In this issue of Blood, Scott et al discuss the optimal fluorescence in situ hybridization (FISH) screening strategy to identify high-grade B-cell lymphoma with MYC and BCL2 and/or BCL6 rearrangements (HGBL-DH/TH) with diffuse large B-cell lymphoma (DLBCL) morphology.1
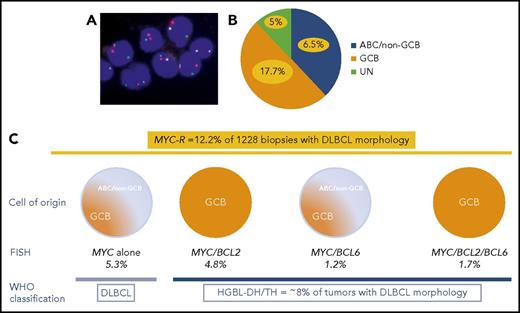
(A) FISH for MYC using MYC break-apart probe in DLBCL. (B) In the study of Scott et al, GCB, ABC, and unclassified DLBCLs represent 50%, 38%, and 12% of all DLBCLs, respectively. MYC-R DLBCL (yellow) accounts for 17.7% of GCB, 6.5% of ABC, and 5% of unclassified DLBCLs. (C) MYC-R was detected in 12.2% of cases with DLBCL morphology, including 5.3% of DLBCLs with MYC sole rearrangement; 4.2% of MYC/BCL2 HGBL-DH; 1.2% of MYC/BCL6 HGBL-DH; and 1.7% of MYC/BCL2/BCL6 HGBL-TH. Overall, HGBL-DH/TH represents ∼8% of cases with DLBCL morphology.
(A) FISH for MYC using MYC break-apart probe in DLBCL. (B) In the study of Scott et al, GCB, ABC, and unclassified DLBCLs represent 50%, 38%, and 12% of all DLBCLs, respectively. MYC-R DLBCL (yellow) accounts for 17.7% of GCB, 6.5% of ABC, and 5% of unclassified DLBCLs. (C) MYC-R was detected in 12.2% of cases with DLBCL morphology, including 5.3% of DLBCLs with MYC sole rearrangement; 4.2% of MYC/BCL2 HGBL-DH; 1.2% of MYC/BCL6 HGBL-DH; and 1.7% of MYC/BCL2/BCL6 HGBL-TH. Overall, HGBL-DH/TH represents ∼8% of cases with DLBCL morphology.
Diffuse large B-cell lymphoma not otherwise specified (DLBCL-NOS) is the most common lymphoma, accounting for ∼30% of adult non-Hodgkin lymphomas.2 DLBCL-NOS is characterized by clinical, histopathological, cytogenetic, and molecular heterogeneity. Major advances have been made in the last decade deciphering the molecular complexity of the disease. Cell-of-origin (COO) classification in germinal center B-cell-like (GCB) and activated B-cell-like (ABC)/non-GCB DLBCL subtypes is required to assess clinical outcome and adjust therapy. COO molecular classification based on gene expression profiling has been translated into routine practice using immunohistochemical algorithms, which classify cases into GCB and non-GCB subtypes, with Hans algorithm being the most frequently used. More recent methods based on RNA transcripts quantification, such as the Lymph2Cx gene expression assay or the RT-MLPA classifier, provide more accurate COO classification into GCB, ABC, and unclassified categories.3,4 Other prognostic markers include MYC gene alterations, which have a key role in the oncogenesis of a subset of DLBCL-NOS. MYC rearrangement (MYC-R) is observed in 5% to 15% of de novo DLBCLs and is associated with a worse prognosis and higher risk of central nervous system involvement.5 DLBCLs with double MYC and BCL2 gene rearrangements (double-hit [DH] lymphomas) represent a peculiar subset of DLBCLs with high-risk clinical features. More recently, MYC protein overexpression (≥40%) in association with BCL2 protein overexpression (≥50%) defining so-called double-protein expressor (DPE) DLBCL has been suggested as an additional poor prognostic marker, accounting for 20% to 35% of DLBCLs and observed mostly in ABC/non-GCB DLBCLs.6
The updated 2017 World Health Organization (WHO) classification requires the identification of all aggressive mature B-cell lymphomas with MYC and BCL2 and/or BCL6 rearrangements as a single category designated HGBL-DH/TH, with the goal of improving our understanding of the disease and to facilitate the development of alternative therapies.2 Thus, the former B-cell lymphoma, unclassifiable, with features intermediate between DLBCL and Burkitt lymphoma category is now replaced by 2 new categories designated HGBL-DH/TH and HGBL-NOS. HGBL-DH/TH represents an aggressive mature B-cell lymphoma with MYC and BCL2 and/or BCL6 rearrangements that may display DLBCL, intermediate, or blastoid morphological features. HGBL-NOS is restricted to tumors with intermediate or blastoid morphology, but without the DH lymphomas, and excludes DLBCL with sole MYC or BCL2 or BCL6 breaks. The updated WHO classification has significant consequences for the diagnostic workup of DLBCL in daily practice because DH/TH DLBCLs do not necessarily display aggressive morphological and/or immunohistochemical features, like starry sky pattern, high mitotic rate, or MYC protein overexpression.5 This raises the question whether every DLBCL should be referred for FISH testing for MYC, BCL2, and BCL6 rearrangements to detect DH status.
Interphase FISH on formalin-fixed paraffin-embedded tissue section is a robust technique, but is time consuming, expensive, and not widely available. However, FISH techniques in recent years have greatly improved with automatization and development of digital imaging technologies. Various strategies have been proposed to restrict FISH testing to GCB subtype, or according to Ki67 proliferative index or MYC protein expression. Some authors suggested limiting FISH to GCB and DPE DLBCLs, which would reduce FISH analysis to 15% of cases.7 However, no consensus has been reached to date, the main reason being the lack of large cohorts of DLBCL patients with COO and FISH data to test various screening strategies.
Scott et al provide data from a large cohort of 1228 de novo DLBCLs, identified in 3 international clinical trials and a population-based registry, to evaluate the incidence of HGBL-DH/TH and the effects of screening strategies based on COO (Lymph2Cx gene expression assay and/or Hans algorithm) and/or DPE. MYC rearrangement (MYC-R) was observed in 12.2% of DLBCLs and included mostly, but not exclusively, GCB DLBCLs. MYC as sole genetic alteration and MYC/BCL6 HGBL-DH included both ABC and GCB DLBCLs, whereas MYC/BCL2 and MYC/BCL2/BCL6 HGBL-DH/TH were exclusively GCB. In total, HGBL-DH/TH represented ∼8% of tumors with DLBCL morphology (see figure).
According to the study by Scott et al, the best method for detecting all HGBL-DH/TH among tumors with DLBCL morphology is to screen all DLBCLs for MYC breaks. When the tumor is positive, it should be further tested for BCL2 and BCL6 gene alterations, which would require that the FISH technique be in pathology laboratories and that reliable MYC probes are used. Alternatively, restricting FISH testing to GCB DLBCLs would reduce FISH testing to half of DLBCLs and would still detect ≥99% HGBL-DH/TH with BCL2 rearrangements. This approach is acceptable for MYC/BCL2 HGBL-DH detection but would miss a considerable number of the uncommon MYC/BCL6 HGBL-DH, where the prognostic value is still controversial.5,8 In addition, this approach would miss DLBCLs with isolated MYC rearrangement and ABC/non-GCB phenotype. A major point of the study is to show that selecting DLBCLs on DPE status and/or COO subtyping results in missing ∼35% of all HGBL-DH.
In summary, the study of Scott et al presents data on the impact of various FISH testing strategies to identify HGBL-DH/TH in tumors with DLBCL morphology. FISH testing for MYC, BCL2, and BCL6 should be incorporated in the routine diagnostic workup of all DLBCLs in an integrated approach together with gene expression assays and next-generation sequencing. If not possible, the optimal strategy is a 2-step approach with testing for MYC first and to perform FISH for BCL2 and BCL6 if there is MYC rearrangement. Other screening strategies to limit the costs should be discussed in each institution depending on the local resources and with the knowledge of the limitations of each strategy as reported in this study.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal