TO THE EDITOR:
The recent Blood article by Schroeder et al demonstrates that plerixafor mobilizes a unique hematopoietic stem and progenitor cell (HSPC) product that is enriched in plasmacytoid dendritic cell (pDC) precursors (pre-pDCs).1 This study further reports that enrichment of these cells in plerixafor-mobilized allografts is associated with lower cumulative incidence of acute graft-versus-host disease (aGVHD) and chronic graft-versus-host disease (cGVHD). In an earlier study, Waller et al reported a protective advantage against aGVHD of pDCs derived from bone marrow (BM) but not granulocyte colony-stimulating factor (G-CSF)-mobilized peripheral blood (PB) graft.2 Studies have shown that allogeneic transplantation with a BM allograft results in lower rates of aGVHD and cGVHD when compared with G-CSF–mobilized PB graft. In a commentary to the Schroeder study, Waller proposes that the relatively rapid onset of mobilization by plerixafor (hours vs days by G-CSF) generates a novel PB graft that immunologically and phenotypically resembles cells harvested from bone marrow.3 He hypothesizes that this unique allograft contains immune subsets, including pre-pDCs and pDCs, which favorably modulate alloreactivity posttransplantation thus resulting in lower GVHD rates.
Recently, the Canadian Blood and Marrow Transplant Group (CBMTG) conducted a phase 3 randomized trial and compared the impact of G-CSF–mobilized PB to BM. In this trial, G-CSF–treated BM donors had a significantly lower frequency of cGVHD,4 similar to that seen in the Schroeder study. A further comprehensive investigation of the donor graft composition found that the lower rate of cGVHD in G-BM correlated most closely with a higher proportion of CD56bright regulatory NK cells (NKreg) represented as the percentage of CD56bright NKreg cells.5 Additional evidence of the importance of this immune-regulatory population is our recent observation of a correlation of low numbers of CXCR3+CD56bright NKreg cells with the onset of cGVHD in adults.6 Based on these observations, we hypothesized that an alternate donor cell population, with a selective increase in NKreg cells by plerixafor, may contribute to the lower rate of cGVHD seen in the recent Schroeder study.
To address this possible mechanism, we recruited 9 healthy adult human volunteers after full consent. This study was approved by the Dalhousie University and University of British Columbia (UBC) research ethical boards. All subjects were between 18 and 60 years of age and were enrolled at the Dalhousie University. All biological analyses were performed at the UBC-affiliated BC Children’s Hospital Research Institute. Five subjects received 1 dose of plerixafor at 240 μg/kg per day subcutaneously and PB and BM samples were harvested prior to administration and at 4 and 24 hours after plerixafor administration. The subsequent 4 participants each received 4 daily doses of G-CSF at 5 μg/kg per day for 4 days, followed by a dose of plerixafor on day 5. To minimize the impact of circadian rhythm, PB and BM samples were collected between 9 am and noon, prior to G-CSF administration, after G-CSF completion, and at 4 and 24 hours after plerixafor administration. We performed a focused analysis on CD56bright NKreg cells in these samples using multiparametric flow cytometry. Nucleated cells isolated from peripheral blood and bone marrow samples were stained with CD3, CD8, perforin, granzyme B, CD56 antibodies conjugated to fluorescein isothiocyanate, phycoerythrin, PB, Flow-Check 770 fluorospheres, and allophycocyanin, respectively. Stained cells were acquired by LSR II cytometer (BD) and the data were analyzed by FlowJo v10. The immunophenotype of NKreg cells is CD3-, CD56bright, perforin- and granzyme-B-.
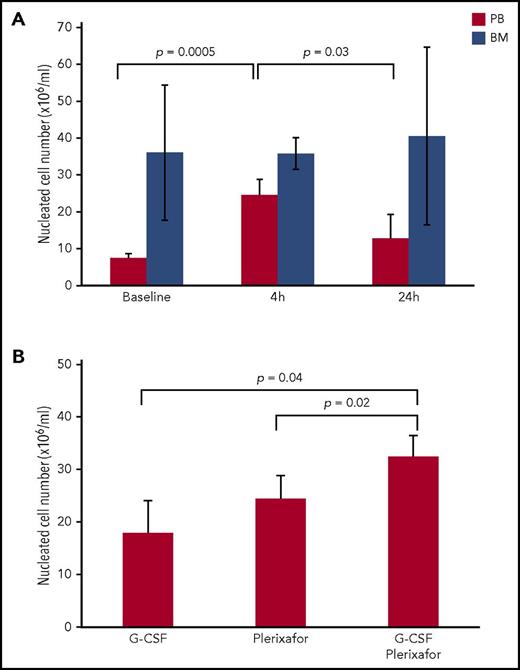
Plerixafor significantly mobilized nucleated cells to PB after 4 hours of treatment, whereas no significant difference was observed after 24 hours of treatment (Figure 1A). Furthermore, longer treatment time resulted in a significantly lower cell number indicating that plerixafor’s action peaked at 4 hours after administration. The magnitude of mobilization observed 4 hours after plerixafor administration was similar to that seen in the previous phase II clinical study.7 Interestingly, plerixafor did not alter the nucleated cell number in the bone marrow (Figure 1A). Four daily injections of G-CSF followed by plerixafor administration significantly mobilized more nucleated cells to PB than either treatment alone (Figure 1B). However, the two agents mobilized similar number of nucleated cells to PB separately. Therefore, plerixafor mobilized nucleated cells to peripheral blood more rapidly than G-CSF. Since the peak action of plerixafor was seen at 4 hours after administration, we selected this time point for further analyses of plerixafor effect on CD56 bright NKreg cells.
Effect of plerixafor and/or G-CSF administration on number of nucleated cells in peripheral blood and bone marrow. (A) Peripheral blood and bone marrow were harvested from 5 subjects prior to (baseline) and at 4 and 24 hours after administration of plerixafor (240 μg/kg). Total nucleated cells were isolated from peripheral blood and bone marrow. Statistical significance was determined by the 2-tailed paired Student t test. (B) Peripheral blood was harvested from subjects (1) after 4-daily administration of G-CSF (5 μg/kg); (2) after 4 hours of plerixafor (240 μg/kg) administration; and (3) after 4-daily injections of G-CSF followed by plerixafor administration. Total nucleated cells were isolated from peripheral blood. Statistical significance was determined by the 2-tailed Student t test.
Effect of plerixafor and/or G-CSF administration on number of nucleated cells in peripheral blood and bone marrow. (A) Peripheral blood and bone marrow were harvested from 5 subjects prior to (baseline) and at 4 and 24 hours after administration of plerixafor (240 μg/kg). Total nucleated cells were isolated from peripheral blood and bone marrow. Statistical significance was determined by the 2-tailed paired Student t test. (B) Peripheral blood was harvested from subjects (1) after 4-daily administration of G-CSF (5 μg/kg); (2) after 4 hours of plerixafor (240 μg/kg) administration; and (3) after 4-daily injections of G-CSF followed by plerixafor administration. Total nucleated cells were isolated from peripheral blood. Statistical significance was determined by the 2-tailed Student t test.
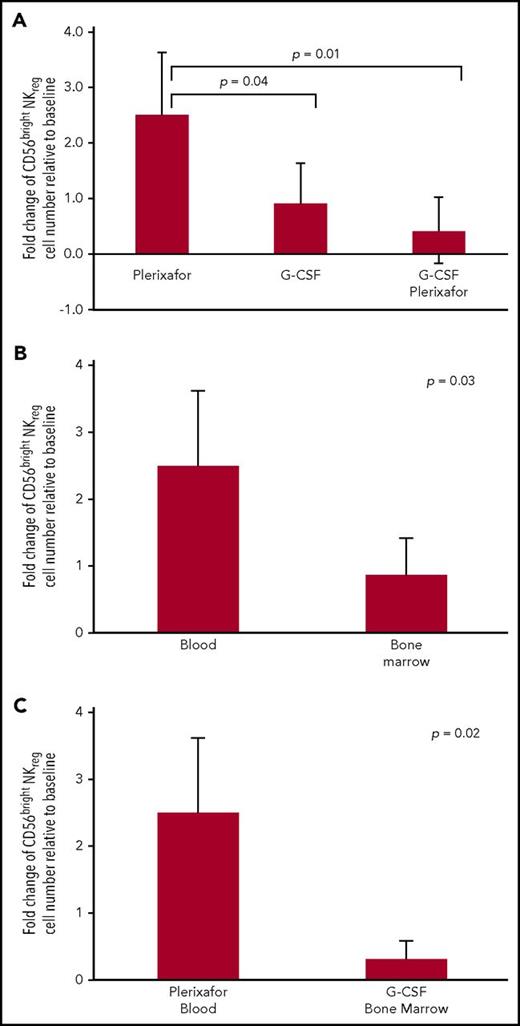
We next examined the effect of plerixafor on mobilizing CD56bright NKreg cells. Plerixafor induced a significantly higher rise of CD56bright NKreg cells in PB than 4 days of G-CSF alone or G-CSF followed by plerixafor (Figure 2A). This result suggested that plerixafor effectively mobilized CD56bright NKreg cells to the peripheral blood. Since the source of allograft influences risk of GVHD, we examined the effect of plerixafor on proportion of CD56bright NKreg cells in peripheral blood and bone marrow. We found a significant increase in proportion of CD56bright NKreg cells in PB relative to BM after plerixafor administration (Figure 2B). The CBMTG clinical trial demonstrates that G-CSF-mobilized bone marrow allograft associates with a lower incidence of GVHD compared with G-CSF-mobilized PB.4 Moreover, G-CSF mobilized bone marrow allograft that contains higher number of CD56bright NKreg cells is associated with lower frequency of GVHD.5 In this study, plerixafor effected a significantly larger increase in CD56bright NKreg cell population in PB than G-CSF stimulated BM allograft (Figure 2C). These results suggested that plerixafor mobilized the highest proportion of CD56bright NKreg cells to peripheral blood compared with bone marrow. One restriction of this study is that we evaluated peripheral blood rather than a peripheral apheresis product and apheresis may alter graft composition.
The effect of plerixafor and G-CSF on CD56brightNKregpopulations in peripheral blood and bone marrow. (A) Number of CD56bright NKreg cells was enumerated from peripheral blood by flow cytometry. Fold change was computed as ratio between number of CD56bright NKreg cells after 4 hours of plerixafor administration, 4-daily administration of G-CSF (5 μg/kg), or 4-daily administration of G-CSF (5 μg/kg) followed by plerixafor administration and baseline. Statistical significant difference was determined by the 2-tailed Student t test. (B) Number of CD56bright NKreg cells was enumerated from blood and bone marrow by flow cytometry. Fold change was computed as ratio between number of CD56bright NKreg cells after 4 hours of plerixafor and baseline. Statistical significant difference was determined by the 2-tailed Student t test. (C) Number of CD56bright NKreg cells was enumerated from peripheral blood and bone marrow by flow cytometry. Fold change was computed as (1) ratio between number of CD56bright NKreg cells after 4 hours of plerixafor and baseline in peripheral blood; (2) ratio between number of CD56bright NKreg cells after 4-daily G-CSF administration and baseline in bone marrow. Statistical significant difference was determined by the 2-tailed Student t test.
The effect of plerixafor and G-CSF on CD56brightNKregpopulations in peripheral blood and bone marrow. (A) Number of CD56bright NKreg cells was enumerated from peripheral blood by flow cytometry. Fold change was computed as ratio between number of CD56bright NKreg cells after 4 hours of plerixafor administration, 4-daily administration of G-CSF (5 μg/kg), or 4-daily administration of G-CSF (5 μg/kg) followed by plerixafor administration and baseline. Statistical significant difference was determined by the 2-tailed Student t test. (B) Number of CD56bright NKreg cells was enumerated from blood and bone marrow by flow cytometry. Fold change was computed as ratio between number of CD56bright NKreg cells after 4 hours of plerixafor and baseline. Statistical significant difference was determined by the 2-tailed Student t test. (C) Number of CD56bright NKreg cells was enumerated from peripheral blood and bone marrow by flow cytometry. Fold change was computed as (1) ratio between number of CD56bright NKreg cells after 4 hours of plerixafor and baseline in peripheral blood; (2) ratio between number of CD56bright NKreg cells after 4-daily G-CSF administration and baseline in bone marrow. Statistical significant difference was determined by the 2-tailed Student t test.
Our group recently published the effect of plerixafor and G-CSF in mobilizing hematopoietic stem and progenitor cells (HSPCs) in the identical group as presented in this paper.8 In that publication, we demonstrated that plerixafor induces the greatest increase in CD34+ cells in peripheral blood (PB) 4 hours after drug administration with or without G-CSF. Notably, this is the exact time-point when plerixafor caused on the highest rise of CD56bright NKreg cell number in PB in this paper. In our previous publication, we evaluated the impact of plerixafor in PB using both three-week long-term in vitro culture and in an in vivo xenotransplant assays. We found that plerixafor optimally mobilizes hematopoietic populations to reconstitute neutrophils and platelets in transplanted immune deficient mice. Thus, plerixafor effectively mobilizes HSPC subsets with rapid myeloid repopulating capacity.
In addition to its efficacy in mobilizing HSPCs, we demonstrated in this study that a single subcutaneous administration of plerixafor effected the highest rise of CD56bright NKreg cell number in peripheral blood of healthy donors when compared with 4-daily G-CSF administration and G-CSF/plerixafor combination. In light of the recent observation that higher proportions of CD56bright NKreg cells in donor grafts significantly correlate with lower cGVHD rates,5 our findings suggest plerixafor would provide a PB donor product enriched in NKreg cells and predicted to be protective against development of cGVHD. Indeed, Schroeder et al demonstrates that plerixafor-mobilized peripheral blood grafts result in lower incidence of acute and chronic GVHD when compared with G-CSF mobilized PB donor product.1 We propose that future donor mobilization strategies should focus on enriching for CD56bright NKreg cells, along with pre-pDCs and pDCs, in the donor product. We also suggest that the unique donor product mobilized by plerixafor is superior in minimizing cGVHD to G-CSF in allogeneic transplantation.
Acknowledgments
This work was supported by an unrestricted grant by Amgen (G-CSF), Genzyme, and the Division of Hematology, Dalhousie University.
Authorship
Contribution: P.P.C.W. analyzed and interpreted results, made figures, and wrote the paper; A.K. performed experiments and analyzed results; S.I. performed experiments; D.J., C.J.E., R.F., and S.C. designed research; and K.R.S. designed research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Kirk R. Schultz, Division of Pediatric Hematology/Oncology/Blood & Marrow Transplant, BC Children’s Hospital, 4480 Oak St, Vancouver, BC V6H 3V4, Canada; e-mail: kschultz@mail.ubc.ca.
REFERENCES
Author notes
P.P.C.W and A.K. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal