Abstract
Traumatic brain injury (TBI)–induced coagulopathy is a common and well-recognized risk for poor clinical outcomes, but its pathogenesis remains poorly understood, and treatment options are limited and ineffective. We discuss the recent progress and knowledge gaps in understanding this lethal complication of TBI. We focus on (1) the disruption of the brain-blood barrier to disseminate brain injury systemically by releasing brain-derived molecules into the circulation and (2) TBI-induced hypercoagulable and hyperfibrinolytic states that result in persistent and delayed intracranial hemorrhage and systemic bleeding.
Trauma is a collective term for a diverse range of conditions that include massive muscle laceration, bone fracture, solid organ injury, and injury to the brain. These conditions differ in their causes, clinical presentations, and treatments, but they inevitably result in bleeding of various levels of severity. Bleeding arrest is therefore not only a physiological response to trauma but also a treatment goal. Trauma-induced uncontrolled hemorrhage is a leading cause of preventable death, accounting for 30% to 40% of trauma fatalities, and is caused by direct injury to the vasculature and secondary coagulopathy.1,2 Coagulopathy associated with extracranial injuries is primarily caused by substantial blood loss (hemorrhagic shock), consumption, hypothermia, and hypoperfusion-induced metabolic acidosis, and it can be further propagated by iatrogenic factors such as fluid resuscitation (hemodilution).3,4 Retrospective and observational studies have consistently shown that coagulopathy is equally prevalent in patients with isolated traumatic brain injury (TBI),5-7 which is increasingly common because falls have surpassed automobile incidents as the leading cause of TBI in civilians.8 A demographic shift in TBI patients from being predominantly young to increasingly older (≥50 years) is attributed to this change.8,9
TBI-induced coagulopathy manifests as disseminated intracranial hemorrhage (Figure 1A), delayed intracranial or intracerebral hematoma (Figure 1B), and systemic bleeding.10-12 It occurs early, often in a prehospital setting, and is closely associated with poor clinical outcomes.8,10,13 The international normalized ratio (INR) and thromboelastogram are laboratory assays that are widely used to diagnose TBI-induced coagulopathy. However, both INR and thromboelastogram are limited in predicting coagulopathy before it develops. New biomarkers and laboratory tests are therefore needed to timely, accurately, and reliably predict the development and severity of TBI-induced coagulopathy so that preventive measures and targeted therapeutics can be developed.
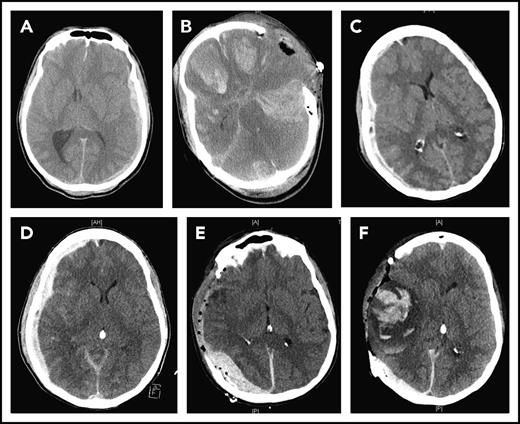
CT images of 2 TBI patients. The first patient suffered from subdural hematoma after a step fall (A) and developed diffused cerebral bleeding immediately after decompressive craniectomy (B). The second patient suffered from progressive subdural hematoma after a car accident (C-D) and developed delayed subdural and intracerebral hematomas after decompressive cranioctomy (E-F).
CT images of 2 TBI patients. The first patient suffered from subdural hematoma after a step fall (A) and developed diffused cerebral bleeding immediately after decompressive craniectomy (B). The second patient suffered from progressive subdural hematoma after a car accident (C-D) and developed delayed subdural and intracerebral hematomas after decompressive cranioctomy (E-F).
The timely, judicious, and balanced use of blood-component and fluid resuscitations has been the management choice for coagulopathic patients with extracranial injuries, especially when hemorrhagic shock is developed. However, their effectiveness for TBI-induced coagulopathy remains to be evaluated, because blood loss in isolated TBI is very limited compared with that in patients with hemorrhagic shock. Plasma transfusion, along with platelets, packed red blood cells, or medications such as desmopressin, has not consistently improved outcomes for patients with severe TBI.14-17
Patients with TBI-induced coagulopathy lack key causal factors for coagulopathy induced by extracranial trauma and hemorrhagic shock: They do not suffer substantial blood loss, fluid resuscitation is often restricted to prevent high intracranial pressure, and they are less likely to develop a hypothermic state during the acute phase,18,19 suggesting that TBI-induced coagulopathy follows a distinct pathogenic pathway. This pathway remains poorly understood. This lack of mechanistic understanding contributes to the fact that TBI-induced coagulopathy has been recognized for decades, but remains a significant risk for poor clinical outcomes because its prevention and treatment options are limited and ineffective.
Here, we highlight recent research progress toward understanding TBI-induced coagulopathy and significant knowledge gaps. Studying TBI-induced coagulopathy also provides a strong case study for how a localized injury spreads to influence systemic hemostasis.
Endothelium and the blood-brain barrier
Systemic coagulopathy often occurs within minutes of TBI,20 suggesting that it is triggered by brain-derived substances that are rapidly released systemically through the disrupted blood-brain barrier (BBB). The BBB is a semipermeable barrier of cerebral microvasculature that consists of endothelial cells, smooth muscle cells, astrocytes, pericytes, and the associated extracellular matrix.21 It controls both passive and transporter-mediated passage of fluid and macromolecules between the blood and the interstitial spaces. The transcellular pathway permits the diffusion of small molecules (<3 nm) following a concentration gradient, and the paracellular pathway transports macromolecules and microvesicles (MVs).22 TBI disrupts the BBB mechanically at the site of injury and increases BBB permeability beyond the injured area through secondary ischemic and inflammatory injuries, leading to the uncontrolled transvascular movements of macromolecules through interendothelial cell junctions (eg, tight junctions).23,24 The ischemic and inflammatory injuries to the BBB are mediated by intracellular signaling through the endothelial cell junction proteins, such as claudins22,25-27 and junctional adhesion molecules.28,29
The BBB disruption allows the vascular leakage of fluid to cause cerebral edema and releases brain-derived substances into the circulation to trigger systemic coagulopathy. Because the systemic dissemination of brain-derived molecules requires sufficient cerebral blood flow, one may ask whether high intracranial pressure, which suppresses the cerebral circulation, could slow the systemic release of brain-derived procoagulant substances, such as cellular MVs.30,31 If this is proven to be the case, decompressive craniectomy or other means of reducing intracranial pressure may inadvertently promote the systemic release of the brain-derived molecules by restoring the cerebral blood flow, following a similar course of ischemic reperfusion injury. In this case, the prophylactic use of MV-scavenging factors, such as lactadherin,32 before surgery may prevent the postoperational development of disseminated intracranial hemorrhage or delayed intracranial or intracerebral hematoma after surgery (Figure 1).
Coagulation
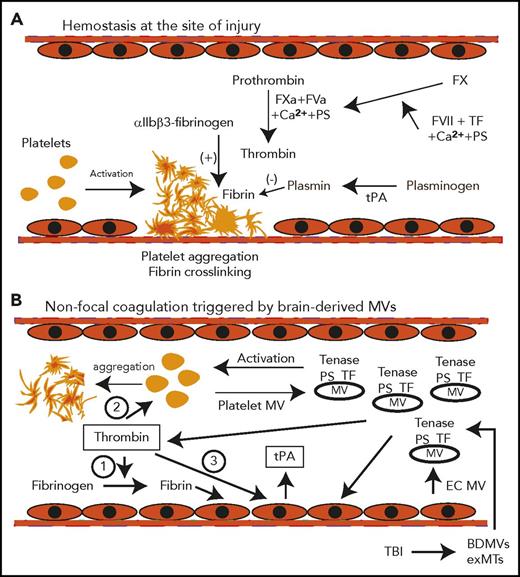
In studies on the time course of TBI-induced coagulopathy, the fibrinolytic product D-dimer and fibrinogen degradation products are first detected within minutes of injury, whereas prolonged prothrombin and partial thromboplastin times are detected late, reaching their peaks ∼3 to 6 hours post-TBI.12,33,34 This time course is consistent with an early transition from a hyper- to a hypocoagulable state. The fact that isolated TBI induces early and systemic coagulopathy raises the question of how a localized brain injury disseminates systemically to trigger the early hypercoagulant and hyperfibrinolytic states. Our recent studies suggest that brain-derived cellular microvesicles (BDMVs) serve as a dissemination factor and a causal factor. We have shown in mouse models that BDMVs are rapidly released into the circulation and induce a systemic hypercoagulable state that quickly develops into consumptive coagulopathy.30 These BDMVs are highly procoagulant because they express abundant tissue factor and phosphatidylserine,30 which are enriched in the brain tissue.35,36 BDMVs were further implicated in triggering TBI-induced coagulopathy when noninjured mice infused with purified BDMVs developed a similar hypercoagulable state.30 Among these BDMVs, 55.2% were extracellular mitochondria (exMTs) that promote coagulation by the surface-exposed mitochondria-specific anionic phospholipid cardiolipin.31 The apoptotic cell-scavenge factor lactadherin (milk fat globule–epidermal growth factor 8)37 prevents TBI-induced coagulopathy and improves the outcome of TBI mice by accelerating the clearance of these procoagulant MVs through anionic phospholipid-mediated phagocytosis.32 These results suggest that the MV-bound tissue factor and anionic phospholipids promote thrombin generation by integrating the coagulation factors V and VIII to assemble the prothrombinase and tenase complexes. As a result, these MVs become circulating platforms to initiate and amplify the coagulation that normally occurs on the surface of activated platelets localized to the site of injury, resulting in nonfocal, exaggerated, and disseminated coagulation (Figure 2).
Schematic pathways for consumptive coagulopathy induced by TBI. (A) Hemostasis is triggered by platelet adhesion and aggregation at the site of vascular injury and stabilized by crosslinked fibrin polymers to form a hemostatic plug. (B) The tissue factor and anionic phospholipid-rich microvesicles (BDMVs and exMTs) from injured brain cells are released into the circulation to provide microplatforms for tenase assembly. Thrombin thus produced (1) cleaves fibrinogen to generate fibrin, (2) activates platelets, and (3) activates endothelial cells to generate platelet and endothelial cell MVs. The blood cell–derived MVs further amplify the coagulation initiated by BDMVs and exMTs. Endothelial cells activated by MVs, thrombin, and fibrin also induce the acute release of tPA, which initiates early fibrinolysis. All of these processes occur in the fluid phase of the blood, without the local vascular injury and blood-derived factors found in trauma patients with hemorrhagic shock. EC, endothelial cells; FVa, activated factor V; FVII, coagulation factor VII; FXa, activated factor X; PS, phosphatidylserine; TF, tissue factor.
Schematic pathways for consumptive coagulopathy induced by TBI. (A) Hemostasis is triggered by platelet adhesion and aggregation at the site of vascular injury and stabilized by crosslinked fibrin polymers to form a hemostatic plug. (B) The tissue factor and anionic phospholipid-rich microvesicles (BDMVs and exMTs) from injured brain cells are released into the circulation to provide microplatforms for tenase assembly. Thrombin thus produced (1) cleaves fibrinogen to generate fibrin, (2) activates platelets, and (3) activates endothelial cells to generate platelet and endothelial cell MVs. The blood cell–derived MVs further amplify the coagulation initiated by BDMVs and exMTs. Endothelial cells activated by MVs, thrombin, and fibrin also induce the acute release of tPA, which initiates early fibrinolysis. All of these processes occur in the fluid phase of the blood, without the local vascular injury and blood-derived factors found in trauma patients with hemorrhagic shock. EC, endothelial cells; FVa, activated factor V; FVII, coagulation factor VII; FXa, activated factor X; PS, phosphatidylserine; TF, tissue factor.
Significant gaps remain in defining this MV-mediated coagulopathy. First, it is not known whether BDMVs alone are sufficient to induce or sustain systemic coagulopathy or whether they trigger a cascade of events to produce more procoagulant MVs from platelets and endothelial cells to propagate the hypercoagulable state. Second, cellular MVs have been primarily defined by their sizes and cellular origins. However, they are also structurally diverse, including membrane vesicles and intracellular granules that carry various cargoes and act differently. For example, exMTs released from injured cells may be metabolically active to generate oxidants, which induce systemic or localized oxidative stress to activate platelets and endothelial cells. Third, MVs generated through active microvesiculation38,39 may differ in activity from those produced through apoptosis or mechanical injury.40,41 An example is that the endosome-derived exosomes produced through microvesiculation have been shown to improve outcomes of TBI by delivering specific cargoes, such as microRNA to targeted cells,42 whereas MVs from mechanically injured cells are procoagulant and proinflammatory.30,31 Classifying MVs not only by their cells of origin but also by their production, structures, and functions is therefore necessary to further define the biological activities of these subcellular MVs.
Fibrinolysis
Fibrinolysis is triggered by fibrin that is generated from thrombin-cleaved fibrinogen.43 Upon fibrin polymer assembly, the αC-domain of fibrin exposes noncompetitive high-affinity sites for tissue-type plasminogen activator (tPA) and plasminogen that are cryptic in fibrinogen.44 tPA proteolytically activates plasminogen to the serine protease plasmin, which cleaves and dissolves fibrin polymers. The D domain also promotes fibrinolysis by undergoing conformational changes to expose low-affinity tPA- and plasminogen-binding sites.45 This fibrinolytic process is kinetically slow and local during normal hemostasis because tPA has limited access to fibrin polymers trapped in a platelet clot,46 and its acute release from endothelial cells is triggered by coagulation products such as thrombin or fibrin.47 However, the sequential changes in laboratory measurements of patients with TBI-induced coagulopathy11 indicate the very early development of a hyperfibrinolytic state, raising the question of whether TBI-induced hyperfibrinolysis is initiated independent of fibrin formation. An interesting hypothesis is that fibrinolysis is triggered, accelerated, and enhanced by fibrin formed on the surface of much smaller and circulating MVs (Figure 2). Consistent with the TBI-induced hyperfibrinolysis, elevated plasma tPA and the fibrinolytic product D-dimer are associated with progressive hemorrhagic injury in patients with TBI.48 In mouse models of TBI, tPA deficiency reduces persistent intracerebral hemorrhage but does not prevent systemic coagulopathy, whereas urokinase deficiency improves both conditions.49
There are fewer reports on changes of fibrinolysis inhibitors during the course of TBI. The plasmin inhibitor α2 macroglobulin decreases significantly by 2 hours and then increases by 4 hours in plasma samples of rats subjected to polytrauma.50 A low-plasma α2 antiplasmin is associated with progressive hemorrhage in patients with TBI.48 Mice deficient in plasminogen activator inhibitor-1 (PAI-1) were found to develop severe intracerebral hemorrhage after TBI.49 Interestingly, the plasma level of PAI-1 was increased, along with elevated plasminogen, plasmin activity, and D-dimers in mice with polytrauma,50 suggesting a trauma-induced imbalance between fibrinolysis and its inhibition. This imbalance is also observed in trauma patients with hyperfibrinolysis, who had a significantly increased level of tPA48 but an unchanged51 or even reduced level of PAI-1 compared with those without hyperfibrinolysis.52 Tranexamic acid, which is a synthetic derivative of the amino acid lysine that reversibly blocks the lysine-binding sites on plasminogen, has been reported to reduce the mortality of trauma patients when it was given within 3 hours of injury in a randomized, double-blind, placebo-controlled trial (CRASH-2).53,54 Its effect on TBI death and disability is currently being investigated through the CRASH-3 trial (NCT01402882). A separate ongoing trial (NCT01990768) also measures the effect of prehospital administration of tranexamic acid on clinical outcomes of moderate to severe TBI. However, coagulopathy is not a primary outcome variable of both trials.
Platelets
TBI-induced changes to platelets and their reactivity are least understood. Platelets from TBI patients and those from rats and pigs subjected to experimental TBI have moderately low counts, are often activated, produce MVs, and express procoagulant activity.55-57 Pulmonary and cerebral microthrombosis is also reported in animal models of TBI.58 The cerebral microthrombi are mostly detected in the pericontusion cortex59,60 and contain not only fibrin61 but also platelets58,60 and von Willebrand factor.60 These phenotypic changes are consistent with a TBI-induced platelet hyperreactivity, but how platelets become activated or hyperreactive after TBI has not been mechanistically examined. Thrombin generated from extrinsic coagulation30 can activate platelets in the acute phase of TBI. Another potential factor that has been studied extensively for its neurotoxicity, but not for platelet activation during the early course of TBI, is platelet-activating factor (1-O-hexadecyl-2-acetyl-sn-glycero-3-phosphocholine). This lipid mediator is enriched in the brain and spinal cord62 and released during cerebral ischemia.63 It activates platelets by initiating intracellular signals to activate phospholipases C and A2, which hydrolyze phosphoinositides (the phosphorylated forms of phosphatidylinositol) to release arachidonic acid,64 a potent platelet activator.
Another observation that has been extensively reported, but not fully explained, is that platelets from TBI patients,65-68 rats,55,68,69 and swines70 that are subjected to experimental TBI respond poorly to adenosine diphosphate and/or arachidonic acid. This poor response is independent of platelet counts, hemorrhagic shock, and tissue hypoperfusion.55,65,68,69 More importantly, it does not appear to be caused by granule depletion of activated platelets.71
In summary, TBI-induced coagulopathy is a well-known and common condition that has been overshadowed by the severity of TBI itself. With continuing advances in TBI treatments, controlling or reversing coagulopathy has been increasingly recognized as an important measure for improving clinical outcomes of TBI. Significant progress has been made in understanding TBI-induced coagulopathy, but gaps remain as discussed here. TBI-induced coagulopathy has been traditionally managed by neurosurgeons and physicians in emergency and critical care departments. Hematologists and investigators in hemostasis should be more involved not only in advancing the mechanistic understanding of TBI-induced coagulopathy and its targeted therapies but also in managing elderly patients.8,9 A demographic shift in TBI patients to be increasingly older (≥50 years) has resulted in more patients with comorbidities or on anticoagulant and antiplatelet medications.8,9 It has been shown that patients on the anticoagulant warfarin and with therapeutic INR at the time of TBI have poor outcomes,72 but the impact of antiplatelet medications has not been consistently reported in the literature.73-76
Acknowledgments
This study is supported by National Institutes of Health, National Institute of Neurological Disorders and Stroke grant NS087296 and National Heart, Lung, and Blood Institute grant HL119391 (J.-f.D.), Natural Science Foundation of China State Key Program Grant 81330029, and Research grants 81271361 and 81271359 (J.Z.).
Authorship
Contribution: J.Z., F.Z., and J.-f.D. analyzed the data from mice subjected to TBI and CT images from TBI patients before and after surgery and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jing-fei Dong, Bloodworks Research Institute, 1551 Eastlake Ave East, Seattle, WA 98102; e-mail: jfdong@psbc.org; and Jianning Zhang, Department of Neurosurgery, Tianjin Medical University General Hospital, Tianjin 300052, China; e-mail: jianningzhang@hotmail.com.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal