In this issue of Blood, Kreitman and colleagues update results of a study of moxetumomab pasudotox in patients with relapsed hairy cell leukemia (HCL), demonstrating a high response rate among the 33 patients reported. Among the 32 patients who had minimal residual disease (MRD) assessment in their bone marrow specimens using flow cytometry, median complete remission (CR) duration was significantly longer in the patients who became MRD negative (median 42.1 months vs 13.5 months in the MRD-persistent patients, P < .001). These results support the argument for validity of MRD monitoring in HCL.1
In hematological cancers, the relevance of MRD, defined as disease persisting after curative intent therapy, is increasing not only due to the development of more sensitive assays able to detect submicroscopic residual disease but also due to the increasing availability of more effective agents able to produce increasingly deep responses.2 However, with progress comes complexity. The difficulty in the identification of the actual disease-sustaining population of cells (commonly referred to as stem cells), the presence of clonal diversity within the leukemic cells, the occurrence of clonal evolution with disease progression, and the ability of some clones to persist long term without predisposing to relapse are a number of issues that have complicated the “simple” concept that eradicating MRD should lead to better outcomes. Furthermore, the availability of a number of assays able to detect MRD and the lack of harmonization and standardization of these assays have hindered the general clinical utility of MRD assessment to predict outcome and to select postremission therapy.3 Clear exceptions to this are diseases such as acute promyelocytic leukemia, whereby there is a specific disease-sustaining molecular aberration, and with a well-established quantitative reverse transcription polymerase chain reaction assay, achieving complete molecular remission has become the standard goal of therapy.4
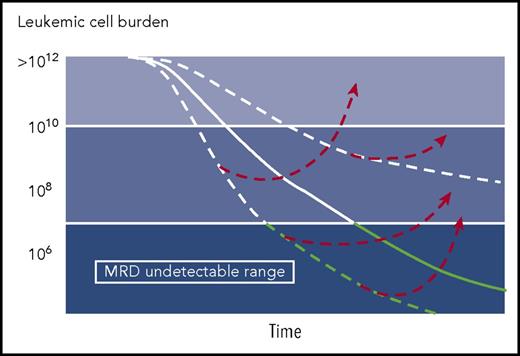
In HCL, persisting disease detected by immunohistochemistry using anti-CD45RO, anti-CD20, and DBA.44 in paraffin-embedded bone marrow sections after initial therapy with nucleoside analogs was associated with a shorter relapse-free survival.5 However, the investigators from Scripps Clinic evaluated bone marrow samples from 19 asymptomatic patients in continuous complete hematological response after initial therapy with a course of cladribine with a median elapsed time from therapy of 16 years and reported the presence of MRD or morphological disease in 10 of 19 (53%) patients, clearly demonstrating the possibility of long-term relapse-free survival while still harboring HCL cells.6 Therefore, the proliferative capacity and the kinetics of relapse are important factors to consider when analyzing MRD data in various leukemias (see figure) and may argue against the need for MRD monitoring in very indolent leukemias such as HCL.
Using 8 weekly rituximab infusions a month after initial cladribine therapy, we have reported eradication of MRD (monitored by flow cytometry in bone marrow specimens performed after the completion of rituximab therapy) in 76% of untreated patients with classic HCL and 64% of patients with relapsed disease.7,8 When peripheral blood flow monitoring was conducted subsequently, 94% of patients achieved a negative MRD status with further follow-up (median time to MRD negative status 2.9 months, range 0.8-18.9 months).8 Among the patients with classic HCL, only 2 patients required subsequent therapy; hence, it was not possible to correlate MRD-negative status with long-term relapse-free survival. Furthermore, only 4 patients had MRD recurrence. Others have reported a very high rate of MRD-negative CR in patients with relapsed and refractory HCL treated with bendamustine and rituximab.9
Clearly, with the development of more effective strategies in HCL such as chemoimmunotherapy, more patients are likely to achieve responses in the MRD undetectable range, which is likely to reduce the overall risk of relapse. Furthermore, novel agents such as moxetumomab pasudotox and BRAF inhibitors such as vemurafenib and dabrafenib (alone or in combination with other agents), that may convert a remission from MRD positive to MRD negative, can be evaluated to reduce relapse risk. Whether such regimens should be used in the initial therapy of patients with HCL remains unclear particularly as the success of therapy with nucleoside analogs has reduced interest in developing new frontline strategies. With the identification of a specific oncogenic molecular aberration in HCL, BRAF V600E mutation,10 molecular monitoring for the determination of MRD in HCL is also plausible. However, these questions can only be answered in the setting of well-designed trials; the rarity of the disease will necessitate multicenter collaborative efforts perhaps guided by the Hairy Cell Leukemia Consortium or other cooperative groups.
Conflict-of-interest disclosure: F.R. received research funding from Medimmune.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal