Key Points
A novel bispecific antibody against CD38 eradicates MM and NHL tumors in murine models.
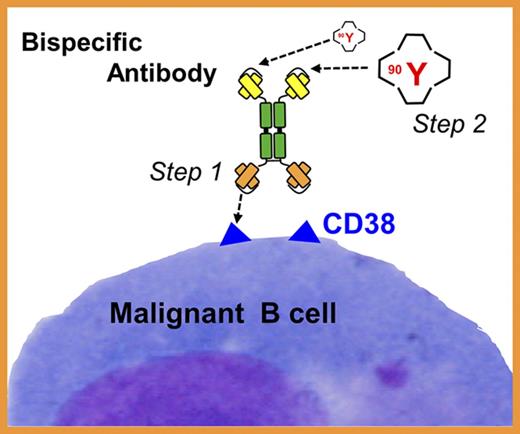
CD38-bispecific antibody pretargeting addresses limitations associated with radioimmunotherapy.
Abstract
Pretargeted radioimmunotherapy (PRIT) has demonstrated remarkable efficacy targeting tumor antigens, but immunogenicity and endogenous biotin blocking may limit clinical translation. We describe a new PRIT approach for the treatment of multiple myeloma (MM) and other B-cell malignancies, for which we developed an anti-CD38–bispecific fusion protein that eliminates endogenous biotin interference and immunogenic elements. In murine xenograft models of MM and non-Hodgkin lymphoma (NHL), the CD38-bispecific construct demonstrated excellent blood clearance and tumor targeting. Dosimetry calculations showed a tumor-absorbed dose of 43.8 Gy per millicurie injected dose of 90Y, with tumor-to-normal organ dose ratios of 7:1 for liver and 15:1 for lung and kidney. In therapy studies, CD38-bispecific PRIT resulted in 100% complete remissions by day 12 in MM and NHL xenograft models, ultimately curing 80% of mice at optimal doses. In direct comparisons, efficacy of the CD38 bispecific proved equal or superior to streptavidin (SA)-biotin–based CD38-SA PRIT. Each approach cured at least 75% of mice at the highest radiation dose tested (1200 µCi), whereas at 600- and 1000-µCi doses, the bispecific outperformed the SA approach, curing 35% more mice overall (P < .004). The high efficacy of bispecific PRIT, combined with its reduced risk of immunogenicity and endogenous biotin interference, make the CD38 bispecific an attractive candidate for clinical translation. Critically, CD38 PRIT may benefit patients with unresponsive, high-risk disease because refractory disease typically retains radiation sensitivity. We posit that PRIT might not only prolong survival, but possibly cure MM and treatment-refractory NHL patients.
Introduction
CD38, a transmembrane glycoprotein with high surface density and uniform expression on multiple myeloma (MM) cells1 and relatively low expression on normal myeloid and lymphoid cells,2 has proven a successful target for monoclonal antibody (mAb)-based immunotherapy in MM.3,4 Very effective agents introduced in the past decade have made complete response (CR) to induction therapy possible in almost half of MM patients.5,6 However, disease eradication remains elusive, creating conditions that strongly favor the persistence and evolution of therapy-resistant malignant plasma cell clones. As a result, the vast majority of the 120 000 people in the United States living with MM7 will ultimately die of progressive disease.8,9 High-dose chemotherapy followed by autologous stem cell transplantation (ASCT) increases CR rates and prolongs disease-free survival,10-14 and the number of ASCTs for MM increases annually, emphasizing the importance of ASCT in current treatment paradigms.13 Yet, disease almost invariably recurs even after ASCT.
Radioimmunotherapy (RIT) promises 2 critical improvements over other MM treatments: less-toxic conditioning for ASCT and the potential to eradicate disease. The radiosensitivity of malignant plasma cells outside of the bone marrow is well documented in clinical settings. Local recurrence of solitary extramedullary plasmacytomas occurs in <10% of cases after external-beam radiotherapy (RT) alone,15 and sustained local disease control and durable symptom relief has been reported for 98% of lesions receiving >10 Gy.16 Furthermore, this excellent efficacy of external-beam RT for extramedullary plasmacytomas occurs even in patients with poor-risk cytogenetics and active MM,17 suggesting that targeted RIT is agnostic to certain high-risk features.
RIT selectively delivers radiation to target cells at disseminated disease sites, facilitating escalation to radiation doses not achievable through external-beam RT. The efficacy of RIT is well established for several hematologic malignancies,18-20 and has been successfully integrated into ASCT-conditioning regimens with a significant improvement in progression-free survival and overall survival among patients with non-Hodgkin lymphoma (NHL) and acute myeloid leukemia when targeting CD20 and CD45 antigens, respectively.18,21-24 In contrast, few studies have examined RIT in MM25-30 and none have explored pretargeted RIT (PRIT), a 2-step process shown in the clinic to be markedly superior to conventional, single-step RIT.31-36 In conventional RIT, a targeting antibody (Ab) is directly labeled with a radioactive molecule. In 2-step PRIT, a nonradioactive targeting Ab is administered first and allowed to localize to tumor sites. The second step, administered after this “cold” Ab has maximally accumulated in the tumor, is a low-molecular-weight radioactive moiety with a high affinity for the Ab. The small size of the second reagent facilitates rapid tumor penetration, rapid capture and retention by the pretargeted Ab, and rapid clearance of unbound radioactive molecules from the blood. This 2-step approach greatly decreases radiation absorption by healthy tissues. The efficacy of PRIT can be further amplified by administering a “clearing agent” (CA) prior to the radioactive reagent. The CA accelerates clearance of any unbound Ab from the bloodstream, greatly reducing the chance of radioactive molecules attaching to unbound Ab.
Unlike many surface antigens, CD38 is stable on the cell surface,37 and this trait combined with high density and uniform expression in MM and NHL make CD38 an excellent target for PRIT. We recently documented striking therapeutic efficacy of PRIT in MM xenograft models using anti-CD38-streptavidin (SA; OKT10-SA) and the β-emitter 90Y.37 Objective remissions were observed within 7 days in 100% of mice treated with 800 to 1200 µCi CD38-SA PRIT, including 100% complete remissions (no detectable tumor in treated mice compared with tumors in control mice that were 2982% ± 1002% of initial tumor volume) by day 23. Despite these dramatic results, concerns with current PRIT approaches persist, including immunogenicity of bacterially derived SA, and the presence of endogenous biotin, which might block binding of radiolabeled biotin.
Here, we describe the production and characterization of a new anti-CD38-bispecific protein that entirely removes biotin binding and SA from PRIT, while maintaining high CD38-binding affinity and high avidity for the radiolabeled second step. We also directly compare therapeutic efficacy of the new CD38-bispecific with the CD38-SA approach. Several PRIT technologies have been developed to facilitate avidity of the radiolabeled second-step reagent for the pretargeted tumor-bound Ab. These include the SA-biotin and bispecific Ab approaches used here, complementary hybridization (Watson-Crick pairing) of phosphorodiamidate morpholino DNA oligomers,38,39 and cyclooctene-modified Abs binding to radiolabeled tetrazine ligands.40,41 Although prior studies show that all PRIT methods are superior to single-step RIT, no head-to-head comparisons have examined which PRIT method is most promising for clinical development of CD38 targeting. We present a comparative analysis of the biodistribution and therapeutic efficacy of the 2 most popular PRIT strategies. Our findings demonstrate that efficacy, reduced immunogenicity, and absence of interference from endogenous biotin all make anti-CD38-bispecific PRIT an excellent candidate for clinical translation.
Methods
Construction of anti-CD38–bispecific Ab and DOTAY-dextran CA
Methods detailing the construction of the 028-Fc-C825 bispecific (anti-CD38 × anti-90Y-labeled 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid [Y-DOTA]) fusion gene, isolation of CHO cell clones stably expressing the 028-Fc-C825–bispecific Ab, purification of the bispecific Ab, and synthesis of the DOTAY-dextran (DYD) CA are described in supplemental Methods (available on the Blood Web site). Construction of the control, 2H7-Fc-C825 (anti-CD20 × anti-Y-DOTA) bispecific was described previously.42
SA-biotin pretargeting reagents
Conjugates of the OKT10 anti-CD38 mAb and SA were synthesized, purified, and characterized as previously published.43,44 A synthetic, dendrimeric CA containing 16 N-acetylgalactosamine residues and a single biotin residue per molecule (NAGB) was obtained from Aletheon Pharmaceuticals (Seattle, WA) for use with the OKT10-SA conjugate.
Radiolabeling of DOTA-biotin with 90Y
90Y (PerkinElmer, Seattle, WA) labeling of 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA)-biotin was performed using 12 mg/mL DOTA-biotin, 500 mmol/L ammonium acetate (pH 5.3), and 90Y heated for 60 minutes at 84°C. After cooling to room temperature, 100 mmol/L diethylenetriaminepentaacetic acid (DTPA) was added, and labeling was efficiency determined using avidin-agarose beads.43
Cell culture
The human MM cell lines H929, U266B1, and RPMI 8226 were obtained from ATCC (Manassas, VA). These lines and the CD38+, CD20− human Burkitt lymphoma (BL) cell line Namalwa were authenticated by DNA profiling (ATCC kit 135-XV), tested for mycoplasma, and maintained in log-phase growth at >95% viability (trypan-blue exclusion) in RPMI 1640 media supplemented with 10% fetal bovine serum (FBS), 50 U/mL penicillin G, and 50 μg/mL streptomycin sulfate, for no more than 6 weeks after thawing.
Flow cytometry–based bifunctional-binding assay
Log-phase growth H929 cells (0.5 × 106 per group) were harvested and washed once with 1 mL of Hank balanced salt solution (HBSS)–2% FBS (HBSS) buffer. For CD38-blocking groups, cells were resuspended in 40 µL of HBSS buffer containing 40 µg of daratumumab (Janssen R&D, Raritan, NJ) and incubated at 4°C for 30 minutes. Then, 2 µg of bispecific fusion protein and 1 µg of Y-DOTA–biotin were added to all groups and cells incubated at 4°C for 30 minutes, followed by 2 washes. Recovered cells were resuspended in 40 µL of HBSS buffer containing 2 µL of phycoerythrin-labeled SA (AnaSpec Inc, Fremont, CA), incubated at 4°C for 30 minutes, washed 3 times, resuspended in 500 µL of phosphate-buffered saline (PBS) buffer containing 1% formaldehyde and analyzed on a Guava Easycyte mini cytometer.
Mouse xenograft models
Female FoxN1Nu athymic nude mice (Envigo, Hayward, CA) were maintained under standard protocols approved by the Fred Hutchinson Cancer Research Center (FHCRC) Institutional Animal Care and Use Committee (IACUC). CD38+ tumor cells (107) were injected subcutaneously in the right flank 7 to 11 days prior to experiments to produce 50- to 80-mm3 tumor xenografts. To attenuate tumor rejection due to natural killer (NK) cell activity, mice were injected intraperitoneally with anti-asialoGM1 Ab (Wako, Richmond, VA) 1 day prior to tumor implantation, 5 days later, and weekly thereafter. All mice were placed on a biotin-deficient diet (TestDiet, Richmond, IN) 7 days prior to PRIT studies.
Blood clearance and biodistribution studies
Groups of 3 to 5 mice bearing H929 flank tumors were injected via the tail vein (IV) with 2.8 nmol (210-420 μg) of 028-Fc-C825 (CD38 bispecific) or 2H7-Fc-C825 (CD20 control bispecific). Optimal Ab dosing was determined in prior experiments.37,42 Twenty-three hours later, mice were injected with 5 µg of DYD CA, followed 1 hour later by 1.2 nM (2 μg) DOTA-biotin labeled with 20 to 40 μCi (0.74-1.48 MBq) of 90Y. For blood clearance studies, retro-orbital blood sampling was performed at serial time points up to 24 hours after 90Y injection. For biodistribution studies, blood samples, tumors, and body organs were harvested at 6 to 120 hours. 90Y dose in each tissue sample was counted on a γ counter and the percentage of injected dose per gram (percent injected dose [ID] per gram) was calculated.45
Dosimetry: estimating absorbed doses of radioactivity in tissues
Mean absorbed doses to organs and tissues were calculated from the activity-over-time curves generated in biodistribution experiments. The calculations estimate organ self-dose plus cross-organ dose by accounting for organ mass, specific absorbed energy fraction, the emission spectrum of the radionuclides, and the β-particle absorbed fractions for small organs.45 The results were expressed as radiation absorbed dose (gray) per unit administered activity (per millicurie).
Therapy studies
Therapeutic efficacy of CD38 bispecific and CD38-SA PRIT were assessed in groups of 8 to 10 mice (sample size determined by power analysis) bearing flank H929 or Namalwa xenografts. Mice were randomized into groups with equivalent mean tumor volumes, and treatments were administered as in “Blood clearance and biodistribution studies,” with the addition of a CD38-SA (OKT10-SA) Ab treatment group that received 50 μg of NAGB as CA. All groups received second-step DOTA-biotin labeled at 600, 1000, or 1200 μCi of 90Y (22.2, 37, or 44.4 MBq). Tumor size and body weight were measured 3 times a week following treatment. Mice were killed when they experienced excessive weight loss, hind-limb paralysis, or exceeded tumor volume limits per IACUC requirements.
Statistical analyses
In murine xenograft studies, treatment effects on tumor growth rate were determined by first calculating tumor growth rate for each mouse as area under the curve of tumor volume over time, standardized for the number of days the mouse was alive. Treatment effects on standardized tumor area under the curve were then determined using analysis of variance. Treatment effects on mouse survival were determined by log-rank comparisons of Kaplan-Meier survival functions. Analyses were performed using JMP 12.2.0 (SAS Institute, Cary, NC) and GraphPad Prism 7 (GraphPad Software, La Jolla, CA).
Results
Engineering, expression, purification, and in vitro testing of the anti-CD38, 028-Fc-C825–bispecific protein
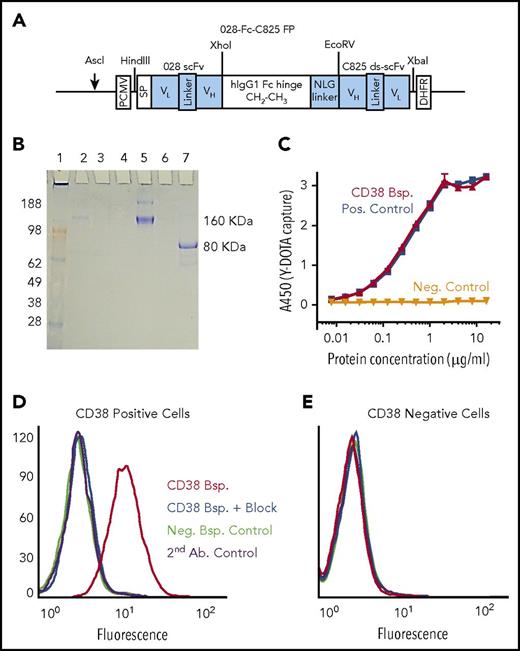
The 028-Fc-C825–bispecific Ab fusion protein, designed to recognize the CD38 surface antigen and the yttrium-DOTA ligand, was constructed by fusing DNA fragments encoding scFv of the 028 anti-CD38 human Ab and the ds-scFv of the affinity-enhanced C825 Ab to both sides of a human immunoglobulin G1 Fc containing a N-linked glycosylation (NLG) linker (Figure 1A). CHO-DG44 cells were transfected with the fusion construct DNA and high-expressing clones were selected using methotrexate. The monomeric bispecific protein spontaneously dimerized to form a 160-kDa molecule, which was purified from culture supernatants by affinity chromatography and characterized by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) (Figure 1B); enzyme-linked immunosorbent assay showed that the bispecific captured the Y-DOTA ligand in a concentration-dependent fashion (Figure 1C). Bifunctional flow cytometry analysis demonstrated that the bispecific bound only to cells expressing CD38, that binding was blocked by unmodified anti-CD38 mAb binding the same epitope, and that the bispecific captured the Y-DOTA ligand (Figure 1D-E). The anti-CD38 mAb OKT10 did not block cell binding of the CD38 bispecific, indicating that the binding epitopes did not overlap (supplemental Figure 1).
Structure and characterization of the CD38-bispecific protein. (A) Schematic of the 028-Fc-C825 bispecific (anti-CD38 × anti-Y-DOTA) Fc fusion gene. An anti-human CD38 028 scFv gene and an yttrium-DOTA capturing C825 disulfide-stabilized scFv (ds-scFv) gene were fused to the human immunoglobulin G1 Fc fragment at the amino and carboxyl ends, respectively. An NLG was incorporated between the Fc and C825 ds-scFv domains, as shown. Relevant restriction enzymes for cloning and linearization are indicated (schematic not drawn to scale). (B) SDS-PAGE analysis of the 028-Fc-C825 fusion protein. Bispecific 028-Fc-C825 fusion polypeptides were expressed in CHO-DG44 cells, where they spontaneously formed dimers via the hinge regions and were secreted into the growth medium. The purification fractions and the 028-Fc-C825 fusion protein (5 µg) were analyzed by electrophoresis on a 4% to 20% 2-morpholinoethanesulfonic acid SDS-PAGE gel (Invitrogen). Lane 1, SeeBlue Plus2 marker proteins in kilodaltons (Invitrogen); lane 2, culture supernatant; lane 3, protein A column flow-through; lane 4, wash; lane 5, the nonreduced 028-Fc-C825 fusion protein (samples boiled); lane 7, the monomeric 028-Fc-C825 fusion protein (samples boiled and reduced with 2-mercaptoethanol); lane 6 is empty. The gel was stained with Coomassie blue. (C) Sandwich enzyme-linked immunosorbent assay demonstrating concentration-dependent binding of the CD38 (028-Fc-C825) bispecific protein (red) to the Y-DOTA ligand. A 96-well plate was coated with 70 µL of the bovine serum albumin (BSA)–Y-DOTA conjugate (1 µg/mL in PBS) and then blocked with 200 µL of 2% BSA in PBS buffer. After washing, the wells were treated with 100 µL of bispecific protein at 16 µg/mL followed by serial dilution as indicated. The plate was further treated with horseradish peroxidase (HRP)–anti-human Fc Ab followed by 3,3′,5,5′-tetramethylbenzidine (TMB). Controls demonstrate that binding to Y-DOTA is dependent on the C825 portion of the bispecific protein: the positive control, CD20 2H7-Fc-C825 bispecific (blue), shows binding to Y-DOTA; the negative control, fusion protein-Fc without C825 (black), does not. (D-E) Bifunctional binding assays of the CD38 (028-Fc-C825) bispecific protein demonstrate targeted binding to CD38+ cells and ligand capture of Y-DOTA–biotin. (D) CD38+ target cells (H929) or (E) CD38− control cells (U266) (0.5 × 106) were incubated in 40 µL of HBSS–2% FBS buffer containing 1 µg of biotin–Y-DOTA ligand and 2 µg of either CD38 (red and blue) or CD20 (green) bispecific proteins, or no protein (purple) for 30 minutes at 4°C. For CD38-blocking controls (blue), cells were preincubated for 30 minutes in buffer containing 40 µg of anti-CD38 Ab. Cells were finally washed and resuspended in 40 µL of buffer plus 2 µL of phycoerythrin-SA, incubated 30 minutes at 4°C, washed 3 times, resuspended in 500 µL of PBS buffer containing 1% formaldehyde, and analyzed by flow cytometry. Bsp., bispecific; DHFR, dihydrofolate reductase; FP, fusion protein; Neg., negative; PCMV, (cytomegalovirus) promoter; Pos., positive; SP, signal peptide.
Structure and characterization of the CD38-bispecific protein. (A) Schematic of the 028-Fc-C825 bispecific (anti-CD38 × anti-Y-DOTA) Fc fusion gene. An anti-human CD38 028 scFv gene and an yttrium-DOTA capturing C825 disulfide-stabilized scFv (ds-scFv) gene were fused to the human immunoglobulin G1 Fc fragment at the amino and carboxyl ends, respectively. An NLG was incorporated between the Fc and C825 ds-scFv domains, as shown. Relevant restriction enzymes for cloning and linearization are indicated (schematic not drawn to scale). (B) SDS-PAGE analysis of the 028-Fc-C825 fusion protein. Bispecific 028-Fc-C825 fusion polypeptides were expressed in CHO-DG44 cells, where they spontaneously formed dimers via the hinge regions and were secreted into the growth medium. The purification fractions and the 028-Fc-C825 fusion protein (5 µg) were analyzed by electrophoresis on a 4% to 20% 2-morpholinoethanesulfonic acid SDS-PAGE gel (Invitrogen). Lane 1, SeeBlue Plus2 marker proteins in kilodaltons (Invitrogen); lane 2, culture supernatant; lane 3, protein A column flow-through; lane 4, wash; lane 5, the nonreduced 028-Fc-C825 fusion protein (samples boiled); lane 7, the monomeric 028-Fc-C825 fusion protein (samples boiled and reduced with 2-mercaptoethanol); lane 6 is empty. The gel was stained with Coomassie blue. (C) Sandwich enzyme-linked immunosorbent assay demonstrating concentration-dependent binding of the CD38 (028-Fc-C825) bispecific protein (red) to the Y-DOTA ligand. A 96-well plate was coated with 70 µL of the bovine serum albumin (BSA)–Y-DOTA conjugate (1 µg/mL in PBS) and then blocked with 200 µL of 2% BSA in PBS buffer. After washing, the wells were treated with 100 µL of bispecific protein at 16 µg/mL followed by serial dilution as indicated. The plate was further treated with horseradish peroxidase (HRP)–anti-human Fc Ab followed by 3,3′,5,5′-tetramethylbenzidine (TMB). Controls demonstrate that binding to Y-DOTA is dependent on the C825 portion of the bispecific protein: the positive control, CD20 2H7-Fc-C825 bispecific (blue), shows binding to Y-DOTA; the negative control, fusion protein-Fc without C825 (black), does not. (D-E) Bifunctional binding assays of the CD38 (028-Fc-C825) bispecific protein demonstrate targeted binding to CD38+ cells and ligand capture of Y-DOTA–biotin. (D) CD38+ target cells (H929) or (E) CD38− control cells (U266) (0.5 × 106) were incubated in 40 µL of HBSS–2% FBS buffer containing 1 µg of biotin–Y-DOTA ligand and 2 µg of either CD38 (red and blue) or CD20 (green) bispecific proteins, or no protein (purple) for 30 minutes at 4°C. For CD38-blocking controls (blue), cells were preincubated for 30 minutes in buffer containing 40 µg of anti-CD38 Ab. Cells were finally washed and resuspended in 40 µL of buffer plus 2 µL of phycoerythrin-SA, incubated 30 minutes at 4°C, washed 3 times, resuspended in 500 µL of PBS buffer containing 1% formaldehyde, and analyzed by flow cytometry. Bsp., bispecific; DHFR, dihydrofolate reductase; FP, fusion protein; Neg., negative; PCMV, (cytomegalovirus) promoter; Pos., positive; SP, signal peptide.
In vivo PK and blood clearance of the CD38-bispecific protein
Pharmacokinetics (PK) analysis of the CD38-bispecific protein required our standard, 2-step PRIT protocol because direct radioiodination of the bispecific protein impaired binding. For PRIT, mice were injected first with the unlabeled protein, followed 23 hours later with CA, then 1 hour later with 90Y-DOTA–biotin. Blood samples were taken at 5 minutes, 30 minutes, and 1, 4, and 24 hours after the 90Y injection. Figure 2 demonstrates that without CA, blood clearance of 90Y was 72.8% ID per gram after 30 minutes and 86.8% after 24 hours. With 5 µg of CA, clearance was 97.8% ID per gram at 30 minutes and 99.3% after 24 hours. We additionally tested increasing doses of CA; all doses produced virtually identical results (eg, clearance using 32 µg of CA was 98.2% ID per gram at 30 minutes and 99.8% after 24 hours; data not shown). We therefore used the 5-µg dose for further experiments.
DYD CA effectively clears circulating CD38-bispecific protein from the bloodstream. Athymic nude mice (n = 3-5 per group) were injected at −24 hours with 1.4 nM CD38-bispecific protein (028-C825), then at −1 hour with 5 µg of CA, and at 0 hours with 90Y-DOTA–biotin. Controls received no CA. Percent ID per gram (%ID/g) was determined from retro-orbital venous samples taken at serial time points starting 5 minutes after the 90Y-DOTA–biotin injection. Error bars = 1 standard error of the mean (SEM).
DYD CA effectively clears circulating CD38-bispecific protein from the bloodstream. Athymic nude mice (n = 3-5 per group) were injected at −24 hours with 1.4 nM CD38-bispecific protein (028-C825), then at −1 hour with 5 µg of CA, and at 0 hours with 90Y-DOTA–biotin. Controls received no CA. Percent ID per gram (%ID/g) was determined from retro-orbital venous samples taken at serial time points starting 5 minutes after the 90Y-DOTA–biotin injection. Error bars = 1 standard error of the mean (SEM).
In vivo biodistributions of radioactivity demonstrate favorable tumor targeting and retention using CD38-bispecific PRIT
Biodistributions of radioactivity in blood, tumors (H929 xenografts), and normal organs were compared between CD38-bispecific PRIT and control (CD20-targeted) bispecific PRIT (Figure 3A). Tumor-bearing mice were injected at −24 hours with unlabeled protein, at −1 hour with CA, then at 0 hour with 90Y-DOTA–biotin. Assessed 24 hours after 90Y-DOTA–biotin injection, tumor-to-normal tissue ratios of absorbed radiation were 16:1 for blood, 14:1 for lung, 12:1 for liver, and 19:1 for kidney for the CD38-bispecific group (Figure 3A). For the control-bispecific group, ratios were <2:1 for the same tissues (Figure 3A). We also evaluated radioactivity biodistributions over time, taking tissues 6, 24, 48, and 120 hours after 90Y-DOTA–biotin injections. These studies confirmed the tumor specificity of CD38-bispecific PRIT, and further demonstrated high retention of radiation in tumors over time (Figure 3B). Control-bispecific PRIT showed no tumor targeting or retention (Figure 3C).
Biodistribution and PK of 90Y-DOTA–biotin using CD38-bispecific PRIT. Athymic nude mice (n = 5 per group) bearing H929 (MM) xenografts (107 cells injected in the right flank) were injected at −24 hours with 2.8 nM pretargeting Ab (either CD38 bispecific = 028-C825, or control bispecific [targeting CD20] = 2H7-C825), then at −1 hour with CA, and at 0 hours with 90Y-DOTA–biotin. (A) Blood, tumor, and normal organ specimens were taken 24 hours after radioactivity injections. (B-C) Comprehensive tissue biodistributions were obtained at sequential time points 6, 24, 48, and 120 hours after 90Y-DOTA–biotin injection, using (B) CD38 or (C) control bispecific PRIT. The panel C description also applies to nontarget tissues in panel B. Error bars = 1 SEM.
Biodistribution and PK of 90Y-DOTA–biotin using CD38-bispecific PRIT. Athymic nude mice (n = 5 per group) bearing H929 (MM) xenografts (107 cells injected in the right flank) were injected at −24 hours with 2.8 nM pretargeting Ab (either CD38 bispecific = 028-C825, or control bispecific [targeting CD20] = 2H7-C825), then at −1 hour with CA, and at 0 hours with 90Y-DOTA–biotin. (A) Blood, tumor, and normal organ specimens were taken 24 hours after radioactivity injections. (B-C) Comprehensive tissue biodistributions were obtained at sequential time points 6, 24, 48, and 120 hours after 90Y-DOTA–biotin injection, using (B) CD38 or (C) control bispecific PRIT. The panel C description also applies to nontarget tissues in panel B. Error bars = 1 SEM.
Dosimetry
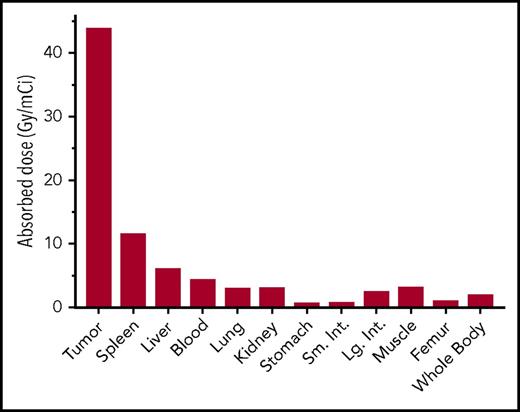
We estimated radiation-absorbed doses to tumors, whole body, and 10 normal tissues from time-activity curves generated in CD38-bispecific biodistribution experiments, using a dosimetry method that calculates both organ self-dose absorbed fractions and β-particle cross-organ dose contributions, per unit of administered activity (Figures 3B and 4).45 Absorbed radiation dose to tumor was 43.8 Gy/mCi, and tumor-to-normal organ ratios of absorbed dose were 51:1 for the femur, 23:1 for the whole body, 15.4:1 for the lung, 15:1 for the kidneys, 10:1 for the blood, and 7.4:1 for the liver (Figure 4).
Dosimetry of 90Y-DOTA–biotin using CD38-bispecific PRIT. Dosimetry, absorbed radiation dose per unit administered activity (Gy/mCi), was calculated for tumor and normal organs during the first 120 hours after radioactivity injections (tissues per Figure 3B). Dosimetry includes organ self-dose absorbed fraction plus β-particle cross-organ absorbed fraction.45 Absorbed radiation dose to tumor was 44 Gy/mCi, in contrast to 6, 3, and 3 Gy/mCi for liver, lung, and kidney, respectively.
Dosimetry of 90Y-DOTA–biotin using CD38-bispecific PRIT. Dosimetry, absorbed radiation dose per unit administered activity (Gy/mCi), was calculated for tumor and normal organs during the first 120 hours after radioactivity injections (tissues per Figure 3B). Dosimetry includes organ self-dose absorbed fraction plus β-particle cross-organ absorbed fraction.45 Absorbed radiation dose to tumor was 44 Gy/mCi, in contrast to 6, 3, and 3 Gy/mCi for liver, lung, and kidney, respectively.
Therapy studies
We studied the efficacy of CD38-bispecific PRIT in 2 xenograft models, and found that optimized PRIT dosing cured at least 75% of mice in every experiment. Athymic mice (n = 8-10 per group) bearing H929 MM or Namalwa NHL xenografts were injected first with cold Ab, then 23 hours later with CA, and then in 1 more hour with 90Y-DOTA–biotin. Previous PRIT studies by our group37,42 indicated that 1200 µCi 90Y provides the most favorable therapeutic ratio, and we first examined efficacy of the CD38-bispecific Ab at 1200 µCi. In the H929 model, treatment with CD38-bispecific PRIT dramatically reduced tumor growth (Figure 5A) and increased survival (Figure 5B) relative to treatment with bispecific control PRIT (1200 µCi) and untreated control groups (P ≤ .0002, CD38 bispecific vs either control). In total, 19 of 20 mice in the 2 control groups died of tumor progression within 30 days (1 untreated mouse showed spontaneous tumor regression), whereas in the CD38-bispecific group 8 mice sustained complete remissions through the end of the study (150 days) and 2 died of tumor progression, both after day 50. In a second CD38+ tumor xenograft model (Namalwa), we added head-to-head comparisons of the CD38-bispecific Ab vs the CD38-SA (OKT10-SA) Ab, each labeled with 1200 µCi 90Y. In this experiment, both CD38 treatments resulted in 100% CRs by day 21, followed by 1 subsequent relapse in the CD38-SA group and zero relapses in the CD38-bispecific group (Figure 6). Thus, at this dose, the 2 CD38 treatments reduced tumor growth and increased survival with high and equivalent efficacy (P < .0001 for either CD38 treatment vs either control, P = .48 for CD38 bispecific vs CD38-SA). To further characterize the dose-response relationship of CD38 PRIT, we also evaluated 600 and 1000 µCi 90Y pretargeted to xenograft tumors. These reduced-dose experiments compared CD38-bispecific PRIT vs CD38-SA PRIT, and both anti-CD38 systems reduced tumor growth (Figure 7A) while improving survival (Figure 7B) in a marginally dose-dependent manner (P < .062, PRIT 600 µCi vs PRIT 1000 µCi). Importantly, at these reduced doses, the CD38-bispecific Ab strongly outperformed CD38-SA, resulting in long-term survival of 61% vs 22%, respectively, with 600 µCi, and 78% vs 47%, respectively, with 1000 µCi (P < .004, CD38 bispecific vs CD38-SA). In contrast, all controls, including mice treated with cold (no radiolabel) CD38 bispecific, cold CD38-SA, or control bispecific at 1000 µCi, died of tumor progression by day 15 (Figure 7, P < .0001, any CD38 PRIT group vs any control group).
Effect of CD38-bispecific PRIT on tumor growth rate and survival of mice bearing H929 (MM) xenografts. Athymic nude mice (n = 10 per group) with H929 xenografts were injected at −24 hours with a pretargeting protein (CD38 bispecific or control [anti-CD20] bispecific), then at −1 hour with CA, and at 0 hours with 1200 µCi 90Y-DOTA–biotin. Tumor volume was monitored 3 times weekly and mice were euthanized when tumor size reached IACUC-mandated limits. For tumor volume graphics, data from euthanized mice were retained until all mice in a group died. (A) Treatment with CD38-bispecific PRIT (red) resulted in 100% CRs during days 15 to 30 and 80% long-term complete remissions. This contrasted with control mice, where 90% of untreated (black) and 100% of control bispecific groups (gray) died of tumor progression by day 27 (P ≤ .0001, CD38 bispecific vs either control group, error bars = 1 SEM). One untreated mouse exhibited spontaneous tumor remission. (B) Kaplan-Meier analysis indicates that CD38-bispecific PRIT significantly improved survival over controls (P ≤ .0001), curing 80% of mice. Cure was defined as no sign of tumor recurrence at day 150.
Effect of CD38-bispecific PRIT on tumor growth rate and survival of mice bearing H929 (MM) xenografts. Athymic nude mice (n = 10 per group) with H929 xenografts were injected at −24 hours with a pretargeting protein (CD38 bispecific or control [anti-CD20] bispecific), then at −1 hour with CA, and at 0 hours with 1200 µCi 90Y-DOTA–biotin. Tumor volume was monitored 3 times weekly and mice were euthanized when tumor size reached IACUC-mandated limits. For tumor volume graphics, data from euthanized mice were retained until all mice in a group died. (A) Treatment with CD38-bispecific PRIT (red) resulted in 100% CRs during days 15 to 30 and 80% long-term complete remissions. This contrasted with control mice, where 90% of untreated (black) and 100% of control bispecific groups (gray) died of tumor progression by day 27 (P ≤ .0001, CD38 bispecific vs either control group, error bars = 1 SEM). One untreated mouse exhibited spontaneous tumor remission. (B) Kaplan-Meier analysis indicates that CD38-bispecific PRIT significantly improved survival over controls (P ≤ .0001), curing 80% of mice. Cure was defined as no sign of tumor recurrence at day 150.
Comparative effects of CD38-bispecific PRIT and CD38-SA PRIT on tumor growth and survival of mice bearing Namalwa (CD38+BL) xenografts. Athymic nude mice (n = 8 per group) with Namalwa xenografts were injected at −24 hours with a pretargeting protein (CD38 bispecific, CD38-SA, or control [anti-CD20] bispecific), then at −1 hour with CA, and at 0 hours with 1200 µCi 90Y-DOTA–biotin. Tumor volumes were monitored 3 times weekly and mice were euthanized when tumor size reached IACUC-mandated limits. For tumor volume graphics, data for euthanized mice were retained until all mice in a group died. (A) CD38-bispecific PRIT (red) and CD38-SA PRIT (blue) each reduced tumor volumes to undetectable levels by day 21, followed by a single tumor recurrence in the CD38-SA group and no recurrences in the CD38-bispecific group (P < .0001, either CD38 PRIT group vs either control group, error bars = 1 SEM). (B) Kaplan-Meier survival analysis. CD38-bispecific PRIT cured 75% of mice (all mortality due to early weight loss), whereas CD38-SA PRIT cured 88% of mice (all mortality due to tumor progression), demonstrating that at this 1200-µCi dose, the 2 CD38 treatments each benefitted survival with high and equivalent efficacy (P < .0001 for either CD38 treatment vs either control, P = .48 for CD38 bispecific vs CD38-SA). Cure was defined as the absence of tumor recurrence through day 150.
Comparative effects of CD38-bispecific PRIT and CD38-SA PRIT on tumor growth and survival of mice bearing Namalwa (CD38+BL) xenografts. Athymic nude mice (n = 8 per group) with Namalwa xenografts were injected at −24 hours with a pretargeting protein (CD38 bispecific, CD38-SA, or control [anti-CD20] bispecific), then at −1 hour with CA, and at 0 hours with 1200 µCi 90Y-DOTA–biotin. Tumor volumes were monitored 3 times weekly and mice were euthanized when tumor size reached IACUC-mandated limits. For tumor volume graphics, data for euthanized mice were retained until all mice in a group died. (A) CD38-bispecific PRIT (red) and CD38-SA PRIT (blue) each reduced tumor volumes to undetectable levels by day 21, followed by a single tumor recurrence in the CD38-SA group and no recurrences in the CD38-bispecific group (P < .0001, either CD38 PRIT group vs either control group, error bars = 1 SEM). (B) Kaplan-Meier survival analysis. CD38-bispecific PRIT cured 75% of mice (all mortality due to early weight loss), whereas CD38-SA PRIT cured 88% of mice (all mortality due to tumor progression), demonstrating that at this 1200-µCi dose, the 2 CD38 treatments each benefitted survival with high and equivalent efficacy (P < .0001 for either CD38 treatment vs either control, P = .48 for CD38 bispecific vs CD38-SA). Cure was defined as the absence of tumor recurrence through day 150.
Comparative dose-response effects of CD38-bispecific PRIT vs CD38-SA PRIT on tumor growth and survival of mice bearing CD38+BL xenografts. This figure presents data from 2 replicate experiments, each using 8 to 10 athymic nude mice per group. In total, n = 18 mice per treatment were injected at −24 hours with a pretargeting protein (CD38 bispecific, CD38-SA, or control [anti-CD20] bispecific), then at −1 hour with CA, and at 0 hours with 600 or 1000 µCi 90Y-DOTA–biotin. An additional n = 10 mice per group received only pretargeting protein (CD38 bispecific or CD38-SA) and CA, with no 90Y-DOTA–biotin. (A) All control mice including untreated (black), pretargeting protein without 90Y (maroon and navy), and control bispecific 1000-µCi groups (gray) experienced rapid tumor progression and died by day 14. All CD38 PRIT mice showed CR by day 11, and subsequent strongly reduced tumor progression relative to controls (P < .0001, any CD38 PRIT group vs any control group). However, CD38-bispecific PRIT (reds) outperformed CD38-SA PRIT (blues), the former showing later and fewer tumor progressions in the 600- and 1000-µCi treatment groups (P < .003, CD38 bispecific vs CD38-SA). (B) Kaplan-Meier survival analyses reflect the tumor volume results, showing greatly improved survival in each CD38 PRIT group relative to each control (P < .0001), and improved survival of CD38-bispecific PRIT mice relative to CD38-SA PRIT mice, across 600- and 1000-µCi levels (P < .004, CD38 bispecific vs CD38-SA).
Comparative dose-response effects of CD38-bispecific PRIT vs CD38-SA PRIT on tumor growth and survival of mice bearing CD38+BL xenografts. This figure presents data from 2 replicate experiments, each using 8 to 10 athymic nude mice per group. In total, n = 18 mice per treatment were injected at −24 hours with a pretargeting protein (CD38 bispecific, CD38-SA, or control [anti-CD20] bispecific), then at −1 hour with CA, and at 0 hours with 600 or 1000 µCi 90Y-DOTA–biotin. An additional n = 10 mice per group received only pretargeting protein (CD38 bispecific or CD38-SA) and CA, with no 90Y-DOTA–biotin. (A) All control mice including untreated (black), pretargeting protein without 90Y (maroon and navy), and control bispecific 1000-µCi groups (gray) experienced rapid tumor progression and died by day 14. All CD38 PRIT mice showed CR by day 11, and subsequent strongly reduced tumor progression relative to controls (P < .0001, any CD38 PRIT group vs any control group). However, CD38-bispecific PRIT (reds) outperformed CD38-SA PRIT (blues), the former showing later and fewer tumor progressions in the 600- and 1000-µCi treatment groups (P < .003, CD38 bispecific vs CD38-SA). (B) Kaplan-Meier survival analyses reflect the tumor volume results, showing greatly improved survival in each CD38 PRIT group relative to each control (P < .0001), and improved survival of CD38-bispecific PRIT mice relative to CD38-SA PRIT mice, across 600- and 1000-µCi levels (P < .004, CD38 bispecific vs CD38-SA).
Toxicity
PRIT using all Abs was well tolerated, with minimal weight loss and recovery to starting weight within 14 days of treatment (supplemental Figure 2). Over all studies (Figures 5-7), 2 of 18 mice treated with CD38 bispecific at 1200 μCi and 2 of 18 treated with CD38 bispecific at 1000 μCi died due to low body weight before day 18. All other deaths in all groups resulted from tumor progression. Anecdotal data suggest that early weight loss and mortality may be ameliorated as follows. Between our final 2 studies, we implemented husbandry changes to reduce external radiation exposure from cage mates and bedding; mortality before the changes was 2 of 8 mice and mortality after was 0 of 10 mice for 1000 μCi CD38-bispecific treatments (groups combined in Figure 7).
Discussion
Combinations of immunomodulatory- and proteasome inhibitor–based therapies frequently induce remission in MM patients, but relapse is nearly inevitable and the need for development of potentially curative treatments remains critical. Our results demonstrate 75% to 88% cure rates using PRIT in 2 CD38-expressing murine xenograft tumor models. These results are consistent with the steep dose-response relationship between radiation and hematologic malignancies. In MM, external-beam radiation can cure isolated plasmacytomas and provide sustained local disease control in 98% of lesions receiving >10 Gy16 ; in follicular NHL, another generally incurable B-cell malignancy, external-beam radiation can also eradicate disease that is limited to a single site of involvement.46,47
RIT offers a delivery model designed to parlay the unique antitumor potency of radiation demonstrated in localized disease to a broader population of patients with multifocal disease by sparing healthy tissues while delivering a targeted radiation payload directly to malignant cells. Single-step RIT has demonstrated some promise in MM,25-30 yet clinical applications have been very limited. Similar to CD20 single-step RIT, enthusiasm may be limited by low tumor-to-normal ratios (eg, <2.4:1 for kidney, lung, and liver in NHL43,48 ). PRIT methods can greatly improve these therapeutic ratios. In MM models, we previously demonstrated that CD38-SA PRIT provides tumor-to-normal tissue dosimetry ratios of 6:1 for kidney, lung, and liver (Figure 5 in Green et al37), and here show that CD38-bispecific PRIT provides ratios of 15:1 for kidney and lung and 7:1 for liver (Figure 4). To our knowledge, we have performed the only studies of PRIT in MM, and only 1 other group has used CD38 targeting for RIT (single-step).30
Recent clinical successes with unmodified anti-CD38 mAb therapy in MM have motivated ongoing clinical studies of this therapy in B-cell NHL. High-density CD38 expression is a common feature of malignant B cells and the predictable growth kinetics associated with NHL cell lines have led to their frequent use in research models for the development of CD38-targeted MM therapy.3 Despite advances in the management of NHL, nearly 30% of these patients die of progressive disease within 5 years of diagnosis.49 As in MM, treatment-refractory NHL typically retains sensitivity to radiation,50,51 making CD38 PRIT a potentially effective treatment of such patients as well. Beyond tumors that share a common B-cell lineage, CD38 is expressed in most NK/T-cell lymphomas,52 where 50% of patients die within 5 years53 and overexpression of CD38 predicts poor outcomes for NK/T-cell lymphoma patients,52 presenting another potential translational application for CD38 PRIT. We respectfully suggest that a broad range of potential indications for CD38 PRIT increases the probability of successful translation into a commercially viable radioimmunotherapeutic. To further evaluate CD38 as a target for RIT, we are conducting a clinical trial using a CD38 mAb directly conjugated to the α-emitter 211At (single-step RIT). We are also developing bispecific fusion protein constructs for α-emitter delivery that will facilitate head-to-head comparisons of α- and β-emitter PRIT.
Although the preclinical efficacy of PRIT appears clear, concerns have been raised regarding clinical translation. SA is a bacterial protein, and PRIT using SA might result in immunogenicity and thus limit the ability to administer multiple cycles of therapy, although 2 factors may mitigate this concern. First, the immunocompromised status of many patients with hematological malignancies limits immunogenic responses, and, second, PRIT is designed to be efficacious following a single dose, as demonstrated in both this manuscript and our previous studies. To reduce these concerns, several methods of modifying SA have been developed,54-57 but approaches that eliminate SA are desirable. To achieve this, we developed the CD38-bispecific fusion protein, which exploits the same principles as the SA-biotin system, but replaces SA with a humanized yttrium-DOTA capturing C825 disulfide-stabilized scFv (Figure 1A). A bispecific construct harboring the same anti-yttrium–DOTA (C825) in an immunocompetent murine model (J.J.O., S.O., D.J.G., A.L.K., O.W.P., Bispecific antibody targeting CD45 and 90Y-DOTA in preclinical murine models of acute myeloid leukemia, unpublished observations) has demonstrated no evidence of toxicity. Reduced immunogenicity may allow for repeat dosing of CD38-bispecific PRIT, and fractionation may offer the opportunity to improve the therapeutic ratio in clinical settings. Our data here, however, suggest that PRIT is effective and well tolerated as a single-dose therapeutic.
A second concern for SA-PRIT is the potential for endogenous biotin in patient blood and tissues to occupy and block SA-binding sites, preventing subsequent binding of the second-step radio-DOTA-biotin reagents. The bispecific approach obviates this concern, as binding of the C825 portion of the bispecific to the DOTA portion of the second-step reagent precludes any possible interference from endogenous biotin.
Our data suggest that bispecific PRIT has superior efficacy when compared with CD38-SA PRIT (Figure 7). The anti-CD38 region of the bispecific protein binds a different epitope of CD38 than the OKT10-SA protein, as demonstrated by the absence of OKT10 mAb blocking of the bispecific CD38 in cell-binding assays (supplemental Figure 1). The binding of separate epitopes raises the possibility that the first-step CD38 targeting constructs have differing therapeutic effects, but we demonstrated that neither construct has antitumor efficacy when administered without radiolabeling (Figure 7). Alternatively, the therapeutic differences may be a consequence of differing in vivo–binding efficiencies. The bispecific and SA treatments were designed such that all mice received an equivalent injected protein dose carrying an identical radiolabel dose, but subsequent tumor binding might differ for 2 reasons. First, it is feasible that CD38-SA binding, and thus PRIT efficacy, was reduced by very low-level blocking by residual endogenous biotin in mouse tissues, despite institution of a biotin-free diet. Second, differences in Ab-ligand interaction may be a function of each construct’s avidity for its respective epitope, resulting in kinetics that may favor the bispecific.
In conclusion, we have characterized a new CD38-bispecific targeting protein for PRIT of MM and NHL. The bispecific Ab is relatively easy to produce, exhibits excellent blood clearance using an inexpensive CA, and shows excellent tumor-to-normal organ ratios of absorbed activity reflected by favorable dosimetry in murine models. Moreover, the CD38 bispecific rapidly reduced disease burden, and at optimal doses ultimately cured 75% to 80% of mice in each of 2 xenograft models with minimal toxicity. CD38-SA was equally effective at the highest radiation dose, but the bispecific’s superiority over a range of doses, combined with its reduced risk of immunogenicity and lack of endogenous biotin interference, make the CD38 bispecific a prime candidate for clinical translation.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from the National Institutes of Health, National Cancer Institute K08CA151682 (D.J.G.), R01CA205248 (D.J.G), K24CA184039 (A.K.G.), K01CA188151 (J.J.O.), K23CA154874 (B.G.T.), P01CA044991 (O.W.P.), R01CA076287 (O.W.P), R01CA136639 (O.W.P.), and R01CA154897 (O.W.P.), and by Multiple Myeloma Opportunities for Research and Education (MMORE), the Brotherton Family Fund, and the David and Patricia Giuliani Family Foundation.
Authorship
Contribution: D.J.G. and O.W.P. designed research, analyzed data, and wrote the paper; S.O. designed and performed research, analyzed data, and wrote the paper; Y.L. performed research, contributed vital new reagents, and analyzed data; M.L.C. performed research and analyzed data; A.L.K., M.N., and M.D.H. performed research; D.K.H., D.S.W., K.D.O., and K.D.W. contributed vital new reagents.; and D.R.F., A.K.G., T.A.G., J.J.O., and B.G.T. analyzed data.
Conflict-of-interest disclosure: A.K.G. consults for, and receives research support paid to the institution from, Gilead and Janssen. B.G.T. and O.W.P. receive Roche/Genentech and Mustang Bio research support paid to the institution and receive royalties from patents held in common with Mustang Bio. K.D.O. and K.S.W. hold US patent 8 648 176 (Engineered Proteins with High Affinity for DOTA Chelates). O.W.P. consults for Bayer, sits on the data safety monitoring board for Bristol-Myers Squibb and Roche, and has ownership interest in Emergent Biosolutions and PhaseRx. The remaining authors declare no competing financial interests.
Correspondence: Damian J. Green, Clinical Research Division, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave N., MS: D3-190, Seattle, WA 98109; e-mail: dgreen@fredhutch.org.


![Figure 3. Biodistribution and PK of 90Y-DOTA–biotin using CD38-bispecific PRIT. Athymic nude mice (n = 5 per group) bearing H929 (MM) xenografts (107 cells injected in the right flank) were injected at −24 hours with 2.8 nM pretargeting Ab (either CD38 bispecific = 028-C825, or control bispecific [targeting CD20] = 2H7-C825), then at −1 hour with CA, and at 0 hours with 90Y-DOTA–biotin. (A) Blood, tumor, and normal organ specimens were taken 24 hours after radioactivity injections. (B-C) Comprehensive tissue biodistributions were obtained at sequential time points 6, 24, 48, and 120 hours after 90Y-DOTA–biotin injection, using (B) CD38 or (C) control bispecific PRIT. The panel C description also applies to nontarget tissues in panel B. Error bars = 1 SEM.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/131/6/10.1182_blood-2017-09-807610/4/m_blood807610f3.jpeg?Expires=1764965815&Signature=X499nvQW2lNzUzxk87Ebm8EOJZNsBRaiF2ZtGZFEqftRyozUS96vZaMwKiVGURGBcyseScGg72DXc~K~z1yFUAs1kGcgm00CCnTewNxC~~8MJmozbfIX3ZTP3SVMVa-R~KqhACQNhhYrpsZ0qpprTXwYp5RMk~Wgk4SEaYpdytNg3CzD3AMJgQkHYQ20zR6fdsCD45A3W2vmejdYwiAtphPaWiG281KQsEp0FiskBJvzHISbsmmUbs2FHPXEnEfCvPwggU9jnvGydFn71UmYkj0TN87VEhMfrQLUW07zyi3ctxBjYi4qQxfPQ1ZWQqJU80AovnyoL0ZRpiQLQGUwIg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 5. Effect of CD38-bispecific PRIT on tumor growth rate and survival of mice bearing H929 (MM) xenografts. Athymic nude mice (n = 10 per group) with H929 xenografts were injected at −24 hours with a pretargeting protein (CD38 bispecific or control [anti-CD20] bispecific), then at −1 hour with CA, and at 0 hours with 1200 µCi 90Y-DOTA–biotin. Tumor volume was monitored 3 times weekly and mice were euthanized when tumor size reached IACUC-mandated limits. For tumor volume graphics, data from euthanized mice were retained until all mice in a group died. (A) Treatment with CD38-bispecific PRIT (red) resulted in 100% CRs during days 15 to 30 and 80% long-term complete remissions. This contrasted with control mice, where 90% of untreated (black) and 100% of control bispecific groups (gray) died of tumor progression by day 27 (P ≤ .0001, CD38 bispecific vs either control group, error bars = 1 SEM). One untreated mouse exhibited spontaneous tumor remission. (B) Kaplan-Meier analysis indicates that CD38-bispecific PRIT significantly improved survival over controls (P ≤ .0001), curing 80% of mice. Cure was defined as no sign of tumor recurrence at day 150.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/131/6/10.1182_blood-2017-09-807610/4/m_blood807610f5.jpeg?Expires=1764965815&Signature=3N39Ss25ZrAcaGqtAVvNfLyicOM2WYCGnidJjWZgdcQVf3fTGCRb~Je3SdlHpc34~ej5ym2RYdTQkVmwtMsKdx8si6y~WthcGunzIF5oyHV-mw0fLxNx3ybdkNN6Ck0TS7bzRVILaKRod1iuHg-MiakenCSh9TtEH7LEHGP78bSxmDYYYnHudHVgDLp2pvGzoWMnRpXAL48hqiIixAKNXpdPiR8mmVHIlS1eivaCG-JcGjHRgFerFJU5iFf-FDpIDJE0y8wiM8g4Qf8YbXNDnloOkuQgApNHXVoxDH9l6O6jRFk7eY5nlu5elq1YYoC~DsRhe-hn7rfBXPL0uJ7-gg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 6. Comparative effects of CD38-bispecific PRIT and CD38-SA PRIT on tumor growth and survival of mice bearing Namalwa (CD38+ BL) xenografts. Athymic nude mice (n = 8 per group) with Namalwa xenografts were injected at −24 hours with a pretargeting protein (CD38 bispecific, CD38-SA, or control [anti-CD20] bispecific), then at −1 hour with CA, and at 0 hours with 1200 µCi 90Y-DOTA–biotin. Tumor volumes were monitored 3 times weekly and mice were euthanized when tumor size reached IACUC-mandated limits. For tumor volume graphics, data for euthanized mice were retained until all mice in a group died. (A) CD38-bispecific PRIT (red) and CD38-SA PRIT (blue) each reduced tumor volumes to undetectable levels by day 21, followed by a single tumor recurrence in the CD38-SA group and no recurrences in the CD38-bispecific group (P < .0001, either CD38 PRIT group vs either control group, error bars = 1 SEM). (B) Kaplan-Meier survival analysis. CD38-bispecific PRIT cured 75% of mice (all mortality due to early weight loss), whereas CD38-SA PRIT cured 88% of mice (all mortality due to tumor progression), demonstrating that at this 1200-µCi dose, the 2 CD38 treatments each benefitted survival with high and equivalent efficacy (P < .0001 for either CD38 treatment vs either control, P = .48 for CD38 bispecific vs CD38-SA). Cure was defined as the absence of tumor recurrence through day 150.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/131/6/10.1182_blood-2017-09-807610/4/m_blood807610f6.jpeg?Expires=1764965815&Signature=q1wxh6lUNySwCCNTyASbXcCaW7rJoiat0rhZX7n4Ke66jgr6xrLZ39JKqoqNYjCUd3-uw72dus11LZLc8wXBrBz-wuVD-r8YugKC6bZOzwPFkOAgYPRKwcQuEN5v4UZCIAuz7~PUrn6Fx0EemQzqULrCd6HPmNJr3WBQboKzfWK3gVQoTlCZT9xNJZLW3NbaqjRR3cNmm2s93Avvm1Wz3--lSXDYxWe9LvlG~0p-1iYDvOzJzDrMo47sBsr078F07HoiWb7dCdWYR8dgO-Ymiotr8Kr2QVpGwp0Zlonm8Le9yz~LiHKEMu9zbZdfffeE-PKtuWkF3uVrhJNrYsZRfw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 7. Comparative dose-response effects of CD38-bispecific PRIT vs CD38-SA PRIT on tumor growth and survival of mice bearing CD38+ BL xenografts. This figure presents data from 2 replicate experiments, each using 8 to 10 athymic nude mice per group. In total, n = 18 mice per treatment were injected at −24 hours with a pretargeting protein (CD38 bispecific, CD38-SA, or control [anti-CD20] bispecific), then at −1 hour with CA, and at 0 hours with 600 or 1000 µCi 90Y-DOTA–biotin. An additional n = 10 mice per group received only pretargeting protein (CD38 bispecific or CD38-SA) and CA, with no 90Y-DOTA–biotin. (A) All control mice including untreated (black), pretargeting protein without 90Y (maroon and navy), and control bispecific 1000-µCi groups (gray) experienced rapid tumor progression and died by day 14. All CD38 PRIT mice showed CR by day 11, and subsequent strongly reduced tumor progression relative to controls (P < .0001, any CD38 PRIT group vs any control group). However, CD38-bispecific PRIT (reds) outperformed CD38-SA PRIT (blues), the former showing later and fewer tumor progressions in the 600- and 1000-µCi treatment groups (P < .003, CD38 bispecific vs CD38-SA). (B) Kaplan-Meier survival analyses reflect the tumor volume results, showing greatly improved survival in each CD38 PRIT group relative to each control (P < .0001), and improved survival of CD38-bispecific PRIT mice relative to CD38-SA PRIT mice, across 600- and 1000-µCi levels (P < .004, CD38 bispecific vs CD38-SA).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/131/6/10.1182_blood-2017-09-807610/4/m_blood807610f7.jpeg?Expires=1764965815&Signature=XU~V5hBLdX2NzPA75L6TH9Ub8C5~o10UoQATyA2sjd3~jEwPiTl2ycZMIUveHBJkc4sQK~h8QBCBoVkHNGRl8jjLX4n72XgDPzNoEMQq9W5wcjqSOJvx3XhFpzcRg6J69RpGNS-CMokqZH6aJMy9j6T9AccWX1R8Gj9DT-1Tc3tXbXNbaASM65rKnvT9V0JXd97djSZKAtzexSnvSZ539HYgFftGmtyTaD6O5golLE2YihxP3Xqz9u4Xg8-Hp2mfxTUGW6BuWg-WIVjkFiTDCApht6hDhOxrHG9k6iDVr-DCHrtmN56SjQ27tIDAUAVR4y9f8YuEEK6J99VUic6DZA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal