was confirmed by the data that S1943D rescued WPB distribution (supplemental Figure 9). As shown in supplemental Figure 10, the peripheral number of WPBs in the S1916D rescued HUVECs was lower than the WT rescued HUVECs under quiescent condition and decreased further after forskolin stimulation, suggesting that the phosphorylation at S1916 promotes WPB exocytosis.
We then tested the role of zyxin in the phosphorylation of the myosin IIa heavy chain. Interestingly, the forskolin-stimulated phosphorylation of myosin IIa at S1916, but not S1943, was abolished in shZyxin-expressing HUVECs (Figure 6E). To identify the upstream kinase responsible for the cAMP-mediated phosphorylation of myosin IIa, using shRNAs, we first screened candidates described in the literature,31 with VWF secretion as a readout. Intriguingly, Casein kinase II (CK2), a tetramer consisting of 2 catalytic subunits (CK2α) and 2 regulatory subunits (CK2β),35 was identified as the most likely potential kinase mediating cAMP-induced VWF secretion (supplemental Figure 11A). Knockdown of both CK2α and CK2β significantly impaired the forskolin-induced phosphorylation of myosin IIa at S1916, but not S1943 (supplemental Figure 11B; Figure 6F). This result indicates that CK2 is responsible for forskolin-induced phosphorylation of the S1916, which is in contrast to the reports in which CK2 is responsible for the phosphorylation of the S1943.36 Consistently, immunoprecipitation showed an interaction between CK2α and myosin IIa (supplemental Figure 11C).
In summary, the phosphorylation of myosin IIa at S1943 is necessary for maintaining the peripheral distribution of WPBs under quiescent condition, whereas the phosphorylation of the S1916 is required for VWF secretion in a zyxin-dependent manner after forskolin stimulation.
Discussion
In this study, we show that myosin IIa is required for peripheral distribution of mature WPB both in human and mouse endothelial cells. Early studies demonstrated that on stimulation with cAMP agonists, only the peripheral WPBs are exocytosed,37,38 suggesting that both the quantity and quality of secreted VWF are tightly controlled. Full maturation of VWF occurs within WPB after their translocation from a perinuclear site of emergence at the trans-Golgi network to the cell periphery. In addition, Rab27a is involved in restricting immature WPB exocytosis.39 Consistent with these observations, we showed that the main pool of Rab27a-positive WPBs underwent successful exocytosis on activation (supplemental Figure 5B-C). Downregulation of myosin IIa led to the translocation of the Rab27a-positive WPBs to the perinuclear region. The bidirectional movement is a feature of WPBs in resting HUVECs.38,40,41 The inhibition of myosin II activity also impaired the peripheral location of WPBs (Figure 2E), indicating that the maintenance of mature WPB positioning requires basal myosin II activity that is at least mediated by phosphorylation of S1943 (Figure 6C). However, it is still unknown how myosin IIa regulates the translocation and positioning of mature WPBs. In the quiescent state, basal activity of myosin IIa mediates the formation of a tense cortical actin network anchored at mature FAs, which may contribute to the maintenance of a peripheral distribution of mature WPBs. Caution should be taken that the impairment of WPB positioning in myosin IIa-deficient ECs may be also caused by the global effect on cytoskeletal integrity resulting from a lack of myosin II activity.
In a very recent study, we showed that cAMP agonists induce zyxin-mediated formation of an actin framework, which is derived from preexisting cortical actin filaments, around exocytosing granules,14 facilitating fusion and secretion. Remarkably, acute inhibition of myosin II activity blocked WPB exocytosis even as an actin framework was formed (Figure 4B). Furthermore, forskolin induced the interaction of zyxin with myosin IIa, and this interaction was required for the formation of a functional actin framework and subsequent exocytosis (Figure 5). Mechanistically, cAMP-activated CK2 phosphorylates myosin IIa at least at S1916, contributing to the formation of an actin framework in a zyxin-dependent manner and in the promotion of WPB exocytosis. It is worth noting that such a fine framework formation is derived from the preexisting cortical actin network before fusion and can be only observed under superresolution microscopy.14 Therefore, it is different from the fusion-induced actomyosin II coat formation described in a previous study, which is de novo generated on the surface of the fused organelle via actin polymerization and can be observed under conventional confocal microscopy.9 Therefore, cAMP-mediated myosin IIa activation facilitates WPB exocytosis via mediating the formation of functional actin framework around exocytosing granules, dependent on the interaction with zyxin.
Despite high-sequence homology, myosin IIa and IIb have different enzymatic properties, subcellular localizations, and cellular roles.42 Our study showed that myosin IIa, but not IIb, mediates the distribution of mature WPBs under quiescent condition by regulating the FA-anchored cortical actin network, although the mechanism for this isoform-specific action needs further study. Interestingly, forskolin induced the interaction of zyxin with myosin IIa, but not IIb (Figure 5A-B), suggesting that myosin IIa plays a major role in regulating the formation of functional actin framework around exocytosing WPBs, although a role of myosin IIb cannot be excluded. The present findings demonstrated the physiological function of myosin IIa in the maintenance of vascular homeostasis via mediating endothelial VWF secretion. Considering the potential of cAMP-mediated secretion in the treatment of patients with von Willebrand disease and mild hemophilia,5 our results may provide an additional therapeutic target for the treatment of related diseases. Interestingly, the mutations in the myh9 gene, which encodes the nonmuscle heavy chain of myosin IIa, result in autosomal dominant bleeding disorders characterized by a macrothrombocytopenia and giant platelets.43,44 It remains to be determined whether mutations in the myh9 gene affect endothelial VWF secretion and contribute to bleeding symptoms, especially in the patients whose hemorrhages are not controlled by the desmopressin treatment.
Interestingly, another myosin motor, myosin Va, has also been shown to be involved in linking WPBs to the peripheral actin cytoskeleton of ECs.39 However, the deletion of MyoVa led to the increase of the secretion of less-mature VWF from ECs in response to calcium agonists such as histamine. Thus, it has been proposed that myosin Va plays a negative role in prevention of the secretion of WPBs that are not fully matured by anchoring WPBs at cortical actin filaments.39 This is in sharp contrast to the function of myosin IIa, which is required for the movement of WPB from the perinuclear site to the cell periphery, and the deficiency of which severely impairs VWF secretion from cAMP-activated ECs.
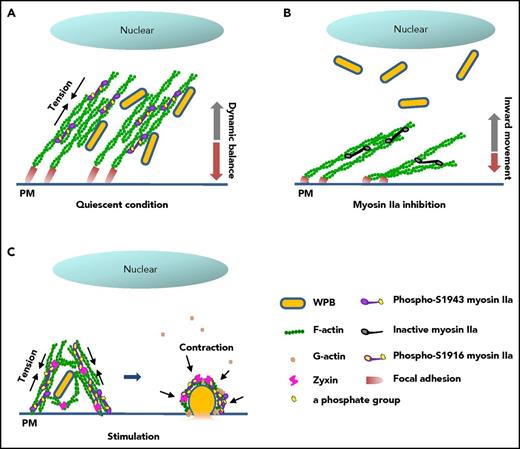
In summary, the data presented in this work suggest that myosin IIa couples the maintenance of mature WPB in a peripheral region to the promotion of VWF secretion in synergy with zyxin via spatiotemporally distinctive actin remodeling, using different phosphorylation sites (Figure 7). Our findings implicate myosin IIa as an important regulator of blood homeostasis under pathophysiological conditions.
Proposed mechanism for the regulation of cAMP-mediated endothelial WPB exocytosis by myosin IIa. (A) In quiescent condition, basal myosin IIa with an activity of phosphorylated-S1943 maintains the tension of the FA-anchored actin cytoskeleton, resulting in a dynamic balance of WPB movement and the distribution of WPBs along peripheral stress fibers. (B) In contrast to A, inhibition of the basal activity of myosin IIa by blebbistatin leads to relaxation of the actin network, and subsequently an inward movement of WPBs to the perinuclear region. (C) On stimulation by cAMP agonist, myosin IIa with high activity of phosphorylation at S1916 promotes the movement of WPBs toward the plasma membrane and subsequent cargo release via an interaction with zyxin in the formation of an actin framework around exocytosing granules.
Proposed mechanism for the regulation of cAMP-mediated endothelial WPB exocytosis by myosin IIa. (A) In quiescent condition, basal myosin IIa with an activity of phosphorylated-S1943 maintains the tension of the FA-anchored actin cytoskeleton, resulting in a dynamic balance of WPB movement and the distribution of WPBs along peripheral stress fibers. (B) In contrast to A, inhibition of the basal activity of myosin IIa by blebbistatin leads to relaxation of the actin network, and subsequently an inward movement of WPBs to the perinuclear region. (C) On stimulation by cAMP agonist, myosin IIa with high activity of phosphorylation at S1916 promotes the movement of WPBs toward the plasma membrane and subsequent cargo release via an interaction with zyxin in the formation of an actin framework around exocytosing granules.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank R. Adams (Max-Planck-Institute for Molecular Biomedicine, Münster, Germany) for providing the mouse line expressing tamoxifen-inducible Cre-recombinase (VEcreERT2) regulated by the vascular endothelial cadherin promoter on a C57BL/6J background, and J. Voorberg (Sanquin Research Laboratory, Amsterdam, The Netherlands) for providing GFP-VWF. The authors are grateful to I. C. Bruce for reading the manuscript and offering valuable comments.
J.L. was supported by research grants from the National Science Funds (No. 91339111, No. 81470298, and Project 31521062), the Major State Basic Research Development Program of China (No. 2012CB945103), and the National Science and Technology Support Project (grant no. 2014BAI02B01).
Authorship
Contribution: P.L. and G.W. carried out the experiments, analyzed data, and wrote the manuscript; Y. He and L.C. designed the experiments and read the manuscript; Y.C., Q.D., X.Han, X.Huang, and Y. Huo performed the experiments; and J.L. designed the experiments and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jincai Luo, Room 234, New Life Science Building, Institute of Molecular Medicine, Peking University, Beijing 100871, China; e-mail: jincailuo@pku.edu.cn.
References
Author notes
P.L. and G.W. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal