Key Points
Loss of antioxidative PON2 causes cardiovascular dysfunction and activates coagulation.
PON2 predominantly controls redox-sensitive endothelial TF-activation pathways.
Abstract
Oxidative stress and inflammation of the vessel wall contribute to prothrombotic states. The antioxidative protein paraoxonase-2 (PON2) shows reduced expression in human atherosclerotic plaques and endothelial cells in particular. Supporting a direct role for PON2 in cardiovascular diseases, Pon2 deficiency in mice promotes atherogenesis through incompletely understood mechanisms. Here, we show that deregulated redox regulation in Pon2 deficiency causes vascular inflammation and abnormalities in blood coagulation. In unchallenged Pon2−/− mice, we find increased oxidative stress and endothelial dysfunction. Bone marrow transplantation experiments and studies with endothelial cells provide evidence that increased inflammation, indicated by circulating interleukin-6 levels, originates from Pon2 deficiency in the vasculature. Isolated endothelial cells from Pon2−/− mice display increased tissue factor (TF) activity in vitro. Coagulation times were shortened and platelet procoagulant activity increased in Pon2−/− mice relative to wild-type controls. Coagulation abnormalities of Pon2−/− mice were normalized by anti-TF treatment, demonstrating directly that TF increases coagulation. PON2 reexpression in endothelial cells by conditional reversal of the knockout Pon2 cassette, restoration in the vessel wall using bone marrow chimeras, or treatment with the antioxidant N-acetylcysteine normalized the procoagulant state. These experiments delineate a PON2 redox-dependent mechanism that regulates endothelial cell TF activity and prevents systemic coagulation activation and inflammation.
Introduction
Blood coagulation is regulated by cellular and molecular pathways that assure a tight balance between bleeding and thrombus formation, whereas enhanced coagulation increases the risk for cardiovascular diseases. Endothelial cells (ECs) are crucial for these processes due to their central role in vasorelaxation, platelet adhesion, leukocyte interaction, and secretion of soluble or presentation of membrane-bound procoagulant and anticoagulant factors.1 Inflammatory conditions may render the endothelium dysfunctional and even prothrombotic through endothelial expression of proinflammatory cytokines, upregulation of procoagulant surface proteins, and concomitant downregulation of anticoagulant mediators.2 Oxidative stress underlies many of these steps. The overproduction of reactive oxygen species (ROS) that exceeds antioxidant defense mechanisms3 can, in a vicious cycle, induce proinflammatory EC reactions, the coagulation cascade,4,5 and platelet activity.6
Inflammation may increase coagulation by stimulating expression of tissue factor (TF), the initiator of the extrinsic coagulation pathway. Upon interaction with and activation of coagulation factor VII (FVII), the TF-FVIIa complex catalyzes the conversion of FX and FIX to their active forms (FXa, FIXa), resulting in the generation of thrombin, fibrin formation, and platelet activation. TF is constitutively expressed in the extravascular compartment, whereas TF is generally absent or expressed at low levels in cells that come in direct contact with blood.7 However, under pathological conditions, proinflammatory mediators can induce TF expression in ECs with a potential risk for thrombosis, which has been demonstrated by several studies, including in human cancer patients, a mouse model of sickle cell disease, and in septic baboons.8-14 Posttranslational mechanisms can convert TF from a state with low to a fully coagulant form in a process termed TF decryption. Although cryptic TF binds FVII/FVIIa with lower affinity and poorly cleaves FX, decrypted TF binds FVII/FVIIa and activates FX efficiently. TF decryption involves redox- and thiol-disulfide–dependent processes that increase the procoagulant activity of TF. Phosphatidylserine (PS) membrane exposure similarly contributes to TF decryption, and both processes are influenced by protein disulfide isomerase, complement activation, and cellular stress pathways.15 Moreover, TF procoagulant activity is controlled by tissue factor pathway inhibitor (TFPI),16 which is expressed by ECs and inhibits the TF-FVIIa complex by forming a quaternary complex with FXa. Interestingly, ROS inactivate TFPI and decrypt TF,17 highlighting the crucial role of endothelial oxidative stress in thrombotic pathologies.
Key molecules that link ROS with coagulation initiation in ECs remain poorly defined. We hypothesized a role for vascular paraoxonase-2 (PON2) protein, known to counteract oxidative stress, inflammation, and atherosclerosis.18-22 In contrast to other PON proteins, PON2 is cell associated and absent from plasma or high-density lipoprotein.23,24 We and others showed that by diminishing mitochondrial ROS, PON2 suppresses apoptosis and increases cellular stress resistance.18,25 Moreover, it counteracts lipid peroxidation, a free radical chain reaction contributing to inflammatory pathologies.26 In support of such functions, PON2 levels are strongly reduced in ECs of atherosclerotic patients22 and Pon2-deficient mice develop atherosclerosis.20,27 Here, we show that Pon2 deficiency in mice provokes EC oxidative stress and dysfunction, leading to TF decryption and platelet activation, collectively resulting in a pronounced procoagulant state. Thus, our study uncovered PON2 as antioxidative factor regulating endothelial TF decryption, platelet reactivity, and blood coagulation.
Methods
Detailed experimental procedures are described in supplemental Methods (available on the Blood Web site).
Mice and approval of animal studies
The Translational Animal Research Center of the Johannes Gutenberg University (JGU) Mainz housed the wild-type (WT) C57Bl/6J and Pon2-deficient mice backcrossed to C57Bl/6J.20 The latter were originally generated by insertion of a loxP-site flanked, β-geo–containing gene trap vector into Pon2 intron-2 that reduced PON2 expression by 95% and provided a genetic reconstitution strategy through cell-type–specific Cre excision of the targeting cassette. We refer to these PON2-deficient mice with PON2 protein levels of <5% (varying depending on tissue; see “Results”) as Pon2−/− mice. WT and Pon2−/− mice established after the backcrossing were analyzed and key experiments were confirmed with littermates of WT and Pon2−/−. To reconstitute PON2 in endothelial and hematopoietic cells, Pon2−/− mice were crossed with Tie2(Tek)-Cre mice (The Jackson Laboratory). The resulting mouse strain carrying 2 targeted Pon2 alleles is hereinafter referred to as Tek-Cre+/−Pon2ECrestored. We compared littermates from crosses that yielded WT and Tek-Cre+/− with untargeted Pon2 alleles as well as Pon2−/− and Tek-Cre+/−Pon2ECrestored mice. All strains were kept at the experimental animal facility with access to water and standard chow diet at libitum. Where indicated, antioxidant N-acetylcysteine (NAC; Sigma-Aldrich) was administered at a concentration of 7 mg/mL in the drinking water. NAC treatment started in the prenatal phase by treating pregnant mothers and continued after birth. To evaluate the contributions of FXa signaling through protease-activated receptor 2 (PAR2) to the procoagulant phenotype of Pon2−/− mice, we crossed these mice with a PAR2 knock-in mutant mouse strain that carried a point mutation, G37I, rendering PAR2 resistant to FXa cleavage.28
Unless otherwise stated, experimental mice were female, 8 to 12 weeks old, and humanely euthanized by intraperitoneal injection of 1% pentobarbital 0.8 mL/25 g body weight. All animal studies were permitted by state authority Landesuntersuchungsamt Rheinland-Pfalz (approval ID 23177-07/G13-1-055).
BM transplantation
Bone marrow (BM) cells of Pon2−/− or WT donor mice were isolated by flushing femoral and tibial bones using RPMI 1640/penicillin-streptomycin/0.1% bovine serum albumin (BSA) followed by BM cell resuspension in Dulbecco modified Eagle medium. Pon2−/− and WT BM chimeras were generated by injecting 5 × 106 freshly isolated BM cells IV into recipient mice of both genotypes 24 hours after lethal irradiation (137Cs, 1 dose of 9 Gy). This dose was confirmed to be lethal after 8 to 10 days under our experimental conditions. BM-transplanted mice were analyzed no earlier than 21 days after BM cell injection and after confirmation of blood cell Pon2 messenger RNA (mRNA) expression by quantitative reverse transcription polymerase chain reaction.
Antibody administration
Rat anti-mouse TF antibody,29 rabbit immunoglobulin G (IgG; Sigma-Aldrich), or solvent (0.9% NaCl) were administered intraperitoneally (2 doses of 0.5 mg per mouse each, 72 hours and 24 hours prior to analysis).
Coagulation assays
Prothrombin time (PT) and activated partial thromboplastin time (aPTT) were measured in plasma prepared by centrifugation (1500g, 20 minutes) of citrated whole blood. PT and aPTT were determined using an Automated Coagulation Analyzer KC 4 Delta (Trinity Biotech); Thromborel S and Pathrombin SL reagent (human) and calcium chloride solution (0.025 mol/L; Siemens Healthcare Diagnostics) were used according to the instructions of the manufacturer. The normal range (mean; 95% confidence interval) of coagulation times in WT mice with these reagents was 9.82 seconds (9.28-10.35 seconds) for PT and 35.59 seconds (33.28-37.89 seconds) for aPTT. For individual coagulation factor activities, plasma samples were diluted in imidazole buffer (1/5 or 1/20) and added to human coagulation factor–deficient plasma (Siemens Healthcare Diagnostics).
Tail-bleeding times were measured by resecting the distal 10 mm of the tail, followed by immersion of the tail in 37°C phosphate-buffered saline at an angle of 45°. Bleeding was followed visually and time to spontaneous cessation was recorded. The operator was typically blinded to mouse genotypes.
Gene expression analyses
Levels of mRNA expression were analyzed according to previously established protocols30 and through custom-made TaqMan or SYBR quantitative polymerase chain reaction arrays, generally applying 2 to 4 housekeeping genes (Gapdh; βActin; Polr2a; Atp5j).
ELISA
Mouse plasma samples and cell culture supernatants were analyzed for cytokine and chemokine levels by chemiluminescence-based multianalyte enzyme-linked immunosorbent assay (ELISA) Q-Plex array (QUANSYS Biosciences). Signal intensities were digitally recorded and evaluated using Q-view software.
Western blotting
Preparation of lysates, sodium dodecyl sulfate–polyacrylamide gel electrophoresis, western blotting, and immunodetection were performed as reported previously.18
TF activity assay
TF activity of whole cells was measured by a commercial chromogenic assay, calculated from a standard curve established by known TF activities, and normalized to the total protein level. Where indicated, ECs were preincubated with 5 µg/mL 21E10 anti-TF29 for 3 hours to demonstrate specificity.
Flow cytometry
Endothelial and platelet surface protein profiles as well as platelet PS exposure were analyzed by flow cytometry using fluorescently conjugated antibodies and fluorescent annexin V conjugate, respectively.
Statistics
GraphPad Prism software was used, applying the 2-tailed Student t test (normally distributed data, skewness <1) or the Mann-Whitney U test (nonnormally distributed data, skewness >1) for comparison of 2 groups. For >2 groups, 1-way analysis of variance (ANOVA) with Bonferroni multiple comparison test or the nonparametric Kruskal-Wallis test with the Dunn multiple comparison test was applied; P values were adjusted to show exact values. Numbers of mice in the experimental groups are indicated in the figures. Data are shown as mean ± standard error of the mean (SEM). P < .05 was considered significant.
Results
Pon2 deficiency causes endothelial oxidative stress, inflammation, and dysfunction
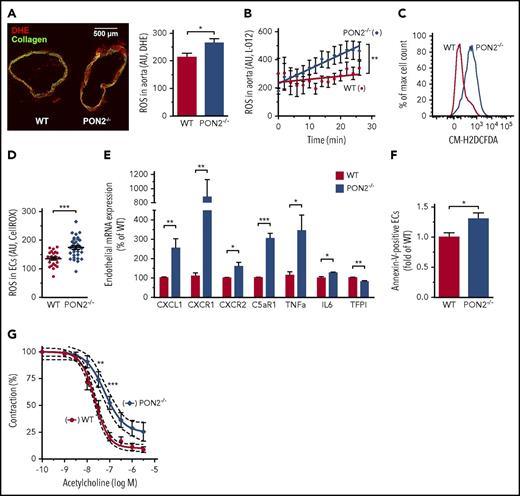
Atherogenesis in Pon2−/− mice did not require a cross with proatherogenic mutant strains, such as apolipoprotein E or low-density lipoprotein (LDL) receptor knockouts. Although macrophages were considered a major contributor to the proinflammatory state in these animals,20 we hypothesized that inflammatory activation and dysfunction of the endothelium might be equally important for the phenotype leading to exacerbated cardiovascular disease in Pon2−/− mice. PON2 diminishes cellular ROS, particularly superoxide, in response to stimulation with different agonists.25,27 We analyzed murine vascular endothelium under steady-state conditions and used 4 independent approaches to analyze basal ROS production in Pon2−/− mice compared with WT. First, we detected superoxide in frozen WT/Pon2−/− aortic sections with dihydroethidium (DHE). Quantification by confocal microscopy revealed increased ROS in the Pon2−/− vasculature (Figure 1A). Similarly, ROS was increased in fresh aortic rings using detection with chemiluminescent superoxide probe L-012 (Figure 1B). We next detected ROS in isolated single cells from fresh aortic tissue with the fluorescent dye CM-H2DCFDA by flow cytometry, which demonstrated a substantial right shift of the peak, revealing enhanced ROS in Pon2−/− vascular cells (Figure 1C). Lastly, microvascular ECs were isolated from lungs by anti-CD31/anti-ICAM2 antibody selection, yielding ∼85% pure ECs (supplemental Figure 1A-C), and were stained with the ROS-sensitive fluorescent dye CellROX-DeepRed. Quantification by confocal microscopy confirmed enhanced ROS production by Pon2−/− ECs (Figure 1D). Thus, under basal conditions Pon2−/− ECs produce significantly more ROS.
Pon2−/−mice have a pro-oxidant, proinflammatory, and dysfunctional endothelium. (A, left) Representative image of cryosectioned aortae form Pon2−/− and WT mice, stained with dihydroethidium (DHE; red) for O2− and analyzed by confocal imaging (green, collagen autofluorescence; scale bar, 500 µm). (Right) Quantification of DHE-fluorescence intensities per area of aortic tissue (n = 19 sections of 3 mice each). *P = .0302; Student t test. (B) L-012 chemiluminescence signal of fresh aortic sections quantified over time for ROS formation (representative graph). Linear regression curve fits were calculated and slopes were analyzed for statistically significant differences. **P = .0053. (C) ROS level of aortic cells determined by H2DCFDA flow cytometry (representative graph). A right shift of the peak indicates higher ROS levels. (D) Primary murine ECs were isolated from lungs, stained with CellRox-DeepRed, and quantified by confocal microscopy (n = 23-33 of 2-3 mice). ***P = .0001; Student t test. (E) mRNA expression of proinflammatory cytokines, chemokines, receptors, and coagulation-regulation factors in ECs isolated from lungs (n = 2-6 assays using 4-12 mice per group and target normalized against Gapdh and Polr2a). *P ≤ .0156; **P ≤ .007; ***P < .0001; Student t test. (F) Lung cells were stained with EC marker lectin and annexin V for quantification of endothelial PS exposure (n = 7-8). *P = .0277; Student t test. (G) Aortic rings of Pon2−/− and WT mice were precontracted with norepinephrine and endothelium-dependent vasodilation induced by increasing concentrations of acetylcholine was measured (n = 6 rings of 3 mice). **P < .01; ***P < .001; 2-way ANOVA with Bonferroni multiple comparison for individual data points and nonlinear regression (R2 = 0.84-0.97) with 95% confidence intervals (dotted lines) showed significant differences between curves.
Pon2−/−mice have a pro-oxidant, proinflammatory, and dysfunctional endothelium. (A, left) Representative image of cryosectioned aortae form Pon2−/− and WT mice, stained with dihydroethidium (DHE; red) for O2− and analyzed by confocal imaging (green, collagen autofluorescence; scale bar, 500 µm). (Right) Quantification of DHE-fluorescence intensities per area of aortic tissue (n = 19 sections of 3 mice each). *P = .0302; Student t test. (B) L-012 chemiluminescence signal of fresh aortic sections quantified over time for ROS formation (representative graph). Linear regression curve fits were calculated and slopes were analyzed for statistically significant differences. **P = .0053. (C) ROS level of aortic cells determined by H2DCFDA flow cytometry (representative graph). A right shift of the peak indicates higher ROS levels. (D) Primary murine ECs were isolated from lungs, stained with CellRox-DeepRed, and quantified by confocal microscopy (n = 23-33 of 2-3 mice). ***P = .0001; Student t test. (E) mRNA expression of proinflammatory cytokines, chemokines, receptors, and coagulation-regulation factors in ECs isolated from lungs (n = 2-6 assays using 4-12 mice per group and target normalized against Gapdh and Polr2a). *P ≤ .0156; **P ≤ .007; ***P < .0001; Student t test. (F) Lung cells were stained with EC marker lectin and annexin V for quantification of endothelial PS exposure (n = 7-8). *P = .0277; Student t test. (G) Aortic rings of Pon2−/− and WT mice were precontracted with norepinephrine and endothelium-dependent vasodilation induced by increasing concentrations of acetylcholine was measured (n = 6 rings of 3 mice). **P < .01; ***P < .001; 2-way ANOVA with Bonferroni multiple comparison for individual data points and nonlinear regression (R2 = 0.84-0.97) with 95% confidence intervals (dotted lines) showed significant differences between curves.
Enhanced ROS production is a potent proinflammatory stimulus leading to endothelial dysfunction.3 We therefore characterized transcript profiles of fluorescence-activated cell sorter–sorted lectin+/CD31+ aortic macrovascular ECs (supplemental Figure 1D). We screened >90 markers of gene regulation, endothelial function, adhesion, apoptosis, inflammation, and coagulation. WT and Pon2−/− livers that contain ECs and are the major site for coagulation factor synthesis were also included. In isolated ECs or liver, several inflammatory mediators were changed in Pon2−/− relative to WT mice (supplemental Figure 2A-B). Analysis of lung microvascular ECs confirmed the increased expression of the mouse interleukin-8 (IL-8) homolog Cxcl1 (KC), its 2 receptors (Cxcr1, Cxcr2), complement factor C5a receptor (C5ar1), Tnf-α, and Il-6, whereas Tfpi was decreased in Pon2−/− compared with WT mice (Figure 1E; for TF expression levels, see Figure 5). No differences were found in several surface proteins on lung ECs, including PECAM-1 (CD31), scavenger receptor CD36, tumor necrosis factor (TNF) receptor-1 (CD120a), von Willebrand factor (VWF), E-Selectin (CD62E), P-Selectin (CD62P), or VCAM1 (CD106) (supplemental Figure 3A; for ICAM1 expression levels, see supplemental Figure 2).
Annexin V binding to Pon2−/− and WT ECs by flow cytometry showed that increased oxidative stress resulted in PS exposure on the outer leaflet of the plasma membrane of Pon2−/− ECs relative to WT (Figure 1F). Underscoring an activated proinflammatory Pon2−/− endothelium leading to endothelial dysfunction, we found that acetylcholine-mediated relaxation of aortas from unchallenged mice was markedly reduced in Pon2−/− aortas compared WT controls (Figure 1G). Thus, although not all markers typically upregulated on highly activated ECs were upregulated by Pon2 deficiency, the lack of Pon2 produced under basal conditions endothelium activation and inflammation increased oxidative stress and pronounced endothelial dysfunction.
Increased cytokine production is triggered by vascular Pon2 deficiency
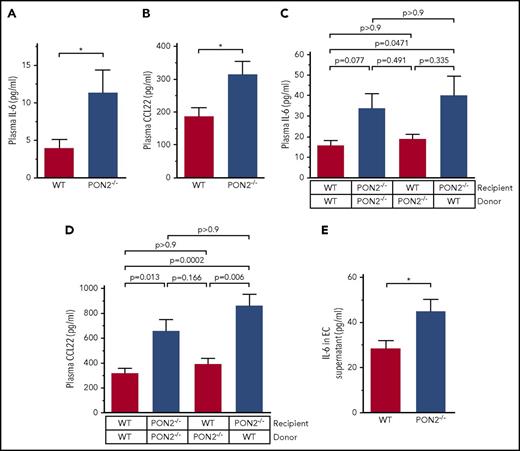
To determine whether the proinflammatory Pon2−/− endothelium causes systemic elevation of cytokines and/or chemokines, we measured plasma levels for inflammatory mediators by multiplex ELISAs (supplemental Figure 3B). Significantly elevated plasma levels of IL-6 and CC-chemokine ligand 22 (CCL22) were found in Pon2−/− relative to WT mice (Figure 2A-B). TNF-α (and interferon-γ) levels were below detection limit and the documented threefold higher mRNA expression in Pon2−/− ECs relative to WT was not reflected in plasma protein levels (Figure 1E).
Pon2 deficiency triggers increased IL-6 and CCL22 release from the vasculature. Plasma concentrations of (A) IL-6 and (B) CCL22 quantified by multianalyte chemiluminescent ELISA array using plasma samples of Pon2−/− and WT mice (n = 7-8). *P ≤ .0377; Mann-Whitney U test. (C-D) IL-6 and CCL22 plasma concentrations in plasma derived from BM chimeras and transplantation controls (n = 6). Adjusted P values are indicated; Kruskal-Wallis test with the Dunn multiple comparison test. (E) IL-6 concentrations in cell-culture supernatant of ECs isolated from Pon2−/− and WT quantified as in panels A-D. *P ≤ .0351; Student t test.
Pon2 deficiency triggers increased IL-6 and CCL22 release from the vasculature. Plasma concentrations of (A) IL-6 and (B) CCL22 quantified by multianalyte chemiluminescent ELISA array using plasma samples of Pon2−/− and WT mice (n = 7-8). *P ≤ .0377; Mann-Whitney U test. (C-D) IL-6 and CCL22 plasma concentrations in plasma derived from BM chimeras and transplantation controls (n = 6). Adjusted P values are indicated; Kruskal-Wallis test with the Dunn multiple comparison test. (E) IL-6 concentrations in cell-culture supernatant of ECs isolated from Pon2−/− and WT quantified as in panels A-D. *P ≤ .0351; Student t test.
To identify the origin of the circulating cytokines, chimeric mice with Pon2 deficiency either in the vessel wall or in hematopoietic cells were generated by BM transplantation. Analysis of Pon2 mRNA levels in hematopoietic cells confirmed the expected reconstitution or deletion of PON2 in the BM chimeras and the transplantation controls (supplemental Figure 4). Transplantation controls showed that total Pon2 deficiency resulted in elevated IL-6 and CCL22 relative to WT transplant controls. IL-6 and CCL22 were also increased in chimeric mice with a Pon2-deficient vascular wall replenished with WT BM, but not if BM cells were Pon2-deficient in an otherwise WT host (Figure 2C-D). These data demonstrate that systemic increases in plasma IL-6 and CCL22 in Pon2−/− mice originate from deregulated redox regulation in the vessel wall and not from BM-derived hematopoietic cells. Isolation of primary ECs furthermore directly showed that Pon2−/− ECs secreted higher levels of IL-6 (Figure 2E), whereas CCL22 was below the detection limit.
Pon2 deficiency activates blood coagulation
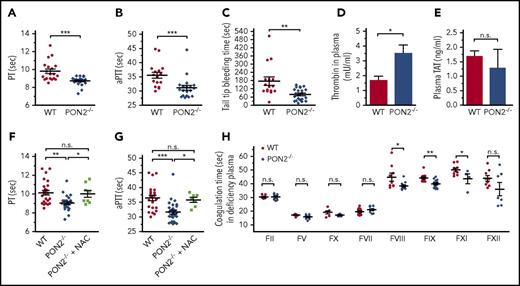
We hypothesized that endothelial activation may trigger blood coagulation and thereby contribute to the cardiovascular phenotype observed in Pon2−/− mice. Indeed, plasma from Pon2−/− mice showed significantly accelerated clotting times in extrinsic TF-initiated PT as well as contact pathway-initiated aPTT, indicating a preactivated coagulation system (Figure 3A-B). Consistently, tail-bleeding times were significantly shorter in Pon2−/− mice (Figure 3C). This phenotype was already observed in young mice (2-10 months) and did not further aggravate in older mice (11-21 months; supplemental Figure 5A-B). Thus, our studies typically used 2- to 3-month-old mice. In line with the shortened coagulation times, basal thrombin levels were elevated in the plasma of Pon2−/− mice (Figure 3D), whereas the plasma thrombin–antithrombin (TAT) III levels of Pon2−/− did not differ from WT mice (Figure 3E).
Pon2−/−mice have an accelerated coagulation. (A) PT, (B) aPTT, and (C) bleeding time of the tail vein of Pon2−/− and WT mice (n = 16-21). ***P ≤ .0008; **P = .0041; Mann-Whitney U test. (D) Basal plasma levels of active thrombin in Pon2−/− and WT mice (n = 2-3). *P ≤ .0138; Student t test. (E) Plasma TAT levels in Pon2−/− and WT mice (n = 2-3). Student t test. (F) PT and (G) aPTT of NAC-treated Pon2−/− mice compared with Pon2−/− and WT mice (n = 7-28). ***P < .001; **P < .01; *P < .05; Kruskal-Wallis test with the Dunn multiple comparison test. (H) Coagulation factor activities of Pon2−/− and WT mice determined by coagulation time measurement in factor-depleted plasma (n = 6-14). **P = .0019; *P ≤ .047; n.s.; Student t test. Scatter plots show results for individual mice, mean and SEM. n.s., not significant.
Pon2−/−mice have an accelerated coagulation. (A) PT, (B) aPTT, and (C) bleeding time of the tail vein of Pon2−/− and WT mice (n = 16-21). ***P ≤ .0008; **P = .0041; Mann-Whitney U test. (D) Basal plasma levels of active thrombin in Pon2−/− and WT mice (n = 2-3). *P ≤ .0138; Student t test. (E) Plasma TAT levels in Pon2−/− and WT mice (n = 2-3). Student t test. (F) PT and (G) aPTT of NAC-treated Pon2−/− mice compared with Pon2−/− and WT mice (n = 7-28). ***P < .001; **P < .01; *P < .05; Kruskal-Wallis test with the Dunn multiple comparison test. (H) Coagulation factor activities of Pon2−/− and WT mice determined by coagulation time measurement in factor-depleted plasma (n = 6-14). **P = .0019; *P ≤ .047; n.s.; Student t test. Scatter plots show results for individual mice, mean and SEM. n.s., not significant.
To verify that the pro-oxidative state of Pon2−/− mice is involved in the accelerated coagulation, PT and aPTT were determined in Pon2−/− mice receiving the antioxidant NAC in the drinking water. NAC-treated compared with untreated Pon2−/− mice showed significantly prolonged PT and aPTT, whereas no significant changes in coagulation parameters were found in NAC-treated WT mice (Figure 3F-H). These data indicated that increased ROS levels in Pon2-deficient mice triggered the procoagulant phenotype.
In addition, measurements of individual coagulation factor activities in coagulation factor–deficient plasma indicated that several factors were preactivated (Figure 3G). No differences were observed for FII or FVII activities between Pon2−/− and WT mice, but FVIII, FIX, and FXI showed increased activities, with FXII, FV, and FX displaying a nonsignificant trend (P = .108, .092, or .090, respectively) in Pon2−/− vs WT mice. Hence, hypercoagulability in Pon2−/− mice is characterized by elevated coagulation factor activity without evidence for coagulation factor depletion that characterizes consumptive coagulopathy.
Increased basal platelet activation in Pon2−/− mice
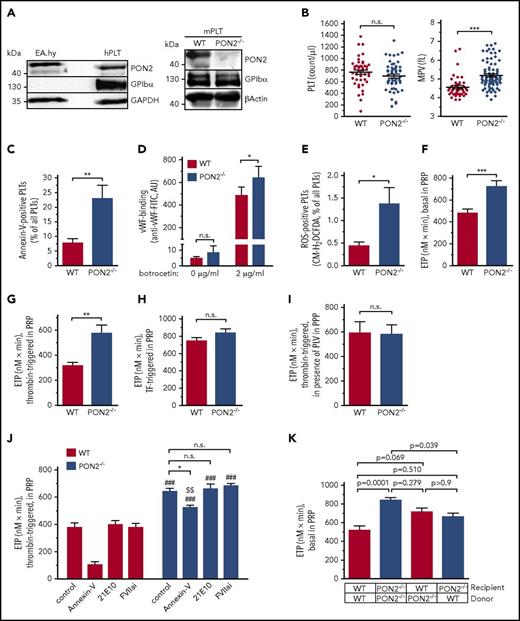
Next, we investigated whether Pon2 deficiency affects platelet activation and function. As platelet PON2 expression has not been analyzed before, we first verified that PON2 protein is indeed readily detectable in human and murine platelets (Figure 4A). Complete blood count analyses revealed that platelet numbers were unchanged; however, platelet volumes were markedly increased in Pon2−/− mice compared with WT (Figure 4B). An increased platelet volume may either result from disorders in which incompletely differentiated young platelets dominate over small mature platelets, or it may indicate platelet activation. Platelet survival times did not differ between WT and Pon2−/− mice (supplemental Figure 6A). Instead, increased platelet procoagulant activity was observed. Basal PS exposure as well as botrocetin-triggered VWF binding was significantly enhanced in Pon2−/− relative to WT mice (Figure 4C-D). As seen with ECs, platelet ROS levels were elevated (Figure 4E), possibly contributing to elevated PS exposure. In line with the latter, basal and thrombin-amplified thrombin generation, expressed as endogenous thrombin potential (ETP) determined by calibrated automated thrombography, were significantly enhanced in platelet-rich plasma (PRP) of Pon2−/− mice (Figure 4F-G), whereas TF-triggered ETP in PRP (Figure 4H) and in platelet-poor plasma (PPP) (supplemental Figure 6B) were not changed. Thrombin-triggered thrombin generation in PPP in the presence of phospholipid vesicles also did not differ between Pon2−/− and WT mice, demonstrating that platelets were required for the difference in thrombin generation in PRP (Figure 4I).
Pon2−/−platelets are procoagulant. (A) Glycoprotein Ibα (GPIbα; platelet marker) and PON2 protein expression in human platelets (hPLT; left panel) and murine platelets (mPLT; right). Endothelial EA.hy 926 (ATCC-No. CRL-2922) served as control. (B) Platelet count (PLT; left graph) and mean platelet volume (MPV; right graph) of Pon2−/− and WT mice determined from whole-blood samples (n = 58-80). ***P < .0001; Student t test. (C) Plasma membrane PS exposure of Pon2−/− and WT platelets displayed by flow cytometric analysis of annexin V binding (n = 7-8). **P = .0071; Student t test. (D) Flow cytometric analysis of VWF binding to platelets under basal conditions and upon botrocetin treatment (n = 5). *P = .0367; Student t test. (E) ROS level of platelets determined by H2DCFDA flow cytometry (n = 3-5). *P = .0192; Student t test. (F-H) Thrombin generation in PRP determined by calibrated automated thrombography. Thrombin generation indicated as ETP, (F) under basal conditions, (G) thrombin-triggered, and (H) TF-triggered (n = 6-13). **P = .0034; ***P = .0007; Student t test. Thrombin-triggered ETP (I) in PPP in the presence of phospholipid vesicles and (J) in PRP upon preincubation with annexin V, anti-TF antibody 21E10 and active site inhibited FVIIa (FVIIai) (n = 6-7). *P < .01 vs indicated group; ###P < .001 vs, respectively, treated WT; $$P < .01 vs WT control; 1-way ANOVA with Bonferroni multiple comparison test. (K) Basal ETP in PRP of BM-transplanted mice (n = 12-13). Adjusted P values are indicated; Kruskal-Wallis test with Dunn multiple comparison test. FITC, fluorescein isothiocyanate; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Pon2−/−platelets are procoagulant. (A) Glycoprotein Ibα (GPIbα; platelet marker) and PON2 protein expression in human platelets (hPLT; left panel) and murine platelets (mPLT; right). Endothelial EA.hy 926 (ATCC-No. CRL-2922) served as control. (B) Platelet count (PLT; left graph) and mean platelet volume (MPV; right graph) of Pon2−/− and WT mice determined from whole-blood samples (n = 58-80). ***P < .0001; Student t test. (C) Plasma membrane PS exposure of Pon2−/− and WT platelets displayed by flow cytometric analysis of annexin V binding (n = 7-8). **P = .0071; Student t test. (D) Flow cytometric analysis of VWF binding to platelets under basal conditions and upon botrocetin treatment (n = 5). *P = .0367; Student t test. (E) ROS level of platelets determined by H2DCFDA flow cytometry (n = 3-5). *P = .0192; Student t test. (F-H) Thrombin generation in PRP determined by calibrated automated thrombography. Thrombin generation indicated as ETP, (F) under basal conditions, (G) thrombin-triggered, and (H) TF-triggered (n = 6-13). **P = .0034; ***P = .0007; Student t test. Thrombin-triggered ETP (I) in PPP in the presence of phospholipid vesicles and (J) in PRP upon preincubation with annexin V, anti-TF antibody 21E10 and active site inhibited FVIIa (FVIIai) (n = 6-7). *P < .01 vs indicated group; ###P < .001 vs, respectively, treated WT; $$P < .01 vs WT control; 1-way ANOVA with Bonferroni multiple comparison test. (K) Basal ETP in PRP of BM-transplanted mice (n = 12-13). Adjusted P values are indicated; Kruskal-Wallis test with Dunn multiple comparison test. FITC, fluorescein isothiocyanate; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
To evaluate whether TF is involved in the enhanced thrombin generation in PRP, the TF-blocking antibody 21E10 or active site-inhibited FVIIa were added to PRP before ETP measurement. TF inhibition did not abolish increased ETP, indicating platelet TF-independent thrombin generation (Figure 4J). Furthermore, as anionic surfaces of activated platelets substantially contribute to thrombin generation and increased PS levels were found on Pon2−/− platelets (Figure 4C), thrombin-triggered thrombin generation was determined in the presence of PS-blocking annexin V. PS inhibition significantly reduced ETP compared with untreated Pon2−/− PRP (Figure 4J). However, annexin V did not completely normalize thrombin generation to the WT level, indicating a platelet-independent effect possibly related to the observed preactivation of the coagulation system. Consistently, Pon2−/− chimeras with WT BM showed only an intermediate reduction of basal and thrombin-triggered thrombin generation (Figure 4K; supplemental Figure 6C), indicating that both platelet-dependent and -independent stimuli trigger platelet coagulant activity in Pon2−/− mice.
Pon2 deficiency causes a prothrombotic state via endothelial TF
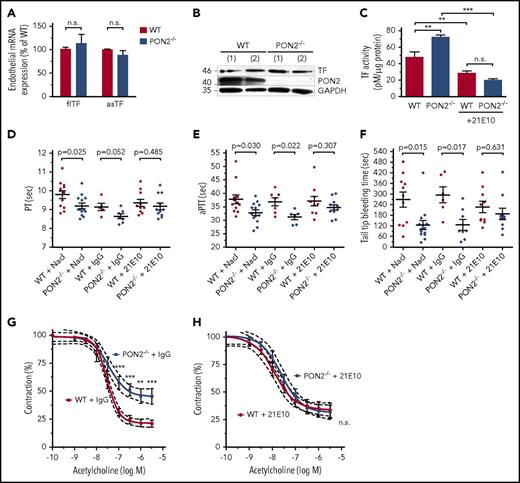
The findings above (see Figures 1 and 2) supported the hypothesis that endothelial effects triggered the prothrombotic state in Pon2−/− mice. Because inflammatory cytokines are known to induce TF in ECs, we next tested whether endothelial TF was involved in coagulation activation observed in Pon2−/− mice. In isolated Pon2−/− ECs, mRNA levels of neither full-length nor alternatively spliced TF were changed and total TF protein levels were indistinguishable from WT (Figure 5A-B). Because TF activity is redox-regulated through protein disulfide isomerase–dependent pathways and PS,31-33 we next tested whether PON2 regulates TF at the level of activity. Indeed, TF activity was significantly increased on ECs from Pon2−/− mice relative to WT and this difference was abolished in the presence of anti-TF 21E10 antibody (Figure 5C). We addressed next whether TF was responsible for the prothrombotic state of Pon2−/− in vivo. We treated mice with 21E10 given 72 and 24 hours prior to blood sampling. Remarkably, this short-term treatment normalized PT, aPTT, and tail-bleeding times in Pon2−/− mice in comparison with saline or IgG-injected mice (Figure 5D-F). Moreover, anti-TF antibody 21E10, but not IgG, largely normalized endothelial dysfunction of Pon2−/− mice (Figure 5G-H). Collectively, these data demonstrate a crucial role for endothelial TF as the primary trigger for the procoagulant state of Pon2−/− mice.
TF activity is enhanced in Pon2−/−ECs and anti-TF treatment normalizes coagulation and endothelial dysfunction in Pon2−/−mice. (A) Full-length TF (flTF) and alternatively spliced TF (asTF) mRNA levels in primary ECs isolated from lungs of Pon2−/− and WT mice (n = 5-7; normalized against Gapdh and β-actin). Student t test. (B) Western blotting for TF protein expression from cells as in panel A (results from 2 representative cell isolations are shown). (C) TF activity under basal conditions or upon treatment with anti-TF antibody 21E10 in lysates from Pon2−/− or WT ECs (n = 4-7). Results are presented as pM normalized total protein (µg). **P < .01; ***P < .001; 1-way ANOVA with Bonferroni multiple comparison test. (D) PT, (E) aPTT, and (F) tail-tip bleeding time of Pon2−/− and WT mice treated with anti-TF 21E10, control IgG, or solvent (0.9% NaCl) (n = 5-14). P values are indicated; Mann-Whitney U test; Student t test. Scatter plots show results for individual mice, mean and SEM. (G-H) Endothelium-dependent vascular relaxation of (G) control IgG-treated and (H) 21E10-treated Pon2−/− and WT mice. Statistical analyses performed as in Figure 1G (n = 4-6). **P < .01; ***P < .001.
TF activity is enhanced in Pon2−/−ECs and anti-TF treatment normalizes coagulation and endothelial dysfunction in Pon2−/−mice. (A) Full-length TF (flTF) and alternatively spliced TF (asTF) mRNA levels in primary ECs isolated from lungs of Pon2−/− and WT mice (n = 5-7; normalized against Gapdh and β-actin). Student t test. (B) Western blotting for TF protein expression from cells as in panel A (results from 2 representative cell isolations are shown). (C) TF activity under basal conditions or upon treatment with anti-TF antibody 21E10 in lysates from Pon2−/− or WT ECs (n = 4-7). Results are presented as pM normalized total protein (µg). **P < .01; ***P < .001; 1-way ANOVA with Bonferroni multiple comparison test. (D) PT, (E) aPTT, and (F) tail-tip bleeding time of Pon2−/− and WT mice treated with anti-TF 21E10, control IgG, or solvent (0.9% NaCl) (n = 5-14). P values are indicated; Mann-Whitney U test; Student t test. Scatter plots show results for individual mice, mean and SEM. (G-H) Endothelium-dependent vascular relaxation of (G) control IgG-treated and (H) 21E10-treated Pon2−/− and WT mice. Statistical analyses performed as in Figure 1G (n = 4-6). **P < .01; ***P < .001.
Endothelial Pon2 deficiency enhances coagulation
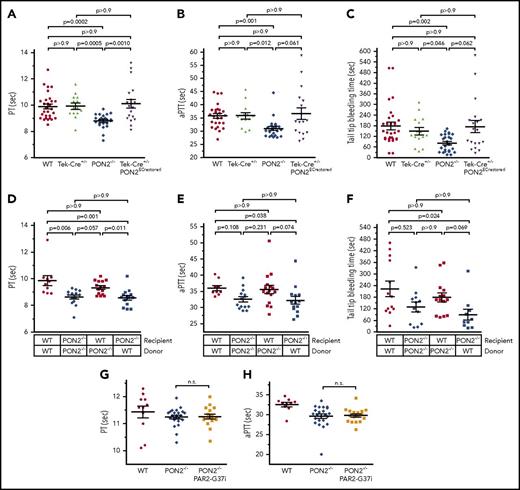
We next used a genetic approach and BM transplantations to demonstrate unequivocally that ECs, rather than the hematopoietic compartment, were responsible for the prothrombotic phenotype of Pon2−/− mice. In this strain, Pon2 expression is reduced by knock-in of a loxP site flanked gene trap vector.20,34 Thus, breeding Pon2−/− mice with Cre recombinase-driver lines can restore Pon2 expression in a cell-type–specific manner. In Cg-Tg(Tek-cre)+/− mice, Cre expression is controlled by the Tie2 (Tek) promoter resulting in early embryonic expression and Cre-mediated recombination in ECs and hematopoietic cells. In isolated ECs or blood cells from Tek-Cre+/−Pon2ECrestored mice, Pon2 expression and activity were restored to ∼30% of WT levels (supplemental Figure 7A-F). A 30% reconstitution is typical for and sufficient in this model, as others have observed a similar Cre-loxP–driven restoration of PON2 in macrophages of Pon2−/−mice. This increase was sufficient to overcome the proatherogenic effects of Pon2 deficiency.34,35 Measurements of PT, aPTT, and tail-bleeding times showed that coagulation abnormalities were largely normalized in Tek-Cre+/−Pon2ECrestored (Figure 6A-C), supporting the hypothesis that ECs mediate the overall prothrombotic phenotype.
The hypercoagulant state in Pon2−/−mice predominantly results from endothelial functions. (A) PT, (B) aPTT, and (C) tail-tip bleeding times Tek-Cre+/−Pon2ECrestored, Tek-Cre+/−, Pon2−/−, and WT mice (n = 12-29). Adjusted P values are indicated; Kruskal-Wallis test with Dunn multiple comparison. Scatter plots show results for individual mice, mean and SEM. (D-F) Similar analyses using BM chimeras and transplantation controls (n = 10-15), demonstrating a normalized coagulation time in WT chimera with Pon2−/−-derived BM. (G) PT and (H) aPTT of Pon2−/−-PAR2G37i, Pon2−/−, and WT mice (n = 10-23); Kruskal-Wallis test with Dunn multiple comparison.
The hypercoagulant state in Pon2−/−mice predominantly results from endothelial functions. (A) PT, (B) aPTT, and (C) tail-tip bleeding times Tek-Cre+/−Pon2ECrestored, Tek-Cre+/−, Pon2−/−, and WT mice (n = 12-29). Adjusted P values are indicated; Kruskal-Wallis test with Dunn multiple comparison. Scatter plots show results for individual mice, mean and SEM. (D-F) Similar analyses using BM chimeras and transplantation controls (n = 10-15), demonstrating a normalized coagulation time in WT chimera with Pon2−/−-derived BM. (G) PT and (H) aPTT of Pon2−/−-PAR2G37i, Pon2−/−, and WT mice (n = 10-23); Kruskal-Wallis test with Dunn multiple comparison.
To further exclude contributions from the hematopoietic compartment, we also generated BM transplantations to restore PON2 exclusively in hematopoietic cells. Similar to nontransplanted mice (Figure 3A-C), coagulation was accelerated in control Pon2−/− mice receiving Pon2−/− BM compared with irradiated WT recipients transplanted with WT BM (Figure 6D-F). In WT recipients, Pon2−/− hematopoietic cells also did not accelerate coagulation. In contrast, coagulation remained enhanced in Pon2−/− mice transplanted with WT BM and no quantitative differences were observed relative to the mutant BM transplantation control. Thus, Pon2 deficiency in the vessel wall is sufficient to cause the prothrombotic state induced by endothelial TF overactivation, whereas platelet-dependent pathways amplify the procoagulant phenotype. To further elucidate how the endothelium contributed to the procoagulant state in Pon2−/− mice, we tested coagulation times of a novel Pon2-deficient mouse strain in which PAR2 signaling by FXa was eliminated. Elimination of PAR2 activation by FXa in Pon2−/−-PAR2 G37I mice had no effect on the increased coagulation activation in Pon2−/− mice (Figure 6G-H), indicating that the procoagulant activation is independent of PAR2-signaling loops.
Discussion
This study demonstrates a crucial function of EC-expressed PON2 in controlling endothelial coagulant properties (Figure 7). Previous studies demonstrated that Pon2−/− mice develop exacerbated atherosclerotic lesions when fed a western diet. This phenotype was reversed when macrophage Pon2 was reexpressed, indicating an important role for PON2 in suppressing proinflammatory LDL with higher levels of lipid hydroperoxides, preserving antiatherogenic capacity of high-density lipoprotein, and attenuating proinflammatory macrophage activation.20,35 Here, we show that PON2 has additional functions in preventing vascular pathologies independent of hematopoietic cells. We demonstrate that Pon2 deficiency causes endothelial dysfunction under unchallenged conditions and provide insight into a crucial role for redox regulation of the endothelium in controlling vessel wall inflammation and coagulation.
Schematic illustration of the proposed EC- and platelet-mediated processes fueling coagulation and vascular inflammation in the setting of Pon2−/−. Endothelial TF procoagulant activity is promoted by Pon2−/−-mediated endothelial redox mechanisms (increased formation of ROS, exposure of PS and lipid peroxidation [Lipid perox]) and reduced endothelial TFPI, leading to generation of thrombin, which mediates fibrin formation and platelet activation. Pon2 knockout in platelets contributes to enhanced coagulation via redox mechanisms. EC-mediated systemic inflammation is established by increased IL-6 levels, potentially contributing procoagulant state as well as vascular inflammation and dysfunction.
Schematic illustration of the proposed EC- and platelet-mediated processes fueling coagulation and vascular inflammation in the setting of Pon2−/−. Endothelial TF procoagulant activity is promoted by Pon2−/−-mediated endothelial redox mechanisms (increased formation of ROS, exposure of PS and lipid peroxidation [Lipid perox]) and reduced endothelial TFPI, leading to generation of thrombin, which mediates fibrin formation and platelet activation. Pon2 knockout in platelets contributes to enhanced coagulation via redox mechanisms. EC-mediated systemic inflammation is established by increased IL-6 levels, potentially contributing procoagulant state as well as vascular inflammation and dysfunction.
Our analyses combine analyses of single-cell functional to systemic mediators and uncover a new pathway in which EC-expressed PON2 controls activation of endothelial TF and thus coagulation. Posttranslational regulation of TF has been associated with redox-based mechanisms including thiol-dependent modifications of the TF allosteric disulfide bond and cell-surface phospholipid composition.33,36 In line with effects on these systems, PON2 can dynamically translocate to the plasma membrane in response to oxidative stress and inhibit peroxidation of plasma membrane lipids mediated by its enzymatic activity,26 potentially in a manner similar to that of PON1 on LDL.37-39 As lipid peroxidation and externalization of PS decrypt TF procoagulant activity,32 PON2 may suppress TF activity by counteracting lipid peroxidation under conditions of oxidative stress and inflammation. This hypothesis is consistent with our findings that, in Pon2−/− mice, TF is regulated on a posttranslational level only and, in this context, TF may be decrypted through a lipid peroxidation-based mechanism that is controlled by the antioxidative effect of PON2.
The primary substrates of PON2 enzymatic activity as well as its specific antioxidative mechanism are largely unknown. Although our previous study suggested that these were independent activities,25 it will be of interest how these are linked in biological processes, including TF decryption. At the inner mitochondrial membrane, PON2 counteracts peroxidation of cardiolipin, likely by preventing mitochondrial ROS production through an interaction with coenzyme Q10 at the mitochondrial respiratory chain.25,27,30 It is an analytic challenge to discriminate whether ROS reduction is a consequence or an effect of decreased lipid peroxidation. PON2 may regulate TF activity by preventing mitochondrial superoxide formation or PS exposure, both processes were addressed in this and previous studies.30 Redox-dependent PS externalization leading to increased TF activity is supported by the finding that enhanced PS exposure provides a TF-dependent procoagulant activity on the cell surface,40 implicated in thrombogenicity of atherosclerotic plaques.41 This suggests an intriguing link to other antiatherogenic functions of PON2, as TF is present in atherosclerotic lesions42 where it not only contributes to plaque thrombogenicity but also to atherogenesis.43
The observed elevated coagulation times of the intrinsic pathway determined by the aPPT could be attributable to the increased activity of FVIII, which can be directly activated by the TF-initiation complex.44 Because the performed coagulation factor–specific activity assays, which also demonstrated increased FIX, FXI, and (as a trend) FXII activity, are FVIII-dependent measurements, TF activation of FVIII may explain the selective increases in activity of these coagulation factors. Acceleration of the extrinsic pathway, as revealed by PT assays, may furthermore be caused by the reduced TFPI expression levels in Pon2−/− mice.
Our study provides evidence that PON2 regulates coagulation in an endothelial-dependent manner. Nevertheless, platelets contribute significantly to Pon2−/−-triggered accelerated coagulation. Thrombin generation in PRP of Pon2−/− mice was markedly enhanced along with other markers for a procoagulant platelet phenotype, and TF was not directly involved in platelet procoagulant activity. Rather, increased oxidative stress in Pon2−/− platelets appears to set the stage for increased platelet activity45 and likely involves increased PS externalization. However, annexin-mediated PS masking on platelets or BM transplantation studies produced an intermediate effect of procoagulant phenotype for platelets. In support of a major role of vessel wall PON2, we showed that only nonhematopoietic PON2 expression normalized the otherwise accelerated coagulation in Pon2−/− mice. Moreover, the measurements of the endogenous thrombin potential in the PRP of BM-transplanted chimeras indicated that Pon2 deficiency in platelets is not sufficient to induce the procoagulant platelet phenotype. Similar to PS inhibition, both the WT chimeras with Pon2−/− blood cells and the Pon2−/− chimeras with WT blood cells showed an intermediate level of generated thrombin in the PRP, supporting the major role vessel-wall–derived procoagulant stimuli. Thus, platelet-intrinsic pathways appear to mainly amplify the coagulation abnormality of TF deregulated in the vessel wall.
Increased coagulation activation also causes vascular dysfunction and a proinflammatory state in Pon2−/− mice. Interestingly, EC-specific TF enhances circulating IL-6 in a mouse model of sickle cell disease, characterized by activation of coagulation and chronic vascular inflammation.14 In this regard, TF functions as dual receptor with distinct contributions to coagulation and inflammatory signaling.31 On the one hand, TF is procoagulant by generating FXa and, on the other hand, the coagulation initiation complex TF-VIIa/FXa on ECs contributes to the pathogenesis of vascular inflammation via autocrine PAR2-dependent signaling that leads to increased circulating IL-6.13,46 Accordingly, the increase in IL-6 levels in Pon2−/− mice is EC-derived and consequently may result from enhanced TF-mediated signaling activity on ECs. Moreover, reduced TFPI expression levels in Pon2−/− mice may contribute to a less pronounced anticoagulant control of the EC TF complex and thus trigger coagulation as well as inflammatory signaling leading to IL-6 induction. To what extent IL-6 signaling is involved in the TF-dependent prothrombotic state and endothelial dysfunction should be addressed in future studies. Of note, the observed endothelial inflammation involving TF occurred with no appreciable changes in plasma TAT complexes. This finding is consistent with other studies demonstrating that TF and thrombin-dependent vascular inflammation can occur without elevated TAT levels47 and that EC-specific TF deficiency in a mouse model of sickle cell disease displays reduced inflammatory cytokines without changes in TAT levels.11 Because TAT levels were unchanged in Pon2-deficient mice, we conclude that global anticoagulation is not affected by PON2 deficiency. Possible changes in local anticoagulation cannot be formally excluded, but TFPI mRNA levels were significantly reduced (Figure 1E) whereas TF activity was increased (Figure 5C). Because anti-TF antibody 21E10 normalized the effects of the mutations, we consider a deregulated TF activity is the dominant effect resulting from PON2 deletion, although a superimposition of altered anticoagulation in the thrombomodulin/activated protein C pathway cannot entirely be excluded from the available data.
Collectively, our study identifies a PON2-dependent redox-based endothelial mechanism that activates EC TF and impacts on systemic coagulation. To what extent the redox-based and inflammation-associated mechanisms in the endothelium also impact other blood cells contributing to the enhanced coagulation in Pon2−/− mice should be further evaluated in future studies. It may also be of interest to address the question of whether PON2 and/or endothelial TF activation may serve a pharmacologically relevant target in anticoagulant and anti-inflammatory strategies.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Jerzy-Roch Nofer (University Hospital Münster, Münster, Germany) for providing phospholipids and Hartmut Kleinert (University Medical Center Mainz, Mainz, Germany) for supplying Taq polymerase.
This work was supported by the Center for Thrombosis and Hemostasis Mainz (BMBF funding allocation ID 01E01003, project TRPA11i), the Center for Translational Vascular Biology Mainz, and the Gerhard and Martha Röttger Foundation. S.H. was additionally supported by the German Research Foundation (DO1289/6-1) and US Department of Defense award W81XWH-12-2-0091. W.R. was supported by the Humboldt Foundation.
Authorship
Contribution: S.H., J.E., and W.R. designed research; S.H., J.E., P.W., J.F.T., K.S., M.D., L.S., and N.X. performed research; K.J., H.L., and W.R. contributed vital new reagents or analytical tools; J.E., S.H., K.J., N.X., P.W., J.F.T., K.S., and M.D. collected data; S.H., J.E., W.R., J.F.T., and K.J. analyzed and interpreted data; J.E. and S.H. performed statistical analysis; and S.H. and J.E. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Sven Horke, Department of Pharmacology, University Medical Center of the Johannes Gutenberg University Mainz, Obere Zahlbacher Str. 67, 55131 Mainz, Germany; e-mail: horke@uni-mainz.de.

![Figure 7. Schematic illustration of the proposed EC- and platelet-mediated processes fueling coagulation and vascular inflammation in the setting of Pon2−/−. Endothelial TF procoagulant activity is promoted by Pon2−/−-mediated endothelial redox mechanisms (increased formation of ROS, exposure of PS and lipid peroxidation [Lipid perox]) and reduced endothelial TFPI, leading to generation of thrombin, which mediates fibrin formation and platelet activation. Pon2 knockout in platelets contributes to enhanced coagulation via redox mechanisms. EC-mediated systemic inflammation is established by increased IL-6 levels, potentially contributing procoagulant state as well as vascular inflammation and dysfunction.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/131/19/10.1182_blood-2017-09-807040/4/m_blood807040f7.jpeg?Expires=1769111948&Signature=vfODsrszZMOHF3C02jO6KfZId1UObHu1Ayr8KKV-Zy4HAfY9~IfiANA0o1EAJ2M5BiV4eG1-9fsMPtD7JgpPnDKN2~VBXuPpbpeuAVxMgXJQJjSfXkcjxZMxXA~HzuRtjZfNUgePl-s477Z30-OJywP3n12Y8tQZVl6fKNmvoGs6gQTFyB93qw9QZxsJd7ouIRC8wtUhTp5aVZk5Ilahl4v9xaKKJcnhaBJLbqV2C6ZGF~G5x--~V3npoEIhGUtqhn6ov81Hq7R7uC1rhFEtLFN1QAS8~nNsrzrU-VzXjxacsSbJg4NxudMe4BjTV8sPK3MHhaOmz7ootti0wxUskg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal