Key Points
Iron released from heme by HO 1 contributes to the pathophysiology of thalassemia.
Inhibition of HO 1 is of therapeutic value for the treatment of thalassemia.
Abstract
Thalassemias are a heterogeneous group of red blood cell disorders, considered a major cause of morbidity and mortality among genetic diseases. However, there is still no universally available cure for thalassemias. The underlying basis of thalassemia pathology is the premature apoptotic destruction of erythroblasts causing ineffective erythropoiesis. In β-thalassemia, β-globin synthesis is reduced causing α-globin accumulation. Unpaired globin chains, with heme attached to them, accumulate in thalassemic erythroblasts causing oxidative stress and the premature cell death. We hypothesize that in β-thalassemia heme oxygenase (HO) 1 could play a pathogenic role in the development of anemia and ineffective erythropoiesis. To test this hypothesis, we exploited a mouse model of β-thalassemia intermedia, Th3/+. We observed that HO inhibition using tin protoporphyrin IX (SnPP) decreased heme-iron recycling in the liver and ameliorated anemia in the Th3/+ mice. SnPP administration led to a decrease in erythropoietin and increase in hepcidin serum levels, changes that were accompanied by an alleviation of ineffective erythropoiesis in Th3/+ mice. Additionally, the bone marrow from Th3/+ mice treated with SnPP exhibited decreased heme catabolism and diminished iron release as well as reduced apoptosis. Our results indicate that the iron released from heme because of HO activity contributes to the pathophysiology of thalassemia. Therefore, new therapies that suppress heme catabolism may be beneficial in ameliorating the anemia and ineffective erythropoiesis in thalassemias.
Introduction
Thalassemias are a heterogeneous group of red blood cell (RBC) disorders characterized by ineffective erythropoiesis, peripheral hemolysis, and anemia.1 Among inherited genetic diseases, β-thalassemia is considered a major cause of morbidity and mortality worldwide. However, there is still no optimal treatment or universally available cure for mild and severe forms of this disease. Three mouse models (th1, th2, and th3) of β-thalassemia have been generated to date. Th3 mice, used in this study, have a deletion of both βmajor and βminor genes.2 Mice homozygous for the deletion (Th3/Th3) die late in gestation and heterozygotes (Th3/+) survive and develop a phenotype similar to β-thalassemia intermedia in humans (anemia, tissue iron overload, and ineffective erythropoiesis).2
Heme is a complex of iron with protoporphyrin IX that is essential for the function of all aerobic cells. In man, most organismal heme (75%-80%) is present in circulating RBC as hemoglobin’s prosthetic group. Senescent RBC is phagocytized by macrophages of the spleen and liver, and the heme catabolized3 by the heme-inducible heme oxygenase 1 (HO-1), yielding iron, bilirubin and carbon monoxide.4,5 HO-1 is a 32.8-kDa membrane-bound enzyme found at highest levels in macrophages of the liver and spleen where HO-1 activity is essential for the recycling of heme-iron.6 Additionally, HO-1 is increased in animal tissues not only by its physiological substrate heme, but also various metals, xenobiotics, endocrine factors, synthetic metalloporphyrins,7 and several agents that cause oxidative damage.8-10
Heme catabolism in the reticuloendothelial system yields approximately 25 mg of iron per day, which is mainly consumed by immature erythroid cells of the bone marrow for hemoglobin synthesis.3 We have recently shown that HO-1 is highly expressed in murine erythroid cells and that, similar to other cell types, its expression is induced by heme.11 In these cells, HO-1 acts as a coregulator of erythroid differentiation, in which the enzyme modulates the “heme-regulatory pool.”11 In erythroid cells, the heme-regulatory pool has been estimated to be in low micromolar levels.12,13 Nevertheless, it has a crucial importance for the regulation of the hemoglobin synthesis because it induces transferrin receptor 1 (TfR1),11,14 HO-1,11 and α/β globin15-17 expression and inhibits iron acquisition from transferrin in erythroid cells.18
Thalassemia results from an imbalanced production of globin chains. In β-thalassemia, there is diminished expression of β-globin genes, resulting in an excess of α-globin chains and vice versa in α-thalassemia.19 The accumulation of excess unmatched globin chains lead to the premature destruction of erythroid precursors in the bone marrow and RBC hemolysis in the peripheral blood.19 Therefore, in thalassemias, there is an increased rate of heme recycling and iron turnover, which directly involves HO-1 activity. HO-1 is widely presented in the literature as a protective enzyme,20-23 because it potentially removes prooxidant “free” heme, generated in stress conditions, and releases biliverdin and bilirubin, which are metabolites with antioxidant properties.24,25 However, the reaction catalyzed by HO-1 also releases iron, which is a potent prooxidant and can induce tissue damage and cell death. Tissue iron overload is one of the main features associated with thalassemias. Additionally, in thalassemia, excess iron delivery to the bone marrow significantly contributes to the shortened RBC lifespan and causes anemia.26 We therefore hypothesize that HO-1–mediated heme-iron recycling aggravates the thalassemic phenotype.
In the present study, we demonstrate that inhibition of HO ameliorates anemia, suppresses ineffective erythropoiesis, and decreases liver iron overload in β-thalassemic (Th3/+) mice. HO inhibition in the erythroid bone marrow cells resulted in decreased heme catabolism and iron release. Our results suggest that HO suppression may improve the pathology of β-thalassemia by decreasing HO-mediated iron release.
Methods
Study approval
All of the animal studies performed in this work were approved by the Lady Davis Institute’s Animal Care Committee following the guidelines of the Canadian Council on Animal Care.
Animals
SnPP preparation
On the day of the injections, tin protoporphyrin IX (SnPP; Frontier Scientific, UT) was freshly dissolved in sodium hydroxide (0.1 M) and then diluted in phosphate-buffered saline (PBS) to a final concentration of 5 mM. HCl (1 N) was added to the 5 mM SnPP solution to obtain a neutral pH. Six-month-old control C57BL/6 (wild-type [Wt]) and Th3/+ littermates were injected (intraperitoneally) 3 times per week with either PBS or SnPP (40 µmol/kg per day) for 4 consecutive weeks. SnPP has been administered previously to both mice27 and humans.28-30 In mice, a 40 µmol/kg per day dose was used in sickle cell mice.27 Because RBC half-life is about 12 days in mice,30 we administered SnPP for 4 weeks; hence, the HO-1 inhibition would be sustained until all circulating RBC are renewed. In humans, SnPP half-life in plasma is about 3.4 hours.28 A similar compound, tin-mesoporphyrin was administered to 2 human patients during 400 days with no side effects, except iron deficiency.29 Therefore, the regimen used in the present work is appropriate.
Total heme measurement
The heme content in tissues was quantified fluorometrically using Sassa’s method31 (see the supplemental Methods on the Blood Web site).
Flow cytometry analysis
Erythropoiesis in bone marrow and spleen cells was analyzed as described previously.32 Briefly, femora and spleen from Wt and Th3/+ mice were collected and their bone marrows extracted. Cells were then incubated for 45 minutes with CD71-fluorescein isothiocyanate and Ter119-APC antibodies (BD Bioscience, CA). Stained cells were then analyzed in the FACSCalibur (BD Bioscience) or sorted according their expression of CD71-fluorescein isothiocyanate and Ter119-APC in the FACSAria Fusion (BD Bioscience).
RBC membrane-associated globin precipitate
Lifespan of fluorescence-labeled RBC
RBC were isolated from the blood of Wt + PBS, Wt + SnPP, Th3/+ + PBS, and Th3/+ + SnPP mice by cardiac puncture and stained in vitro with carboxyfluorescein diacetate succinimidyl ester (CFSE; Molecular Probes, Invitrogen, CA), as described previously.35 Fluorescence-labeled RBC (1 × 109 in 100 µL PBS) were injected into the tail veins of Wt mice and their fluorescence tracked for 25 days.
59Fe recycling studies
59Fe recycling studies were performed as before,36 with a few changes, which are described in further detail in the supplemental Methods. Briefly, 59Fe-labeled RBC were opsonized and injected, intraperitoneally, in the “Wt + PBS, “WT + SnPP, Th3/+ + PBS, and Th3/+ + SnPP treated mice. After 7 hours, the mice were euthanized, perfused, and the radioactivity of blood, liver, spleen, kidneys, heart, and femurs measured in a Packard Cobra γ counter (PerkinElmer, CA).
Mouse colony-forming assay
Freshly isolated bone marrow cells were plated in 1.2 mL of methylcellulose media (StemCell Technologies) according to the manufacturer’s instructions. M3334 and M3534 media were used for the detection of colony forming unit-erythroid (CFU-E) and burst forming unit-erythroid (BFU-E) colonies. In selected cultures, SnPP at a final concentration of 50 μM was added at the beginning of the cultivation. CFU-E and BFU-E colonies were scored by standard morphologic criteria on day 2 and 7, respectively under an inverted microscope (Invertoskop, Carl Zeiss, Oberkochen, Germany).
Statistical analysis
Statistical analysis was performed using Statistica 7.0 software (StatSoft, Inc., OK). The analysis of data with more than 2 groups was performed applying analysis of variance. Differences between the groups were determined using Duncan’s test.
Results
HO-1 expression is increased in the liver of Th3/+ mice
Despite the central role of HO-1 in heme-iron recycling,3-6 its expression levels have never been assessed in Th3/+ mice. We found HO-1 messenger RNA (mRNA) and protein-levels to be increased in Th3/+ livers compared with Wt controls (Figure 1A-B). HO-1 staining of the liver confirmed the increase in its expression in Th3/+ mice (Figure 1C). In contrast, HO-1 mRNA and protein levels in the kidney and heart in the Th3/+ mice are similar to those seen in Wt animals (supplemental Figure 1A-D).
HO-1 expression is increased in the liver and decreased in Ter119+cells of the bone marrow and the spleen of Th3/+mice. (A) Quantitative reverse transcription polymerase chain reaction (qRT-PCR) analysis of HO-1 mRNA expression in the liver of Wt and Th3/+ mice. The results are presented as fold of change relative to the Wt sample (n = 3). (B) Western blot analysis of HO-1 protein expression in the liver of Wt and Th3/+ mice (n = 3). (C) Representative pictures (20× magnification; scale bar, 200 µM) of immunohistochemical staining against HO-1 in the liver from Wt and Th3/+ mice. (D) qRT-PCR analysis of HO-1 mRNA expression in Ter119− and Ter119+ cell populations isolated from the bone marrow of Wt and Th3/+ mice. The results are presented as fold change relative to Wt Ter119− cells (n = 3). (E) Western blot analysis of HO-1 protein expression in Ter119+ bone marrow samples (n = 3). (F) Total heme levels in Ter119− and Ter119+ cells isolated from the bone marrow of Wt and Th3/+ mice (n = 6). (G) Western blot analysis of HO-1 protein expression in Ter119+ spleen samples (n = 3). (H) Total heme levels in Ter119− and Ter119+ cells isolated from the spleen of Wt and Th3/+ mice (n = 6). *P < .05, ***P < .001.
HO-1 expression is increased in the liver and decreased in Ter119+cells of the bone marrow and the spleen of Th3/+mice. (A) Quantitative reverse transcription polymerase chain reaction (qRT-PCR) analysis of HO-1 mRNA expression in the liver of Wt and Th3/+ mice. The results are presented as fold of change relative to the Wt sample (n = 3). (B) Western blot analysis of HO-1 protein expression in the liver of Wt and Th3/+ mice (n = 3). (C) Representative pictures (20× magnification; scale bar, 200 µM) of immunohistochemical staining against HO-1 in the liver from Wt and Th3/+ mice. (D) qRT-PCR analysis of HO-1 mRNA expression in Ter119− and Ter119+ cell populations isolated from the bone marrow of Wt and Th3/+ mice. The results are presented as fold change relative to Wt Ter119− cells (n = 3). (E) Western blot analysis of HO-1 protein expression in Ter119+ bone marrow samples (n = 3). (F) Total heme levels in Ter119− and Ter119+ cells isolated from the bone marrow of Wt and Th3/+ mice (n = 6). (G) Western blot analysis of HO-1 protein expression in Ter119+ spleen samples (n = 3). (H) Total heme levels in Ter119− and Ter119+ cells isolated from the spleen of Wt and Th3/+ mice (n = 6). *P < .05, ***P < .001.
Expression of HO-1 in erythroid cells of the bone marrow and spleen is decreased in Th3/+ mice
Using the erythroid surface marker Ter119,37 we isolated Ter119− and Ter119+ cells from the bone marrow of Wt and Th3/+ mice. Compared with Wt controls, we observed that HO-1 mRNA and protein expression are decreased in Ter119+ cells of Th3/+ mice (Figure 1D-E). We also found that the total heme content is decreased in Ter119+ cells of Th3/+ mice (Figure 1F). Th3/+ mice have an enlarged spleen because of increased extramedullary erythropoiesis.38 Similarly, Ter119+ cells from the spleen have decreased HO-1 protein expression (Figure 1G) and heme content (Figure 1H). Ter119+ cells can be further differentiated, according their expression of TfR1 (CD71), in CD71highTer119high (basophilic), and CD71lowTer119high (orthochromatic) erythroblasts. After separating those cell types from the mouse bone marrow and the spleen, we observed that HO-1 mRNA expression is significantly decreased in Th3/+ basophilic erythroblasts when compared with Wt cells at the same stage of differentiation in both organs (supplemental Figure 2A-B).
HO inhibition ameliorates anemia in Th3/+ mice
Metalloporphyrins, such as SnPP, have been used as inhibitors of HO on the basis of their structural similarity to heme; this makes them useful HO competitive inhibitors. In the group of metalloporphyrins (ie, iron-/zinc-/tin/chromium-protoporphyrins), SnPP was found to be the most potent HO inhibitor.39,40 Therefore, we selected SnPP and investigated its effect in Th3/+ mice. SnPP administration (40 µmol/kg per day, 3 times/week during 4 weeks; see our “Methods” section) efficiently inhibited HO-1 activity in mice as demonstrated by the significant reduction in direct bilirubin levels in Th3/+ mice (supplemental Figure 3A). The Th3/+ mice also exhibited a significant increase in RBC counts, hemoglobin (Hb) and hematocrit (HCT) levels, after SnPP treatment (Figure 2A-C). However, mean corpuscular volume (MCV) and mean corpuscular hemoglobin (MCH) were reduced following SnPP treatment of the Th3/+ mice (Figure 2D-E). Of note, a similar effect was seen in Wt mice after SnPP injections (Figure 2D-E).
SnPP injections rescue anemia and reduce spleen size, reticulocyte levels, serum iron concentration, transferrin saturation, and EPO levels in Th3/+mice. (A) RBC, (B) Hb, (C) HCT, (D) MCV, and (E) MCH indices were determined on Wt + PBS (n = 12), Wt + SnPP (n = 12), Th3/+ + PBS (n = 11), and Th3/+ + SnPP (n = 12) mouse groups by automated analysis. (F) Representative images of spleens isolated from Wt and Th3/+ mice injected either with PBS or SnPP. (G) Spleen weight index of Wt + PBS (n = 9), Wt + SnPP (n = 9), Th3/+ + PBS (n = 8), and Th3/+ + SnPP (n = 7) mice. (H) Flow cytometry analysis of thiazole orange staining of peripheral blood obtained from Wt and Th3/+ mice injected either with PBS or SnPP (n = 6). Analysis of (I) serum iron and (J) transferrin saturation in Wt + PBS (n = 5), Wt + SnPP (n = 5), Th3/+ + PBS (n = 5), and Th3/+ + SnPP (n = 4) mice. (L) Enzyme-linked immunosorbent assay of EPO in serum from Wt and Th3/+ mice injected with PBS or SnPP (n = 11). *P < .05, ***P < .001.
SnPP injections rescue anemia and reduce spleen size, reticulocyte levels, serum iron concentration, transferrin saturation, and EPO levels in Th3/+mice. (A) RBC, (B) Hb, (C) HCT, (D) MCV, and (E) MCH indices were determined on Wt + PBS (n = 12), Wt + SnPP (n = 12), Th3/+ + PBS (n = 11), and Th3/+ + SnPP (n = 12) mouse groups by automated analysis. (F) Representative images of spleens isolated from Wt and Th3/+ mice injected either with PBS or SnPP. (G) Spleen weight index of Wt + PBS (n = 9), Wt + SnPP (n = 9), Th3/+ + PBS (n = 8), and Th3/+ + SnPP (n = 7) mice. (H) Flow cytometry analysis of thiazole orange staining of peripheral blood obtained from Wt and Th3/+ mice injected either with PBS or SnPP (n = 6). Analysis of (I) serum iron and (J) transferrin saturation in Wt + PBS (n = 5), Wt + SnPP (n = 5), Th3/+ + PBS (n = 5), and Th3/+ + SnPP (n = 4) mice. (L) Enzyme-linked immunosorbent assay of EPO in serum from Wt and Th3/+ mice injected with PBS or SnPP (n = 11). *P < .05, ***P < .001.
SnPP reduces splenomegaly, reticulocytosis, serum iron, transferrin saturation, liver iron, and EPO levels in Th3/+ mice
HO inhibition by SnPP significantly reduced spleen size (Figure 2F-G), decreased the percentage of reticulocytes (Figure 2H) and diminished serum iron and transferrin saturation (Figure 2I-J) in Th3/+ mice. Serum ferritin was increased in Th3/+ mice, but it did not change after SnPP treatment, suggesting that elevated serum ferritin is not related to heme catabolism (supplemental Figure 3B). In thalassemia, the ineffective erythropoiesis and consequent tissue hypoxia induce kidney erythropoietin (EPO) production.41 Importantly, SnPP administration led to a significant decrease in serum EPO levels in Th3/+ mice when compared with vehicle-treated controls (Figure 2L).
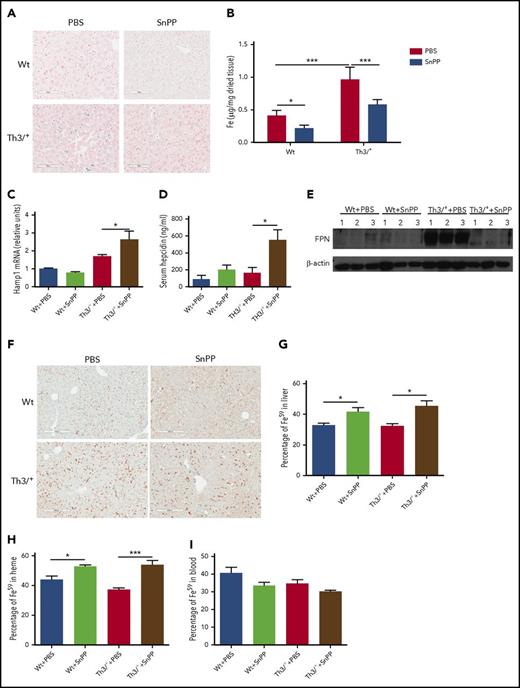
Tissue iron overload is observed in patients with thalassemia intermedia, with the liver being the first organ to be affected.42 Similarly, Th3/+ mice display liver iron overload; Perls’ Prussian blue staining and the ferrozine assay revealed that SnPP injections significantly decreased the nonheme iron levels in the livers of Th3/+ mice (Figure 3A-B).
SnPP injections decrease nonheme iron levels and heme-iron recycling in the liver of Th3/+mice. (A) Representative pictures (20× magnification; scale bar, 200 µM) of Perls’ Prussian blue staining in Wt and Th3/+ mice injected with either PBS or SnPP. (B) Ferrozine assay of nonheme iron content in the liver isolated from mice treated as follows: Wt + PBS (n = 6), Wt + SnPP (n = 6), Th3/+ + PBS (n = 5), or Th3/+ + SnPP (n = 5). (C) qRT-PCR analysis of HAMP mRNA expression in the liver of Wt and Th3/+ mice injected with either PBS or SnPP (n = 3). (D) Enzyme-linked immunosorbent assay of hepcidin in the serum of Wt and Th3/+ mice injected with either PBS or SnPP (n = 6). (E) Western blot analysis of FPN protein expression in samples isolated from the liver of Wt and Th3/+ mice injected with either PBS or SnPP (n = 3). (F) Representative images (20× magnification; scale bar, 200 µM) of immunohistochemical staining of FPN in livers from Wt or Th3/+ mice injected with PBS or SnPP. (G) Percentage of 59Fe in the liver of Wt and Th3/+ mice injected with either PBS or SnPP (n = 3). (H) Percentage of 59Fe in the heme fraction in the liver of Wt and Th3/+ mice injected with either PBS or SnPP (n = 3) (I) Percentage of 59Fe in total peripheral blood of Wt and Th3/+ mice injected with either PBS or SnPP (n = 3). Percentage was calculated based on the total 59Fe recovered for each mouse. *P < .05, ***P < .001.
SnPP injections decrease nonheme iron levels and heme-iron recycling in the liver of Th3/+mice. (A) Representative pictures (20× magnification; scale bar, 200 µM) of Perls’ Prussian blue staining in Wt and Th3/+ mice injected with either PBS or SnPP. (B) Ferrozine assay of nonheme iron content in the liver isolated from mice treated as follows: Wt + PBS (n = 6), Wt + SnPP (n = 6), Th3/+ + PBS (n = 5), or Th3/+ + SnPP (n = 5). (C) qRT-PCR analysis of HAMP mRNA expression in the liver of Wt and Th3/+ mice injected with either PBS or SnPP (n = 3). (D) Enzyme-linked immunosorbent assay of hepcidin in the serum of Wt and Th3/+ mice injected with either PBS or SnPP (n = 6). (E) Western blot analysis of FPN protein expression in samples isolated from the liver of Wt and Th3/+ mice injected with either PBS or SnPP (n = 3). (F) Representative images (20× magnification; scale bar, 200 µM) of immunohistochemical staining of FPN in livers from Wt or Th3/+ mice injected with PBS or SnPP. (G) Percentage of 59Fe in the liver of Wt and Th3/+ mice injected with either PBS or SnPP (n = 3). (H) Percentage of 59Fe in the heme fraction in the liver of Wt and Th3/+ mice injected with either PBS or SnPP (n = 3) (I) Percentage of 59Fe in total peripheral blood of Wt and Th3/+ mice injected with either PBS or SnPP (n = 3). Percentage was calculated based on the total 59Fe recovered for each mouse. *P < .05, ***P < .001.
Hepcidin expression increases and heme iron recycling decreases in the livers of Th3/+ mice after inhibition of HO
HO inhibition was associated with a significant increase in mRNA for hepcidin (known to block iron release from cells3 ) in the liver of Th3/+ mice when compared with vehicle-treated controls (Figure 3C). Hepcidin plasma levels were also increased after SnPP injections in Th3/+ mice (Figure 3D). In agreement with the increases in hepcidin, FPN protein levels were found to be decreased in the liver of Th3/+ mice after SnPP treatment (Figure 3E). The immunohistochemical staining clearly indicated that FPN expression was decreased in the liver of Th3/+ mice after HO inhibition (Figure 3F).
It has been previously demonstrated that HO-1 is required for iron recycling in mice.6 We therefore investigated whether the SnPP-mediated inhibition of HO disrupted heme-iron recycling in Wt and Th3/+ mice. We generated 59Fe-labeled RBC that were opsonized and intraperitoneally injected in Wt and Th3/+ mice treated during 4 weeks with PBS or SnPP. Seven hours after the injection, the mice were euthanized and 59Fe radioactivity measured in the harvested tissues. We observed that SnPP treatment led to a significant increase in the percentage of 59Fe in the liver (Figure 3G), and there was a trend to decrease the amount of 59Fe present in blood of Wt and Th3/+ mice (Figure 3I). When we analyzed the distribution of the 59Fe between heme and nonheme fractions in the liver, we found that SnPP administration caused a significant increase in the percentage of 59Fe incorporation into heme (Figure 3H). Altogether, our data strongly suggest that SnPP treatment efficiently inhibits heme-iron recycling in both Wt and Th3/+ mice.
HO suppression rescues erythroid cell defects and decreases erythroferrone expression in Th3/+ bone marrow
As shown in Figure 3, we observed a marked protective effect of SnPP, likely through a decrease in heme-iron recycling in Th3/+ mice. Next, we investigated the effect of HO inhibition in the bone marrow of SnPP-treated animals. Flow cytometry examination based on the cell surface CD71 and Ter119 markers showed a decrease in the percentage of basophilic erythroblasts in the bone marrow of SnPP-injected Th3/+ mice when compared with PBS-injected Th3/+ controls (Figure 4A). Similarly, the spleens of Th3/+ mice injected with SnPP also displayed the decrease in basophilic erythroblasts (Figure 4A).
HO-1 suppression rescues erythroid cell differentiation, suppresses erythroblast expansion, and reduces Fam132b expression in the bone marrow of Th3/+mice. (A) Representative dot plots of flow cytometry analysis of CD71 (y-axis) and Ter119 (x-axis) expression and its quantification in the bone marrow and the spleen from Wt and Th3/+ mice injected with either PBS or SnPP (n = 3). (B) Flow cytometry analysis of Ter119− and Ter119+ cells present in the bone marrow (n = 3) and the spleen (n = 5) of Wt and Th3/+ mice injected with either PBS or SnPP. (C) qRT-PCR analysis of Fam132b mRNA expression in bone marrow isolated from Wt and Th3/+ mice injected with either PBS or SnPP. The results are presented as fold change relative to Wt + PBS samples (n = 3). *P < .05, ***P < .001.
HO-1 suppression rescues erythroid cell differentiation, suppresses erythroblast expansion, and reduces Fam132b expression in the bone marrow of Th3/+mice. (A) Representative dot plots of flow cytometry analysis of CD71 (y-axis) and Ter119 (x-axis) expression and its quantification in the bone marrow and the spleen from Wt and Th3/+ mice injected with either PBS or SnPP (n = 3). (B) Flow cytometry analysis of Ter119− and Ter119+ cells present in the bone marrow (n = 3) and the spleen (n = 5) of Wt and Th3/+ mice injected with either PBS or SnPP. (C) qRT-PCR analysis of Fam132b mRNA expression in bone marrow isolated from Wt and Th3/+ mice injected with either PBS or SnPP. The results are presented as fold change relative to Wt + PBS samples (n = 3). *P < .05, ***P < .001.
β-thalassemia is associated with ineffective erythropoiesis that is characterized by an expansion of erythroblasts (“erythroid hyperplasia”) along with their premature death in the bone marrow.38,41 As a manifestation of the erythroid cell expansion, Th3/+ mice exhibit an increase in the percentage of Ter119+ cells in both the bone marrow and spleen (Figure 4B). Importantly, this expansion was markedly suppressed by SnPP administration (Figure 4B). Erythroblasts are known to produce erythroferrone (encoded by Fam132b gene),43 which was described as the factor responsible for the suppression of hepcidin expression in the mouse liver.44 In comparison with WT PBS-injected mice, the expression of Fam132b mRNA was increased in the bone marrow cells of similarly treated Th3/+ mice (Figure 4C). This increase in Fam132b mRNA was almost fully restored to control levels after SnPP administration to the Th3/+ mice (Figure 4C), which is consistent with the increase in hepcidin levels found in the SnPP-treated Th3/+ mice (Figure 3C-D).
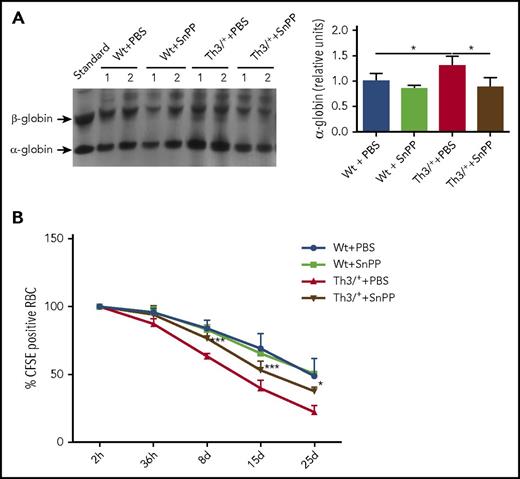
HO inhibition leads to a decrease in α-globin precipitation and an increase in RBC lifespan in Th3/+ mice
In β-thalassemias, unmatched α-globin chains accumulate and precipitate, causing cell damage and premature death of the bone marrow erythroid precursors.45 To confirm the precipitation of α-globin chains, we performed electrophoresis of RBC membranes in a triton-acetic acid-urea gel.33,34 Our data show that SnPP administration decreases in the α-globin precipitation (Figure 5A). To determine if the reduction in the α-globin precipitation has any impact on erythrocyte survival, RBC from Wt and Th3/+ animals, injected either with PBS or SnPP, were labeled with the fluorescent probe CFSE and injected into Wt animals, where the fluorescence of the RBC was tracked in the blood for 1 month. The results, depicted in Figure 5B, show a significant increase in the survival of the RBC derived from Th3/+ mice injected with SnPP when compared with those injected with PBS. Additionally, we observed that PBS-injected Th3/+ mice had an increase in the percentage of apoptotic cells in the bone marrow when compared with Wt mice injected with PBS (supplemental Figure 4A-B). The fraction of apoptotic cells in the Th3/+ mice was significantly decreased after SnPP injection (supplemental Figure 4A-B).
SnPP administration causes a decrease in α-globin precipitation and improves erythroid cell survival in Th3/+mice. (A) Representative image and densitometry quantification of a Coomassie blue-stained triton-acetic acid-urea gel loaded with membrane samples isolated from RBC from Wt and Th3/+ mice treated with either PBS or SnPP. (B) Flow cytometry analysis of CFSE-positive RBC derived from Wt + PBS, Wt + SnPP, Th3/+ + PBS, and Th3+ + SnPP groups and injected into Wt animals (n = 4). *P < .05, ***P < .001.
SnPP administration causes a decrease in α-globin precipitation and improves erythroid cell survival in Th3/+mice. (A) Representative image and densitometry quantification of a Coomassie blue-stained triton-acetic acid-urea gel loaded with membrane samples isolated from RBC from Wt and Th3/+ mice treated with either PBS or SnPP. (B) Flow cytometry analysis of CFSE-positive RBC derived from Wt + PBS, Wt + SnPP, Th3/+ + PBS, and Th3+ + SnPP groups and injected into Wt animals (n = 4). *P < .05, ***P < .001.
SnPP treatment increases the heme-regulatory pool in Ter119+ cells derived from the bone marrow of Th3/+ mice
We also investigated whether the effect of SnPP seen in the Th3/+ bone marrow is only due to the suppression of heme-iron recycling in the liver or if it could also be the result of direct HO inhibition in the bone marrow erythroblasts. Changes in HO activity can be indirectly inferred from alterations in ferritin protein levels because ferritin translation is stimulated by HO-released iron.46 Similarly, the heme-regulatory pool in erythroid cells can be assessed based on changes in the phosphorylation levels of the translation initiation factor, eIF2α.17 An increase in the heme-regulatory pool decreases the activity of the heme regulatory inhibitor, which leads to a reduction in the phosphorylation of its target, eIF2α.17 We performed western blot analysis of ferritin, eIF2α-P, and total eIF2α in Ter119+ cells derived from the bone marrow of Th3/+ mice that were injected with PBS or SnPP (Figure 6A). We observed a decrease in ferritin and eIF2α-P protein levels in Ter119+ cells isolated from SnPP-injected Th3/+ mice when compared with PBS-injected Th3/+ mice. These data are consistent with an inhibition of HO activity and an increase in the heme-regulatory pool levels in the bone marrow erythroid cells (Figure 6A).
HO-1 inhibition increases the heme-regulatory pool, but does not affect in vitro erythroid colony formation in Th3/+mice bone marrow cells. (A) Western blot analysis of ferritin, phosphorylated eIF2α (eIF2α-P), and total eIF2α protein levels in Ter119+ cells isolated from the bone marrow of Th3/+ mice injected with either PBS or SnPP. Results were normalized to the total levels of eIF2α (n = 3). Bone marrow cells from Wt (n = 3) and Th3/+ mice (n = 3) were plated in methylcellulose media supplemented with EPO in the presence or absence of SnPP. (B) Number of BFU-E colonies detected in cultures of bone marrow samples derived from Wt and Th3/+ mice. (C) Number of CFU-E colonies detected in cultures of bone marrow samples derived from Wt and Th3/+ mice. *P < .05, ***P < .001.
HO-1 inhibition increases the heme-regulatory pool, but does not affect in vitro erythroid colony formation in Th3/+mice bone marrow cells. (A) Western blot analysis of ferritin, phosphorylated eIF2α (eIF2α-P), and total eIF2α protein levels in Ter119+ cells isolated from the bone marrow of Th3/+ mice injected with either PBS or SnPP. Results were normalized to the total levels of eIF2α (n = 3). Bone marrow cells from Wt (n = 3) and Th3/+ mice (n = 3) were plated in methylcellulose media supplemented with EPO in the presence or absence of SnPP. (B) Number of BFU-E colonies detected in cultures of bone marrow samples derived from Wt and Th3/+ mice. (C) Number of CFU-E colonies detected in cultures of bone marrow samples derived from Wt and Th3/+ mice. *P < .05, ***P < .001.
SnPP treatment has no effect on in vitro colony formation of bone marrow erythroid progenitors isolated from Th3/+ mice
To evaluate the effect of HO-1 inhibition on erythropoiesis itself, hematopoietic progenitors isolated from bone marrow were plated in EPO-supplemented methylcellulose media in the presence or absence of 50 μM SnPP. SnPP treatment had no effect on colony-forming capacity of BFU-Es and CFU-Es in the cultures derived from Th3/+ mice and Wt mice (Figure 6B-C). However, a significant increase in the number of CFU-E colonies was observed in Th3/+ mice compared with Wt mice (Figure 6C), which is in agreement with ineffective erythropoiesis in their bone marrow. These results indicate that the in vivo effect of SnPP in Th3/+ mice is more likely related to the inhibition of iron recycling rather than directly influencing erythropoiesis.
Discussion
In thalassemias, the imbalance between the globin subunits is central to the pathophysiology of the disease, causing ineffective erythropoiesis and medullary as well as intravascular hemolysis. Disruption of organismal heme homeostasis, caused by the premature death of erythroid cells, can be expected to induce the expression of HO-1.
The expression of HO-1 has never been investigated in the context of thalassemia. Using a Th3/+ mouse model, we have observed that HO-1 mRNA and protein levels significantly increased in the livers of Th3/+ mice when compared with Wt controls (Figure 1A-C). This can be explained by the ineffective erythropoiesis and consequent shorter lifespan of Th3/+ RBC (Figure 5B), which would be expected to increase the heme-iron recycling in the liver of Th3/+ animals.
We have previously demonstrated that HO-1 is expressed in mouse bone marrow (in vivo), and more highly expressed during the differentiation of erythroid progenitors in vitro.11 We have also established that, similar to other cell types, erythroid HO-1 is induced by heme.11 Here, we have shown that HO-1 expression is lower in Ter119+ cells of the bone marrow and spleen of Th3/+ mice when compared with Wt controls (Figure 1D,E,G). Th3/+-Ter119+ bone marrow and spleen cells have lower total intracellular heme levels (Figures 1F and 2H), which is consistent with the downregulation of HO-1 expression when heme production is suppressed.11 Interestingly, our data suggest that the reduction of HO-1 expression in both the bone marrow and the spleen is restricted to the erythroblasts in the basophilic stage (supplemental Figure 2A-B). The increase of HO-1 in splenic Th3/+ orthochromatic erythroblasts is surprising and likely caused by relatively high uncommitted heme levels at this stage (supplemental Figure 2B).
Recent studies have suggested that decreasing iron absorption and/or iron mobilization from the liver stores (both reducing plasma transferrin saturation) may be of therapeutic value for patients with β-thalassemia.47 Such a limitation of iron availability to erythroid cells has been associated with an improvement of both anemia and ineffective erythropoiesis in β-thalassemic mouse models.48-52 A decrease in plasma transferrin saturation can be achieved by increasing the levels of plasma hepcidin that, in turn, reduces intestinal iron absorption and iron release from the heme recycling macrophages of the liver and spleen.53 Overexpression of hepcidin, by targeting the hepcidin repressor, Tmprss6,49-52 or using minihepcidins,48,54 have shown some success in reversing the thalassemic phenotype in mouse models.
The accumulating evidence supports the view that in β-thalassemias excessive plasma iron plays a detrimental effect on RBC survival and the consequent anemia,48-52,54 suggesting that HO activity may contribute to the pathogenesis of the disease. In our experiments, we inhibited HO by injecting Wt and Th3/+ mice with the HO competitive inhibitor, SnPP (supplemental Figure 3A). After 4 weeks of injections, SnPP significantly reduced splenomegaly and reticulocytosis in Th3/+ mice (Figure 2F-H). Additionally, HO inhibition ameliorated anemia, as indicated by the increase in RBC counts as well as Hb and HCT levels in SnPP-injected Th3/+ mice (Figure 2A-C). SnPP treatment caused a reduction in plasma iron levels and transferrin saturation (Figure 2I-J) that can be expected to limit iron availability for erythropoiesis.53 The reduction in MCV and MCH also supports the idea of iron restriction after SnPP injections in both Wt and Th3/+ mice (Figure 2D-E). The increase in the lifespan of Th3/+ RBC after SnPP treatment in Th3/+ mice (Figure 5B) likely balances the decrease in Hb production.
Iron is responsible for the posttranscriptional regulation of FPN mRNA.55 SnPP administration resulted in a decrease in liver iron content in Th3/+ mice when compared with controls (Figure 3A-B). This explains, at least in part, the reduction in FPN protein levels observed in the liver of SnPP-treated Th3/+ mice (Figure 3E-F). Additionally, the increase in hepcidin mRNA in the liver and plasma (Figure 3C-D) contributes to the reduction of FPN expression in the liver (Figure 3E-F) and decreases iron export after HO inhibition.
In thalassemia, ineffective erythropoiesis is caused by a failure in the maturation of the erythroid progenitors, leading to anemia.56,57 In Th3/+ mice, ineffective erythropoiesis is observed in both the bone marrow and the spleen as indicated by an accumulation of erythroid cells at the basophilic stage (Figure 4A). There is also an increased apoptosis and oxidative stress in the bone marrow (supplemental Figure 4A-D) and shorter RBC lifespan in the peripheral blood (Figure 5B). The defective maturation of erythroid cells causes a decrease in RBC counts and Hb levels in Th3/+ mice (Figure 2A-B). The diminished tissue oxygenation from insufficient Hb levels leads to an increase in EPO production (Figure 2L) that expands erythropoiesis58-60 ; this explains the increase of the Ter119+ cell population in both the bone marrow and the spleen of Th3/+ mice (Figure 4B).
The expansion of the erythroblast population causes the increase in Fam132b mRNA expression in Th3/+ mice (Figure 4C). Erythroferrone was shown to be EPO-responsive and produced by the erythroblasts of the bone marrow.43 SnPP treatment of Th3/+ mice ameliorated anemia (Figure 2A-C), increased RBC lifespan (Figure 5B), decreased apoptosis, and reduced oxidative stress (supplemental Figure 4A-D), most likely reducing tissue hypoxia leading to the decrease in plasma EPO levels (Figure 2L). Consequently, the expanded Th3/+ Ter119+ cell population was dramatically decreased in the bone marrow and the spleen after SnPP treatment (Figure 4B). The SnPP-mediated impediment of erythroblast expansion resulted in the decrease of Fam132b expression (Figure 4C). At a systemic level, the suppression of erythroferrone led to an increase in hepcidin levels (Figure 3C-D), preventing iron mobilization from stores (Figure 3E-G).
These discussed systemic changes in the iron distribution caused by HO inhibition in Th3/+ mice are for the most part the result of the local suppression of heme-iron recycling in the liver. We observed that SnPP treatment led to an increase in the percentage of 59Fe present in the liver (Figure 3G). SnPP administration suppressed heme-iron recycling in the liver, as suggested by the observation that most of the 59Fe accumulated in the liver after SnPP injections (Figure 3G) is in the form of heme (Figure 3H). Decreased ability of SnPP-treated Wt and Th3/+ mice to mobilize iron from opsonized RBC means that less 59Fe is available to produce new RBC. This conclusion is compatible with our finding that there is a tendency toward decreased blood 59Fe in Wt and Th3/+ mice after treatment with SnPP (Figure 3I). In contrast to our observation in the liver, the SnPP injections decreased the 59Fe in the spleen, whereas its percentage in heme remained unchanged in Th3/+ mice (supplemental Figure 5A-B). These observations are in agreement with a recent report, which suggests that the liver and not the spleen is the main site for heme-iron recycling in mice.61 The fact that SnPP treatment decreases 59Fe in the spleen could possibly be explained by a reduction of erythropoiesis in this organ.
Our results revealed that SnPP inhibits HO activity, as demonstrated by decreased heme degradation as well as iron release, causing decreased ferritin expression11,46 in Ter119+-Th3/+ bone marrow cells (Figure 6A). Moreover, HO inhibition is confirmed by the decrease of eIF2α phosphorylation in these cells (Figure 6A). eIF2α is phosphorylated by a heme regulatory inhibitor, whose activity is inhibited when the heme-regulatory pool is increased.17 Despite the inhibition of heme catabolism and an increase in the heme-regulatory pool, we did not observe any changes in TfR1 levels (supplemental Figure 6A-C). Finally, we did not detect any changes in the mRNA expression of the alleged heme-exporter, Flvcr1a,62 in the bone marrow of Wt and Th3/+ mice after SnPP injections (supplemental Figure 7).
Additionally, we examined the effect of SnPP on the development of erythroid colonies. We did not observe any change in the numbers of BFU-E and CFU-E colonies formed when HO was inhibited (Figure 6B-C). As mentioned previously, the decrease in iron availability for erythroblasts improves the overall RBC survival in thalassemia.48-52,54 Our data support that suppression of heme-iron recycling is the main factor contributing to the reduction of the ineffective erythropoiesis in Th3/+ mice.
SnPP is one of the various types of metalloporphyrins (MPs), in which iron is replaced by another metal.63 SnPP was used in clinical studies with human adults, where it was shown to efficiently inhibit HO activity in normal adults28 and suppress hyperbilirubinemia in patients with biliary cirrhosis and Gilbert syndrome.64 Long-term administration of another MP, tin-mesoporphyrin, was shown to cause iron deficiency anemia in 2 human patients with Crigler-Najjar type I syndrome.29 Therefore, SnPP or other MP could be of therapeutic value for the treatment of thalassemias in humans.
In the present work, we have demonstrated for the first time that anemia in Th3/+ mice can be ameliorated by inhibition of heme catabolism. Inhibition of HO reverses the ineffective erythropoiesis and increases Th3/+-RBC lifespan. Hence, HO inhibition has a strong potential to be used as a therapeutic approach for the treatment of thalassemias.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors are grateful to Alex Sheftel for reading the manuscript and his useful comments and to Stefano Rivella for kindly providing us with Th3/+ mice.
This work was funded by grants from the Canadian Institutes of Health Research (MOP-14100 and MOP-126064) (D.G.-S., A.H., C.F., and P.P.) the Czech Science Foundation (grant GA15-13732S) (M.H. and Z.S.).
Authorship
Contribution: D.G.-S., A.H., and M.H. designed and conducted experiments, analyzed data, and wrote the manuscript; C.F. and Z.S. conducted experiments and analyzed data; K.P. analyzed data; and P.P. conceived the study, designed experiments, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Prem Ponka, Department of Physiology, McGill University, 3755 Chemin Cote-Ste-Catherine, Montreal, Quebec, H3T1E2, Canada; e-mail: prem.ponka@mcgill.ca.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal