Key Points
Moxetumomab pasudotox eradicated HCL MRD in >50% of CRs, even by the most sensitive measure, bone marrow aspirate flow cytometry.
Elimination of MRD was significantly associated with prolonged CR duration.
Abstract
Anti-CD22 moxetumomab pasudotox achieved 46% complete remissions (CRs) in previously reported phase 1 testing in relapsed/refractory hairy cell leukemia (HCL; n = 28). The importance of minimal residual disease (MRD) after CR in HCL is unknown. A 21-patient extension cohort received 50 µg/kg every other day for 3 doses in 4-week cycles. These patients plus 12 previously reported at this upper dose level received 143 cycles without dose-limiting toxicity. The combined 33-patient cohort achieved 64% CR and 88% overall response rates, with median CR duration of 42.4 months. Of 32 50-µg/kg patients evaluable for MRD by bone marrow aspirate flow cytometry (most stringent assessment), median CR duration was 13.5 (4.9-42.4) months in 9 MRD-positive CRs vs 42.1 (24.0-69.2) months in 11 MRD-negative CRs (P < .001). Among MRD-negative CRs, 10 patients had ongoing CR, 9 without MRD, at end of study. To our knowledge, moxetumomab pasudotox is the only nonchemotherapy regimen that can eliminate MRD in a significant percentage of HCL patients, to enhance CR duration. Repeated dosing, despite early neutralizing antibodies, increased active drug levels without detectable toxicity from immunogenicity. The activity and safety profiles of moxetumomab pasudotox support ongoing phase 3 testing in HCL. This trial was registered at www.clinicaltrials.gov as #NCT00586924.
Introduction
Anti-CD22 recombinant immunotoxin moxetumomab pasudotox achieved 46% complete remission (CR) and 86% overall response rates (ORR) in 28 patents with relapsed/refractory hairy cell leukemia (HCL) treated at 5 to 40 µg/kg (n = 16) and 50 µg/kg (n = 12) every other day for 3 doses in 4-week cycles, without dose-limiting toxicities.1 Grade 2 hemolytic uremic syndrome (HUS) with reversible grade 1 thrombocytopenia and creatinine elevations occurred in 1 patient each at 30 and 50 µg/kg. The present study includes 21 additional patients in an expanded 50-µg/kg cohort.
Study design
Study design (#NCT00586924) was described previously.1 Flow cytometry is described in supplemental Table 1 (available on the Blood Web site).2 Groups of numeric data were compared nonparametrically by Wilcoxon rank-sum or Mann-Whitney U test. Groups with 2 outcomes were compared by Fisher’s exact test. All P values were numeric and 2-sided. Data analyses used SAS (SAS Institute, Cary, NC) version 8.2 or later. The study protocol, protocol amendments and administrative changes, and subject informed consent documents were submitted for review to the institutional review board or independent ethics committee for each investigational site.
Results and discussion
Patients’ characteristics and toxicity
Patient ages (40-77 years), male-to-female ratio (5:1), median prior purine analog courses (2-3), percentage of patients with prior rituximab (50% to 67%), maximum spleen size (145-150 mm), and hematologic parameters were similar regarding dose level (supplemental Table 2). None of the 88 cycles administered to the 21 in the extension cohort were associated with dose-limiting toxicity or HUS (details of toxicities, see supplemental Tables 3-6). In the combined 50-µg/kg cohort (n = 33), 4 patients died of disease progression after relapsing and going off-study, including 1 with a best response of minimal residual disease (MRD)–positive CR who developed grade 2 HUS after 5 moxetumomab pasudotox cycles. After relapsing, these 4 were treated off-protocol with pentostatin-rituximab (n = 2), rituximab (n = 1), and rituximab-bendamustine (n = 1) and died 2 to 29 (median, 17) months after last dose of moxetumomab pasudotox. In 9 patients evaluated for lymphocyte subsets before and after treatment (supplemental Figure 1), circulating HCL cells cleared in all patients, but normal B cells decreased <50% in 3 and increased in other patients (supplemental Figure 1A). Total T cells and CD4+ and CD8+ T-cell subsets increased in all except 1 with normal pretreatment and posttreatment CD8 counts (supplemental Figure 1B-D). Thus, anti-CD22 moxetumomab pasudotox spared T cells and, with its short half-life, avoided prolonged B-cell depletion.
Response
At 50 µg/kg, moxetumomab pasudotox achieved 71% CR and 91% ORR in the 21-patient extension cohort, and 64% CR and 88% ORR in the 33-patient combined cohort (supplemental Table 7). Among 21 CRs, MRD was detected in 8 (40%) only by bone marrow aspirate (BMA) flow cytometry, in 1 by both BMA and blood flow cytometry, undetectable by both in 11 (55%), and not evaluated in 1 (supplemental Table 8). CR was unrelated to number of prior purine analog courses, prior rituximab, or baseline spleen size. However, at 50 μg/kg, the CR rate was 0% in 4 patients with prior splenectomy vs 72% in 29 others (P = .012). Time to major response (CR or partial response; supplemental Figure 2) was shorter at 50 than 5 to 40 μg/kg (median 4.4 vs 8.7 weeks; P = .05). At 50 μg/kg, time to CR was 4 to 110 (median 17) weeks. For 6 patients with longest time to CR (11-25 months), positive bone marrow at end of treatment became negative during follow-up, despite no intervening therapy. Thus, in the absence of the myelotoxicity expected from chemotherapy, patients responded quickly and achieved CR, usually at the first response assessment bone marrow performed after resolution of cytopenias.
MRD and durability of CR
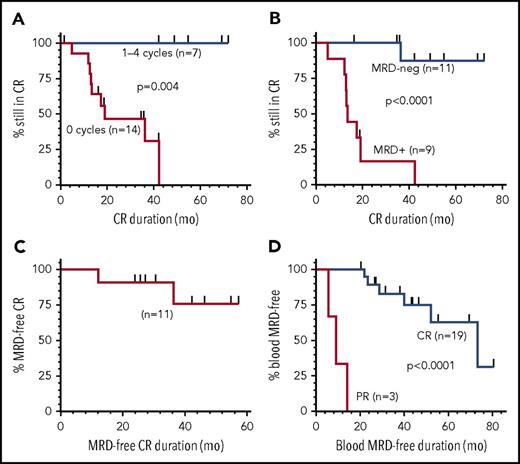
Median duration of the 21 CRs at 50 µg/kg was 42.4 months; none of 7 relapsed after consolidation cycles, whereas 9 of 14 without consolidation relapsed at a median 13.5 months (range, 4.9-42.4) (Figure 1A; P = .004). Of the 20 CRs evaluated for MRD, median CR duration was 35.3 months; 11 (55%) were MRD negative, and 10 remained in CR at end of study, with median CR duration 42.2 months (range, 16.3-72.1). Nine (45%) CRs were MRD+, with median CR duration 13.5 months (range, 4.9-42.4) and 8 relapses (Figure 1B; P < .0001). As shown in Figure 1C, median duration of MRD-negative CR has not been reached, with 9 of 11 MRD-negative patients remaining MRD negative for a median of 42.1 months (range, 24.0-69.2). Thus, moxetumomab pasudotox at 50 μg/kg, particularly with consolidation cycles, cleared MRD in more than half of CRs. MRD clearance was associated with improved CR duration, consistent with MRD studies of other leukemias.3,4 Blood MRD was analyzed because most HCL patients are not followed with posttreatment bone marrow assessments. Of 29 evaluated at 50 μg/kg, 22 (76%) clearing MRD in blood had higher CR (19 of 22 vs 1 of 7, P = .001) and ORR (22 of 22 vs 4 of 7, P = .01) compared with those not clearing blood. Median blood MRD-negative duration, shown in Figure 1D, was 73.4 months (range, 8.1-80.6) for 19 CRs vs 8.9 months (range, 5.6-14) for 3 partial responders (P < .001). Thus, achieving CR was associated with clearing MRD in blood and remaining blood MRD free.
Duration of CR. CR duration is shown for patients at 50 μg/kg by consolidation cycles received (A) and by those evaluable for MRD (B). Durations of MRD-negative CR (C) and MRD negativity in blood for patients achieving CR or partial response are shown (D). MRD evaluation at the National Cancer Institute used multicolor flow cytometry (detection level of 0.002% to 0.006%) in blood and BMA (see supplemental Table 1 for details). MRD was also determined by immunohistochemistry of the bone marrow biopsy. Bone marrows were typically performed after cytopenias resolved consistent with CR, at end of treatment, annually at 0.5 to 2.5 years after best response, and every 2 years thereafter. Flow cytometry of blood was performed precycle, at end of treatment, every 6 months after best response until 2.5 years, and annually thereafter. Time to achieve and duration of response were calculated using Kaplan-Meier, with curves compared by log-rank. PR, partial response.
Duration of CR. CR duration is shown for patients at 50 μg/kg by consolidation cycles received (A) and by those evaluable for MRD (B). Durations of MRD-negative CR (C) and MRD negativity in blood for patients achieving CR or partial response are shown (D). MRD evaluation at the National Cancer Institute used multicolor flow cytometry (detection level of 0.002% to 0.006%) in blood and BMA (see supplemental Table 1 for details). MRD was also determined by immunohistochemistry of the bone marrow biopsy. Bone marrows were typically performed after cytopenias resolved consistent with CR, at end of treatment, annually at 0.5 to 2.5 years after best response, and every 2 years thereafter. Flow cytometry of blood was performed precycle, at end of treatment, every 6 months after best response until 2.5 years, and annually thereafter. Time to achieve and duration of response were calculated using Kaplan-Meier, with curves compared by log-rank. PR, partial response.
Pharmacokinetics and immunogenicity of moxetumomab pasudotox
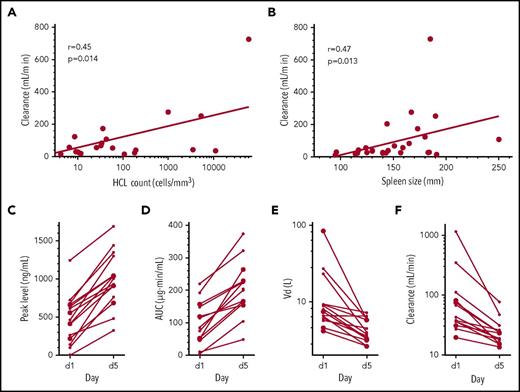
From the dose-escalation phase, we reported dose-dependent plasma levels and areas under the concentration-time curve (AUCs).1 To determine the pharmacokinetics at a fixed dose, we analyzed plasma levels from 29 patients at 50 µg/kg. Peak levels increased 213% during cycle 1 and 121% during cycle 2 from days 1 to 5 (P < .001 and P = .002, respectively; supplemental Tables 9 and 10), suggesting rapid reduction in the CD22+ disease sink effect during treatment. Significant increases in half-life and AUC, and decreases in volumes of distribution and clearances, were also observed (P < .001 to .009). Clearance on day 5 of cycle 1, evaluated at that time point because plasma levels were often too low to measure on day 1, correlated with tumor burden measured by either circulating HCL cells (r = 0.45, P = .01) or spleen size (r = 0.47, P = .01; Figure 2A-B). In Figure 2C-F, pharmacokinetic parameters are shown on days 1 and 5 of cycles beginning immediately after obtaining serum with ≥50% neutralization5-8 of 200 ng/mL, making patients ineligible for future cycles.
Pharmacokinetic parameters, tumor burden, and immunogenicity. In patients receiving 50 µg/kg, clearance during cycle 1 day 5 was related to hairy cell count (A) and spleen size (B). At this dose level, peak levels (C), AUC (D), volumes of distribution (Vd; E), and clearance (F) on days 1 and 5 are shown for cycles given to 15 patients who had >50% neutralization (median, 93%) of 200 ng/mL documented from serum samples just before day 1. These patients were evaluated during cycles 2 (n = 3), 3 (n = 5), 4 (n = 4), 5 (n = 2), and 6 (n = 1) and received that cycle, because the result was known only after completion of the cycle, but were excluded from further retreatment. For the extension cohort enrolled at National Cancer Institute, immunogenicity was determined by in vitro cytotoxicity.1 Enrollment or retreatment for the extension cohort required ≤50% neutralization of 200 ng/mL of moxetumomab pasudotox. Approximately 17% of relapsed HCL/HCL variant patients have >50% neutralization of 200 ng/mL and would be ineligible for enrollment. Pharmacokinetic analyses were previously described.5-8
Pharmacokinetic parameters, tumor burden, and immunogenicity. In patients receiving 50 µg/kg, clearance during cycle 1 day 5 was related to hairy cell count (A) and spleen size (B). At this dose level, peak levels (C), AUC (D), volumes of distribution (Vd; E), and clearance (F) on days 1 and 5 are shown for cycles given to 15 patients who had >50% neutralization (median, 93%) of 200 ng/mL documented from serum samples just before day 1. These patients were evaluated during cycles 2 (n = 3), 3 (n = 5), 4 (n = 4), 5 (n = 2), and 6 (n = 1) and received that cycle, because the result was known only after completion of the cycle, but were excluded from further retreatment. For the extension cohort enrolled at National Cancer Institute, immunogenicity was determined by in vitro cytotoxicity.1 Enrollment or retreatment for the extension cohort required ≤50% neutralization of 200 ng/mL of moxetumomab pasudotox. Approximately 17% of relapsed HCL/HCL variant patients have >50% neutralization of 200 ng/mL and would be ineligible for enrollment. Pharmacokinetic analyses were previously described.5-8
Peak levels and AUCs increased relative to day 1, and the volumes of distribution and clearances decreased. Supplemental Table 11 lists additional immunogenicity data. Enzyme-linked immunosorbent assays documented decreases in soluble CD22, with reduction of HCL burden (supplemental Table 12; supplemental Figure 3). Thus, moxetumomab pasudotox at 50 µg/kg achieved cytotoxic plasma levels that increased with rapid resolution of tumor burden and were not completely blocked when neutralizing antibodies were first detectable. In fact, these early levels of neutralizing antibodies could be at least partially titrated out by repeated dosing.
In conclusion, the extension cohort at 50 μg/kg shows for the first time that eradication of MRD by moxetumomab pasudotox in blood and marrow can be achieved in most CRs and is associated with prolonged CR duration. These data compare favorably to studies of treatments for multiply relapsed HCL, including vemurafenib, which achieves MRD+ CR rates of 35% to 42% with relapse at a median 19 months,9 and combinations of rituximab with purine analogs, which achieve MRD-negative CRs but with prolonged myelotoxicity.10-13 A multicenter pivotal study of moxetumomab pasudotox is ongoing in relapsed/refractory HCL.
Presented in part at the 2015 American Society of Clinical Oncology Annual Meeting, Chicago, IL, 29 May to 2 June 2015; and at the 57th annual meeting of the American Society of Hematology, Orlando, FL, 5-8 December 2015.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the patients and their families for participating in this study; the trial site clinical research teams and staff; and Isoke Sawyer, Meina Liang, Yu Gu, Fatemeh Tavakkoli, Jie Yang, Nai Shun Yao, and the MedImmune study team. Hong Zhou performed the soluble CD22 assays. Neutralization immunogenicity bioassays were performed by David Waters and Timothy McNickle and analyzed by Barbara Debrah. Flow cytometry data were analyzed in part by Constance Yuan. The clinical research staff at the National Cancer Institute involved in patient testing included Sonya Duke, Lesley Mathews, Rita Mincemoyer, Elizabeth Maestri, Natasha Kormanik, Amanda Wiggins, Theresa Yu, Laura Wisch, and Julie Feurtado. Peloton Advantage LLC (Parsippany, NJ) provided editorial assistance, which was funded by MedImmune.
This research was supported in part by the Intramural Research Program of the National Institutes of Health (NIH), the National Cancer Institute (NCI) Center for Cancer Research, a cooperative research and development agreement between the NIH, NCI, and MedImmune. This clinical study was sponsored by MedImmune.
Authorship
Contribution: R.J.K., M.S.T., D.J.F., W.H.W., M.C.L., and I.P. designed the study; R.J.K., M.S.T., T.R., and S.C. were study investigators; R.J.K., M.S.T., T.R., and S.C. provided study patients; R.J.K., M.S.T., T.R., and S.C. collected and assembled the data; R.J.K., M.S.-S., L.S., G.G., M.C.L., and I.P. contributed to data analysis and interpretation; R.J.K., D.J.F., G.G., M.C.L., and I.P. prepared the manuscript; and all authors critically reviewed and revised the manuscript and granted approval for submission of this manuscript.
Conflict-of-interest disclosure: I.P., D.J.F., and R.J.K. are coinventors on immunotoxin patents assigned to the government of the United States, as represented by the Secretary of the Department of Health and Human Services on behalf of the National Institutes of Health, which are licensed by Medimmune. L.S., G.G., and M.C.L. are employees of MedImmune. The remaining authors declare no competing financial interests.
Correspondence: Robert J. Kreitman, National Institutes of Health, Building 37/5124b, 9000 Rockville Pike, Bethesda, MD 20892; e-mail: kreitmar@mail.nih.gov.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal