Key Points
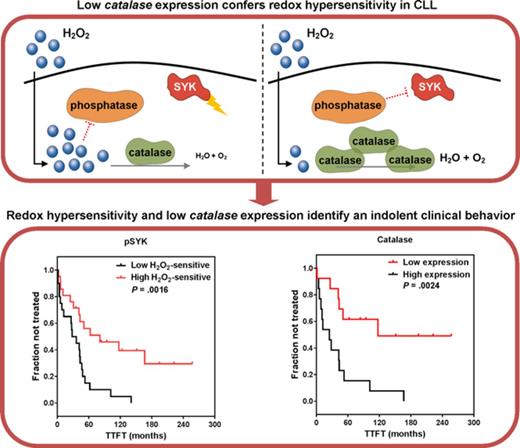
Low catalase expression confers hypersensitivity to external redox cues.
Differential redox profiles are associated with divergent clinical behaviors in CLL.
Abstract
B-cell receptor (BCR) signaling is a key determinant of variable clinical behavior and a target for therapeutic interventions in chronic lymphocytic leukemia (CLL). Endogenously produced H2O2 is thought to fine-tune the BCR signaling by reversibly inhibiting phosphatases. However, little is known about how CLL cells sense and respond to such redox cues and what effect they have on CLL. We characterized the response of BCR signaling proteins to exogenous H2O2 in cells from patients with CLL, using phosphospecific flow cytometry. Exogenous H2O2 in the absence of BCR engagement induced a signaling response of BCR proteins that was higher in CLL with favorable prognostic parameters and an indolent clinical course. We identified low catalase expression as a possible mechanism accounting for redox signaling hypersensitivity. Decreased catalase could cause an escalated accumulation of exogenous H2O2 in leukemic cells with a consequent greater inhibition of phosphatases and an increase of redox signaling sensitivity. Moreover, lower levels of catalase were significantly associated with a slower progression of the disease. In leukemic cells characterized by redox hypersensitivity, we also documented an elevated accumulation of ROS and an increased mitochondrial amount. Taken together, our data identified redox sensitivity and metabolic profiles that are linked to differential clinical behavior in CLL. This study advances our understanding of the redox and signaling heterogeneity of CLL and provides the rationale for the development of therapies targeting redox pathways in CLL.
Introduction
Chronic lymphocytic leukemia (CLL), the most prevalent leukemia in Europe and North America, is characterized by the expansion of a population of mature B cells that accumulate in the bone marrow, lymphoid tissues, and blood.1,2 Clinical presentation, natural course of the disease, and response to treatment are all extremely variable, with some patients having indolent disease and others experiencing a more accelerated course, treatment resistance, and a dismal outcome.3,4 Several biological parameters have been shown to be associated with clinical outcomes in patients with CLL, including the presence or absence of somatic mutations within the immunoglobulin variable heavy chain genes (IGHV), specific chromosomal abnormalities and gene mutations, the expression of the ZAP70 tyrosine kinase, and CD38 antigen.5 Although the reason for this clinical disparity is not fully understood, B-cell receptor (BCR) signaling, which is central to B-cell development, is considered a key determinant of variable clinical behavior and is a target for therapeutic interventions in CLL.6-10
BCR stimulation by antigen induces increase of intracellular calcium; activation of receptor proximal protein tyrosine kinases, such as spleen tyrosine kinase (SYK); and the tyrosine phosphorylation of several proteins of the BCR signaling network.11 However, BCR stimulation also recruits negative regulators such as protein tyrosine phosphatases (PTPs) that counterbalance protein kinase activity.12,13 Initiation, transmission, and strength of the BCR signaling are indeed regulated by the interplay between kinases and PTPs, and the full activation of the signaling cascade requires not only activation of kinases but also inhibition of PTPs.12,13 PTPs may be reversibly inhibited through oxidation of the catalytic cysteine by hydrogen peroxide (H2O2), which is the primary reactive oxygen species (ROS) produced by B cells. Hydrogen peroxide is, in turn, generated by calcium-dependent NADPH oxidases (NOX), which are recruited and activated in B cells after BCR stimulation.12-15 Thus, BCR-induced hydrogen peroxide acts as a positive feedback that plays an important role in the amplification of the BCR signal.
Increased ROS levels have been detected in various cancers, where they activate protumorigenic signals; enhance cell survival, proliferation, and chemoresistance; and cause DNA damage and genetic instability.16-18 However, escalated levels of ROS can also promote antitumorigenic signals, resulting in an increase of oxidative stress and induction of cancer cell death.16,19-21 For this reason, therapies aimed at either reverting the increased ROS levels or further escalating ROS may be potentially effective against cancer.22
CLL cells accumulate higher levels of ROS than normal B cells.23 ROS levels are extremely variable across patients’ samples, and higher ROS levels are associated with favorable prognostic features and a slower disease progression.24 Moreover, augmented levels of ROS confer increased sensitivity to anticancer agents that induce apoptosis in leukemia cells.25 Altogether, escalated levels of ROS seem to account for a lesser aggressive behavior of CLL cells. However, it is still unclear how CLL B cells sense and respond to redox cues and what effect they have on disease.
In this study, we used phosphospecific flow cytometry to characterize redox signaling sensitivity in prognostic subsets of leukemic cells and to investigate possible mechanisms regulating response of leukemic cells to redox cues in CLL.
Methods
Patients
Peripheral blood mononuclear cell (PBMC) samples from 42 untreated patients with CLL and from 9 age-matched healthy donors (HDs) were collected and cryopreserved at the Hematology Unit, Azienda Ospedaliera Universitaria Integrata, Verona, Italy, on approval from the local Ethics Committee (Comitato Etico per la Sperimentazione, Azienda Ospedaliera Universitaria Integrata). In accordance with the Declaration of Helsinki, all patients and donors provided written informed consent. Details on inclusion criteria and characteristics of patients at diagnosis are summarized in supplemental Methods and supplemental Table 1, available on the Blood Web site.
Cell preparation
PBMCs were isolated by Ficoll-hypaque centrifugation (Lymphoprep; Nicomed, Oslo, Norway) and stored in liquid nitrogen. Cell viability assessment and B-cell purification are detailed in supplemental Methods.
Cell treatments
After 24-hour rest at 37°C, PBMCs were treated in bulk for 10 minutes with 3.3 mM H2O2 (Sigma-Aldrich, Milan, Italy) and/or 20 μg/mL goat F(ab′)2 anti-human immunoglobulin M (IgM; SouthernBiotech, Birmingham, AL), as detailed in supplemental Methods. H2O2 efficacy in inhibiting PTPs was assessed using a tyrosine phosphatase assay (supplemental Information). When indicated, before modulation with 3.3 mM H2O2, PBMCs were incubated with the enzyme inhibitors 3-amino-1,2,4-triazole (ATZ; 1 mM), mercaptosuccinic acid (MCA; 100 μM), and sodium diethyldithiocarbamate (DDTC; 70 μM; all from Sigma-Aldrich) for 24 hours at 37°C; the antioxidant N-acetylcysteine (NAC; 5 mM), exogenous catalase (1000 U/mL; both from Sigma-Aldrich), or the SYK inhibitor PRT-060318 (2 μM; Selleck Chemicals, Munich, Germany) for 30 minutes.
Phosphospecific flow cytometry
Phosphospecific flow cytometry was performed as previously described.7,8 Briefly, cells were fixed and permeabilized with PerFix Expose kit (Beckman Coulter, Miami, FL), according to the manufacturer’s protocol. Permeabilized cells were stained with fluorochrome-conjugated antibodies. For staining phosphoproteins, the following antibodies were used: anti-pSYK-PECy7 (pY352)/ZAP70 (pY319) (clone 17A/P-ZAP70), anti-pp38-PE (pT180/pY182, clone 36/p38), anti-pNF-κB-PECy7 p65 (pS529, clone K 10-895.12.50), anti-pJNK-AlexaFluor647 (pT183/pY185, clone N9-66; all from BD Biosciences, San Jose, CA), and anti-pERK1/2-AlexaFluor488 (pT202/pY204, clone D13.14.4E; Cell Signaling Technology, Danvers, MA). The complete list of antibodies used is summarized in supplemental Table 2. Although antibody against pSYK recognizes the activating tyrosine residue in SYK (pY352) and ZAP70 (pY319), in CLL, phosphorylation of the activating tyrosine in ZAP70 is highly inefficient and negligible when compared with phosphorylation of SYK.26 Therefore, we can assume that phosphorylation events recognized in our study mostly refer to SYK phosphorylation at Y352 site.
Approximately 30 000 gated events were acquired for each sample on a FACSCanto II cytometer (Becton Dickinson, Franklin Lakes, NJ). Samples were run in duplicate. Flow cytometry data processing and analysis are described in supplemental Methods.
Quantitative reverse transcription polymerase chain reaction
Real-time quantification for CAT (catalase), GPX1 (glutathione peroxidase1), SOD1 (Cu,Zn-superoxide dismutase), SOD2 (Mn-superoxide dismutase), and gp91phox (Nox subunit) was performed as detailed in supplemental Methods.
Antioxidant enzymes
Protein expression of catalase, GPX, CuZnSOD, and MnSOD was determined using monoclonal antibodies and flow cytometry, as described in supplemental Methods.
Reduced thiols
Levels of reduced thiols were detected by ThiolTracker Violet (Thermo Fisher Scientific), according to the manufacturer’s instructions, as described in supplemental Methods.
Antioxidant capacity
Cell antioxidant capacity was measured in cell lysates (5 × 106 cells), using a colorimetric Antioxidant Assay Kit (Cayman Chemical, Ann Arbor, MI), following the manufacturer’s instructions.
Cellular ROS
Total and mitochondrial ROS were, respectively, measured using 2′,7′-dichlorodihydrofluorescein diacetate (Thermo Fisher Scientific) or MitoSOX Red mitochondrial superoxide indicator (Thermo Fisher Scientific), according to the manufacturer’s instruction, as described in supplemental Methods.
Mitochondria labeling
Mitochondria were labeled with MitoTracker Green (Thermo Fisher Scientific), according to the manufacturer’s instruction, as described in supplemental Methods.
Statistical analysis
Student t test, 2-sample Wilcoxon signed-rank sum test, Mann-Whitney U test, or Kruskal-Wallis test were used as appropriate. Time-to-first-treatment (TTFT) was calculated from the date of diagnosis to the date of initial therapy.4,27 Patients who did not receive any treatment during follow-up were censored at their last follow-up date. TTFT curves estimated using the Kaplan-Meier method were compared using the log-rank (Mantel-Cox) test. Time-to-events models for TTFT were generated using Cox proportional hazards regression, and the proportional hazard assumption was tested using the Schoenfeld’s test. Cutoffs for grouping patients on the basis of signaling sensitivity to H2O2 and enzyme expression were calculated as described in supplemental Information.
Differences were considered statistically significant for P values ≤ .05. Graphing and statistical analyses were performed using GraphPad Prism software (v.7.03; GraphPad Software Inc., La Jolla, CA). Gaussian kernel density estimates and univariate/multivariate time-to-event analyses were performed using Mathematica software (v.11.1.1.0; Wolfram Research Inc., Champaign, IL).
Results
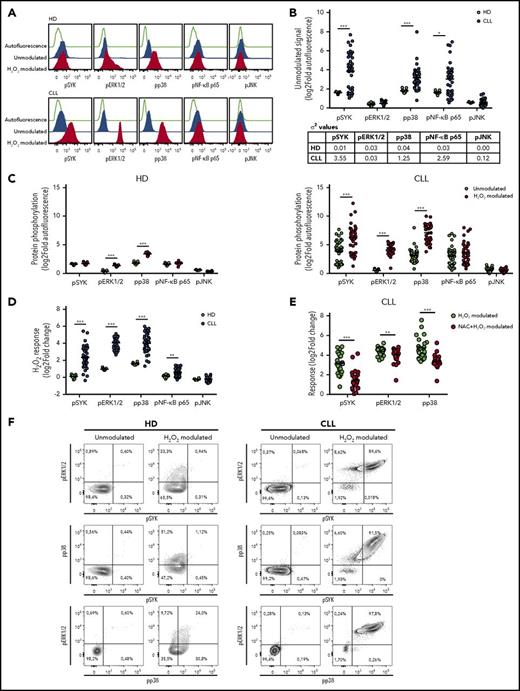
Redox sensitivity distinguishes leukemic from HD B cells
The phosphorylation levels of 5 proteins downstream of the BCR signaling (namely, SYK, ERK1/2, p38, NF-κB p65, and JNK) were analyzed at the single-cell level in 42 CLL cell samples, using phosphospecific flow cytometry. Phosphorylation of the BCR signaling proteins was measured in the basal condition (ie, unmodulated) and under stimulation with H2O2 (ie, H2O2 modulated). Circulating B cells from HDs were analyzed as controls (Figure 1A). Although the basal phosphorylation statuses of signaling proteins were highly heterogeneous among CLL samples (σ2 ≥ 1.25 for pSYK, pp38, and pNF-κB p65; Figure 1B), CLL B cells showed more elevated average basal levels of pSYK, pp38, and pNF-κB p65 than HD B cells (Figure 1B). H2O2 induced a significant increase in the average phosphorylation of ERK1/2 and p38 in both HD and CLL B cells, whereas a significant increase of SYK phosphorylation was observed only in B cells from patients with CLL (Figure 1A,C). Remarkably, redox sensitivity, measured as the log2-fold change in phosphorylation between H2O2-modulated and unmodulated conditions, was significantly higher in CLL than HD B cells for SYK, ERK1/2, p38, and NF-κB p65 (Figure 1D). Redox signaling responses were unrelated to basal phosphorylation levels for each signaling protein except JNK (supplemental Figure 5). Pretreating CLL cells with the antioxidant NAC before stimulation with H2O2 resulted in a significant reduction of redox signaling sensitivity (Figure 1E; supplemental Figure 6).
Redox sensitivity of leukemic and HD B cells. Samples were thawed and rested for 24 hours before modulation with 3.3 mM H2O2 for 10 minutes, or left unmodulated. (A) Representative flow cytometry histograms of BCR phosphoproteins in basal conditions (unmodulated) or after H2O2 modulation in B cells from HDs (n = 9) and patients with CLL (n = 42), compared with autofluorescence signals. (B) Unmodulated levels of signaling protein phosphorylation in B cells from HDs and patients with CLL. Protein phosphorylation was measured as log2 of relative median fluorescence intensity (log2-fold autofluorescence). The table indicates values of variance (σ2) for each phosphoprotein among HD and CLL samples. (C) Signals of phosphoproteins (log2-fold autofluorescence) in the unmodulated condition and after H2O2 modulation in B cells from HDs (left) and patients with CLL (right). (D) Comparison of response to H2O2 modulation, measured as the log2-fold change in phosphorylation between H2O2-modulated and unmodulated conditions, between B cells from HDs and patients with CLL. (E) Responses of pSYK, pERK1/2, and pp38 to H2O2 modulation, measured as log2-fold change, in the absence or presence of 5 mM NAC (n = 24). Lines indicate mean value. Comparisons were performed using the Student t test. *P ≤ .05; **P ≤ .01; ***P ≤ .001. (F) Representative contour plots of pSYK, pERK1/2, and pp38 in basal conditions (unmodulated) or after H2O2 modulation in B cells from HDs and patients with CLL.
Redox sensitivity of leukemic and HD B cells. Samples were thawed and rested for 24 hours before modulation with 3.3 mM H2O2 for 10 minutes, or left unmodulated. (A) Representative flow cytometry histograms of BCR phosphoproteins in basal conditions (unmodulated) or after H2O2 modulation in B cells from HDs (n = 9) and patients with CLL (n = 42), compared with autofluorescence signals. (B) Unmodulated levels of signaling protein phosphorylation in B cells from HDs and patients with CLL. Protein phosphorylation was measured as log2 of relative median fluorescence intensity (log2-fold autofluorescence). The table indicates values of variance (σ2) for each phosphoprotein among HD and CLL samples. (C) Signals of phosphoproteins (log2-fold autofluorescence) in the unmodulated condition and after H2O2 modulation in B cells from HDs (left) and patients with CLL (right). (D) Comparison of response to H2O2 modulation, measured as the log2-fold change in phosphorylation between H2O2-modulated and unmodulated conditions, between B cells from HDs and patients with CLL. (E) Responses of pSYK, pERK1/2, and pp38 to H2O2 modulation, measured as log2-fold change, in the absence or presence of 5 mM NAC (n = 24). Lines indicate mean value. Comparisons were performed using the Student t test. *P ≤ .05; **P ≤ .01; ***P ≤ .001. (F) Representative contour plots of pSYK, pERK1/2, and pp38 in basal conditions (unmodulated) or after H2O2 modulation in B cells from HDs and patients with CLL.
To assess whether phosphorylation events were occurring simultaneously in each cell, we evaluated in individual B cells the relationships between phosphorylation responses that significantly increased on H2O2 modulation (ie, pSYK, pERK1/2, pp38). H2O2 induced the coordinated phosphorylation of ERK1/2 and p38 only in a fraction of the HD cell population, whereas responses of pERK1/2 and pp38 to H2O2 occur simultaneously with the upstream phosphorylation of SYK in most individual CLL cells (Figure 1F). SYK was phosphorylated in concert with ERK1/2 and p38 across individual CLL samples (supplemental Figure 7), thus suggesting that H2O2 triggers an interconnected network of signaling pathways that is common across patients with CLL. Therefore, CLL cells exhibit a specific redox sensitivity pattern with an overall higher and more heterogeneous redox response than HD B cells.
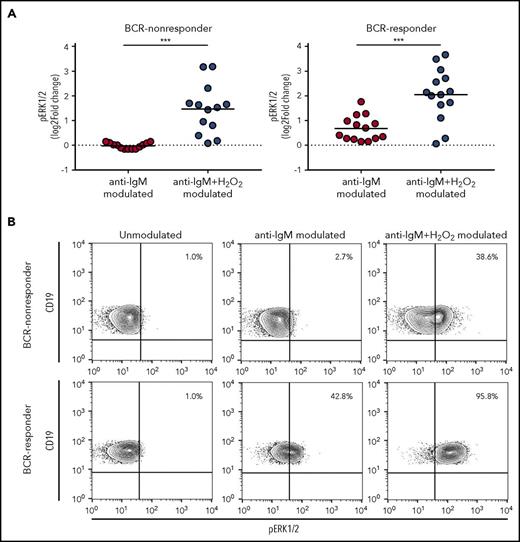
H2O2 modulation potentiates BCR signaling
We examined the relationship between redox sensitivity and BCR signaling, measured as ERK1/2 phosphorylation in response to anti-IgM modulation, in the CLL subsets characterized by different redox sensitivity. ERK1/2 response was expressed as the log2-fold change in phosphorylation between anti-IgM-modulated and unmodulated conditions.7 An overall H2O2 sensitivity index for each patient with CLL was obtained by summing the log2-fold change response to H2O2 for pSYK, pERK1/2, and pp38, which significantly increased in CLL cells on H2O2 modulation (Figure 1A,C). The median of the new distribution was used to discriminate between high and low H2O2-sensitive CLL. As shown in supplemental Figure 8, no differences were detected between anti-IgM ERK1/2 response and H2O2 sensitivity.
To assess whether H2O2 could perturb the signaling response induced by BCR engagement, we measured ERK1/2 response to anti-IgM in the presence or absence of H2O2. BCR-responder and BCR-nonresponder CLL was referred to the median value of the ERK1/2 response distribution. The presence of H2O2 significantly potentiated signaling response of CLL cells to anti-IgM modulation in BCR-nonresponder, as well as the BCR-responder CLL (Figure 2), thus extending data from previous studies.28,29
Influence of H2O2modulation on BCR signaling. (A) CLL cell samples were stimulated with 10 µg/mL anti-IgM for 10 minutes in the absence or presence of 3.3 mM H2O2, and signaling response was measured as the log2-fold change of ERK1/2 phosphorylation between modulated and unmodulated conditions. Samples were grouped in BCR-nonresponder and BCR-responder, referring to the median value of ERK1/2-response to anti-IgM. Comparison of signaling responses was performed using the Student t test. Lines indicate the mean value. ***P ≤ .001. (B) Representative contour plots of pERK1/2 in unmodulated condition or after modulation with anti-IgM, in the absence or presence of H2O2.
Influence of H2O2modulation on BCR signaling. (A) CLL cell samples were stimulated with 10 µg/mL anti-IgM for 10 minutes in the absence or presence of 3.3 mM H2O2, and signaling response was measured as the log2-fold change of ERK1/2 phosphorylation between modulated and unmodulated conditions. Samples were grouped in BCR-nonresponder and BCR-responder, referring to the median value of ERK1/2-response to anti-IgM. Comparison of signaling responses was performed using the Student t test. Lines indicate the mean value. ***P ≤ .001. (B) Representative contour plots of pERK1/2 in unmodulated condition or after modulation with anti-IgM, in the absence or presence of H2O2.
Redox hypersensitivity is associated with favorable prognostic parameters and a slower clinical progression
To investigate whether CLL cells from different prognostic classes could differentially respond to external redox cues, redox sensitivity was considered in relationship to standard prognostic parameters. Phosphorylation response of SYK to H2O2 was significantly higher in the patient subsets defined by M-IGHV, CD38, and ZAP70-negative expression (Figure 3A). Moreover, redox pSYK and pp38 hypersensitivity was associated with early-stage (Binet stage A) disease, whereas redox pSYK and pERK1/2 hypersensitivity was more frequently detected in patients who did not require treatment during the follow-up (Figure 3A).
Association of redox sensitivity with biological and clinical characteristics and clinical progression. (A) Responses of BCR signaling proteins, measured as the log2-fold change in phosphorylation between H2O2-modulated and unmodulated conditions, were associated with biological and clinical parameters (ie, IGHV status [n = 42], CD38 expression [n = 42], ZAP70 expression [n = 42], cytogenetic mutations [n = 41], Binet stage [n = 42], and requirement of treatment during follow-up [n = 42]). Differences in sample sizes depend on availability of biological and clinical information. Lines indicate mean values. Student t test and 1-way ANOVA test were used for comparisons, as appropriate. *P ≤ .05; **P ≤ .01. (B) Kaplan-Meier curves of TTFT for subgroups of patients with CLL defined by pSYK (n = 41), pERK1/2 (n = 41), or the sum of pSYK, pERK1/2, and pp38 (n = 41) responses to H2O2 (each measured as log2-fold change). High and low pSYK and pERK1/2 values (log2-fold change) were defined using threshold values of the probability density function of log2-fold phosphoprotein data (see supplemental Methods and supplemental Figure 4). P values are from the logrank test. High and low summing value was defined using the median value of the distribution. The dot plot shows pSYK, pERK1/2, and pp38 responses to H2O2 for each individual patient, as described in Figure 1C. High and low values used to compute the Kaplan-Meier curves are highlighted in red and blue, respectively. Lines indicate median values.
Association of redox sensitivity with biological and clinical characteristics and clinical progression. (A) Responses of BCR signaling proteins, measured as the log2-fold change in phosphorylation between H2O2-modulated and unmodulated conditions, were associated with biological and clinical parameters (ie, IGHV status [n = 42], CD38 expression [n = 42], ZAP70 expression [n = 42], cytogenetic mutations [n = 41], Binet stage [n = 42], and requirement of treatment during follow-up [n = 42]). Differences in sample sizes depend on availability of biological and clinical information. Lines indicate mean values. Student t test and 1-way ANOVA test were used for comparisons, as appropriate. *P ≤ .05; **P ≤ .01. (B) Kaplan-Meier curves of TTFT for subgroups of patients with CLL defined by pSYK (n = 41), pERK1/2 (n = 41), or the sum of pSYK, pERK1/2, and pp38 (n = 41) responses to H2O2 (each measured as log2-fold change). High and low pSYK and pERK1/2 values (log2-fold change) were defined using threshold values of the probability density function of log2-fold phosphoprotein data (see supplemental Methods and supplemental Figure 4). P values are from the logrank test. High and low summing value was defined using the median value of the distribution. The dot plot shows pSYK, pERK1/2, and pp38 responses to H2O2 for each individual patient, as described in Figure 1C. High and low values used to compute the Kaplan-Meier curves are highlighted in red and blue, respectively. Lines indicate median values.
To assess the potential link between redox sensitivity and disease behavior, measured as TTFT, we examined time-to-event modeling using signaling data in response to H2O2, IGHV status, ZAP70, and CD38 expression; cytogenetic alterations; and Binet clinical stage in 41 patient samples. Univariate time-to-event analysis showed that low pSYK (P = .0025) and pERK1/2 (P = .0436) responses to H2O2 (Figure 3B), UM-IGHV (P< .0001), ZAP70-positive (P = .0050), and Binet stage B-C (P< .0001) were significantly associated with shorter TTFT. Consistent with Myklebust et al,30 grouping patients into low and high responses to H2O2 on the basis of the sum of pSYK, pERK1/2, and pp38 responses allowed a better stratification of different TTFT than using individual phosphoproteins (Figure 3B). Comprehensive multivariate hazards models using significant parameters, as resulted from the univariate analysis, showed that pSYK or pERK1/2 responses to H2O2 do not influence clinical outcome independent of standard prognostic parameters (data not shown). Then, we investigated a possible role for H2O2-induced pSYK in promoting antitumor signals, such as cell death. Analysis of cell viability after treatment with H2O2 in the presence of the SYK inhibitor PRT-060318 showed that SYK inhibition does not influence H2O2-induced cell death (supplemental Figure 9)
The next step was to investigate potential mechanisms underlying different redox sensitivity in CLL cells.
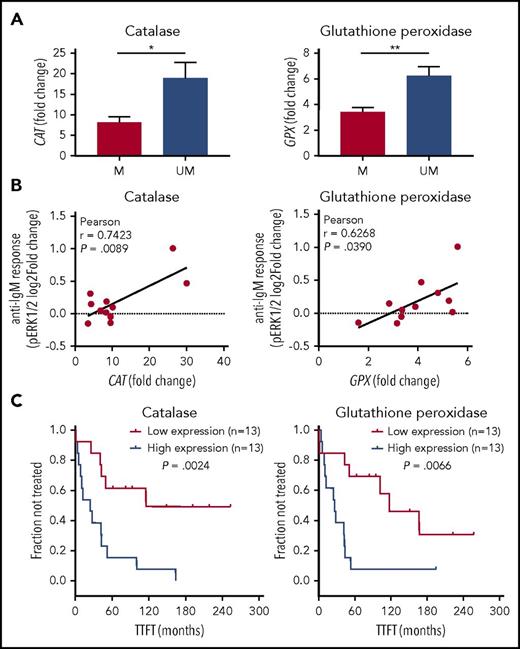
Redox hypersensitivity is associated with lower expression levels of catalase and glutathione peroxidase
We hypothesized that redox hypersensitivity could be the result of an increased accumulation of exogenous H2O2 within the cells, which would induce a higher inhibition of phosphatases with the consequent shift of the enzymatic balance toward phosphorylation. One possibility is that higher accumulation of exogenous H2O2 is a consequence of reduced antioxidant capacity of the cells. Therefore, we analyzed major cellular antioxidant systems, enzymatic and nonenzymatic, and compared them between the 2 CLL subsets characterized by different redox sensitivity, measured by the sum of pSYK, pERK1/2, and pp38 H2O2 responses. Samples exhibiting high sensitivity to H2O2 expressed lower levels of catalase and glutathione peroxidase (GPX), which convert hydrogen peroxide to molecular oxygen (Figure 4A). In contrast, expression levels of superoxide dismutase, both copper-zinc (CuZnSOD) and manganese (MnSOD) types, which catalyze the dismutation of the superoxide anions to hydrogen peroxide, as well as thiols, which represent the major nonenzymatic antioxidant scavenging ROS, showed no significant differences between CLL cells with different redox sensitivity (Figure 4A). Overall, antioxidant capacity of high H2O2-sensitive CLL appeared decreased by trend (Figure 4A). The expression of catalase, GPX, CuZnSOD, and MnSOD was confirmed at protein level (Figure 4B).
Association of redox sensitivity with antioxidant cell systems. (A) Patients were grouped on the basis of high and low H2O2-sensitive values, referred to the median signal values of the combined sum of pSYK, pERK1/2, and pp38, each measured as log2-fold change. Comparisons between high and low H2O2-sensitive groups for relative gene expression of CAT (catalase; n = 26), glutathione peroxidase (GPX; n = 26), SOD1 (Cu,Zn-superoxide dismutase, CuZnSOD; n = 26), SOD2 (Mn-superoxide dismutase, MnSOD; n = 26), reduced-thiol levels (detected by ThiolTracker probe; n = 18), and cell antioxidant capacity (n = 8) were performed using the Student t test. Data are expressed as mean ± SEM. *P ≤ .05; **P ≤ .01. (B) Flow cytometry histograms showing protein expression of catalase, GPX, CuZnSOD, and MnSOD in a representative low H2O2-sensitive sample (n = 3) and a representative high H2O2-sensitive sample (n = 3). Dashed lines represent autofluorescence signals.
Association of redox sensitivity with antioxidant cell systems. (A) Patients were grouped on the basis of high and low H2O2-sensitive values, referred to the median signal values of the combined sum of pSYK, pERK1/2, and pp38, each measured as log2-fold change. Comparisons between high and low H2O2-sensitive groups for relative gene expression of CAT (catalase; n = 26), glutathione peroxidase (GPX; n = 26), SOD1 (Cu,Zn-superoxide dismutase, CuZnSOD; n = 26), SOD2 (Mn-superoxide dismutase, MnSOD; n = 26), reduced-thiol levels (detected by ThiolTracker probe; n = 18), and cell antioxidant capacity (n = 8) were performed using the Student t test. Data are expressed as mean ± SEM. *P ≤ .05; **P ≤ .01. (B) Flow cytometry histograms showing protein expression of catalase, GPX, CuZnSOD, and MnSOD in a representative low H2O2-sensitive sample (n = 3) and a representative high H2O2-sensitive sample (n = 3). Dashed lines represent autofluorescence signals.
We compared catalase or GPX with IGHV status and detected that levels of catalase and GPX were significantly lower within CLL with M-IGHV (Figure 5A). Moreover, both catalase and GPX levels were positively correlated with BCR signaling, measured as pERK1/2 response to BCR modulation (Figure 5B). Then, we investigated potential correlations between catalase or GPX expression levels and disease behavior. As shown in Figure 5C, Kaplan-Meier curves showed that low levels of catalase and GPX were significantly associated with a longer TTFT.
Association of catalase or GPX levels with IGHV status, BCR signaling, and clinical behavior. (A) Expression levels of catalase or GPX in mutated (M) and unmutated (UM) IGHV CLL (n = 26). *P ≤ .05; **P ≤ .01 (B) Association between phosphorylation responses to BCR modulation and catalase or GPX expression levels. BCR signaling in response to anti-IgM stimulation was measured as the log2-fold change of ERK1/2 phosphorylation between anti-IgM-modulated and unmodulated conditions (n = 11). For each comparison is indicated the Pearson correlation coefficient along with the probability value for the null hypothesis of no correlation. (C) Kaplan-Meier curves of TTFT for subgroups of patients with CLL, defined by expression levels of catalase or GPX (n = 26). High and low enzyme expression values were referred to the median expression values. P values are from the logrank test.
Association of catalase or GPX levels with IGHV status, BCR signaling, and clinical behavior. (A) Expression levels of catalase or GPX in mutated (M) and unmutated (UM) IGHV CLL (n = 26). *P ≤ .05; **P ≤ .01 (B) Association between phosphorylation responses to BCR modulation and catalase or GPX expression levels. BCR signaling in response to anti-IgM stimulation was measured as the log2-fold change of ERK1/2 phosphorylation between anti-IgM-modulated and unmodulated conditions (n = 11). For each comparison is indicated the Pearson correlation coefficient along with the probability value for the null hypothesis of no correlation. (C) Kaplan-Meier curves of TTFT for subgroups of patients with CLL, defined by expression levels of catalase or GPX (n = 26). High and low enzyme expression values were referred to the median expression values. P values are from the logrank test.
These data suggest that differences in the antioxidant machinery may underlie divergent redox signaling sensitivity of BCR proteins, as well as clinical behavior in patients with CLL.
Perturbing catalase activity influences redox sensitivity
To assess a role for catalase and GPX in the redox sensitivity of CLL cells, first we blocked activity of catalase, GPX, or CuZnSOD in CLL cells before stimulation with H2O2. Cell treatment with ATZ, which specifically inhibits catalase activity, resulted in a further increase of protein phosphorylation without affecting signal transduction induced by BCR engagement (supplemental Figure 10). In contrast, blocking GPX with MCA induced a slight phosphorylation reduction. Inhibition of CuZnSOD with DDTC before H2O2 treatment had no effects on H2O2-induced phosphorylation of the BCR signaling proteins (Figure 6A-B). Remarkably, preincubation with exogenous catalase before the addition of H2O2 completely reversed protein phosphorylation induced by H2O2 (Figure 6C-D).
Signaling response to H2O2modulation in the presence of antioxidant enzyme inhibitors or exogenous catalase. (A) CLL cells were pretreated with the catalase inhibitor ATZ (1 mM), the GPX inhibitor MCA (100 µM), the CuZnSOD inhibitor DDTC (70 µM), or vehicle for 24 hours and then modulated with 3.3 mM H2O2 for 10 minutes. Response to H2O2 modulation was measured as sum of log2-fold change of pSYK, pERK1/2, and pp38 and then converted to percentage with respect to vehicle response. (B) Representative contour plots of pSYK expression in response to H2O2 modulation in presence of the catalase inhibitor ATZ, the GPX inhibitor MCA, the CuZnSOD inhibitor DDTC, or vehicle. (C) CLL cells were pretreated with 1000 U/mL exogenous catalase from bovine liver for 30 minutes and then modulated with 3.3 mM H2O2 for 10 minutes. Response to H2O2 modulation was measured as sum of log2-fold autofluorescence of pSYK, pERK1/2, and pp38. (D) Representative contour plots of pSYK expression in response to H2O2 modulation in presence of exogenous catalase, or vehicle. Comparisons were performed using the paired Student t test. Data are expressed as mean ± SEM. *P ≤ .05; ***P ≤ .001.
Signaling response to H2O2modulation in the presence of antioxidant enzyme inhibitors or exogenous catalase. (A) CLL cells were pretreated with the catalase inhibitor ATZ (1 mM), the GPX inhibitor MCA (100 µM), the CuZnSOD inhibitor DDTC (70 µM), or vehicle for 24 hours and then modulated with 3.3 mM H2O2 for 10 minutes. Response to H2O2 modulation was measured as sum of log2-fold change of pSYK, pERK1/2, and pp38 and then converted to percentage with respect to vehicle response. (B) Representative contour plots of pSYK expression in response to H2O2 modulation in presence of the catalase inhibitor ATZ, the GPX inhibitor MCA, the CuZnSOD inhibitor DDTC, or vehicle. (C) CLL cells were pretreated with 1000 U/mL exogenous catalase from bovine liver for 30 minutes and then modulated with 3.3 mM H2O2 for 10 minutes. Response to H2O2 modulation was measured as sum of log2-fold autofluorescence of pSYK, pERK1/2, and pp38. (D) Representative contour plots of pSYK expression in response to H2O2 modulation in presence of exogenous catalase, or vehicle. Comparisons were performed using the paired Student t test. Data are expressed as mean ± SEM. *P ≤ .05; ***P ≤ .001.
These findings establish a functional link between catalase and redox sensitivity and support the hypothesis that a lower catalase activity, a condition that would determine the accumulation of exogenous hydrogen peroxide within leukemic cells, can determine redox hypersensitivity in CLL.
Redox hypersensitivity is associated with higher levels of ROS and higher mitochondrial amounts
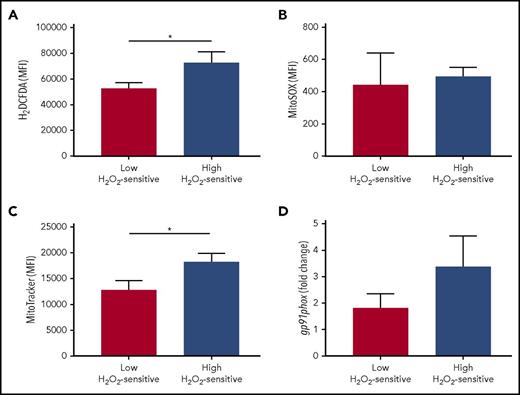
In addition to determining redox hypersensitivity, a reduced antioxidant activity might also induce a higher accumulation of endogenous ROS in leukemic cells. To assess a possible association between redox sensitivity and ROS levels, we compared cellular and mitochondrial levels of ROS in the patient groups defined by high and low sensitivity to H2O2. Total cellular levels of ROS, but not specific mitochondrial superoxide, were significantly increased in high H2O2-sensitive CLL cells (Figure 7A-B). Mitochondria and the NOX family of enzymes are the best-characterized intracellular sources of ROS15 ; therefore, to identify the possible source of ROS, we compared mitochondrial amounts and NOX catalytic subunit (gp91) expression in the patient groups defined by high and low sensitivity to H2O2. We detected higher amounts of mitochondria in high H2O2-sensitive CLL cells (Figure 7C) while observing a trend toward association between high levels of gp91expression and high-H2O2-sensitivity CLL (Figure 7D).
Association of redox sensitivity with ROS levels, mitochondrial amounts, and NADPH-oxidase. Patients were grouped on the basis of high and low H2O2-sensitive values, referred to the median signal values of the combined sum of pSYK, pERK1/2, and pp38 responses (each measured as log2-fold change). Comparisons between the high and low H2O2-sensitive groups for (A) total cell ROS levels (detected by 2′,7′-dichlorodihydrofluorescein diacetate probe; n = 18), (B) mitochondrial-specific ROS levels (detected by MitoSOX probe; n = 17), (C) mitochondrial amounts (detected by MitoTracker probe; n = 18), and (D) relative gene expression of the gp91phox NOX subunit (n = 26) were performed using the Student t test. Data are expressed as mean ± SEM. *P ≤ .05.
Association of redox sensitivity with ROS levels, mitochondrial amounts, and NADPH-oxidase. Patients were grouped on the basis of high and low H2O2-sensitive values, referred to the median signal values of the combined sum of pSYK, pERK1/2, and pp38 responses (each measured as log2-fold change). Comparisons between the high and low H2O2-sensitive groups for (A) total cell ROS levels (detected by 2′,7′-dichlorodihydrofluorescein diacetate probe; n = 18), (B) mitochondrial-specific ROS levels (detected by MitoSOX probe; n = 17), (C) mitochondrial amounts (detected by MitoTracker probe; n = 18), and (D) relative gene expression of the gp91phox NOX subunit (n = 26) were performed using the Student t test. Data are expressed as mean ± SEM. *P ≤ .05.
Discussion
This study shows that sensitivity of BCR signaling proteins to redox cues is of clinical relevance in CLL, as redox hypersensitivity is associated with a lesser aggressive behavior of the disease. We identified low catalase expression as a possible mechanism accounting for redox hypersensitivity. The key advances of this study are defining redox hypersensitivity linked to lower antioxidant capability as intrinsic characteristic of CLL with an indolent clinical course, and gaining deeper insights into mechanisms controlling redox and signaling heterogeneity of CLL.
According to previous reports,7,29 our data document a tonic, antigen-independent signaling in CLL.30-32 Inactivation of phosphatases by H2O2, the redox species that can be endogenously produced in various cells and tissues in response to diverse signals,33 increases the levels of tonic BCR signaling in B cells from patients with CLL, indicating that negative regulatory phosphatase activity is generally required to control tonic BCR signaling in leukemic cells. Redox sensitivities are reverted by the antioxidant NAC, thus supporting a mechanistic link between ROS and phosphorylation of BCR proteins. Phosphorylation responses of ERK1/2 and p38 to H2O2 occur simultaneously with the upstream phosphorylation of SYK in each CLL cell, as well as across individual CLL. Although a kinetic analysis of signaling would be necessary to reveal the temporal sequence of signaling protein activation, these data suggest that downstream signals triggered by H2O2 may depend on the level of SYK phosphorylation. As in previous studies on primary healthy B cells,29,34 the same level of H2O2 is ineffective in phosphorylating SYK in normal B cells, whereas it induces minimal activation of ERK1/2 and p38 signaling proteins. Remarkably, in CLL cells, H2O2 potentiates signal transduction triggered by the BCR engagement. Taken together, these results suggest that exogenous H2O2 interrupts phosphatase activity and perturbs tonic as well as induced BCR signaling in CLL.
CLL is characterized by a highly variable natural clinical course.2 Multiple defects in the dysregulated response to signals from the microenvironment can confer a selective survival advantage to CLL cells with a worse prognosis.35 The elucidation of mechanisms regulating these responses is of preeminent importance to understand CLL pathogenesis and to develop strategies for improving clinical management of the disease. In this study, we show that signaling sensitivity to H2O2 is highly variable among patients with CLL, and that redox hypersensitivity identifies a subgroup of treatment-naive patients with mutated IGHV genes and a slower clinical progression. Intriguingly, CLL with mutated IGHV derives from a postgerminal center B-cell subset,36 which has been shown to exhibit redox signaling hypersensitivity.37 These findings suggest that mechanisms underlying redox sensitivity could be still functional in M-CLL despite oncogenic transformation. Moreover, our findings are in agreement with previous data demonstrating that in CLL, in vitro apoptotic proficiency, in response to chemotherapeutic agents, is associated with a higher signaling response to H2O2.29 Taken together, these data point to redox hypersensitivity as a feature of CLL with a lower selective growth advantage.
SYK hyperactivity has been described as inducing cell death in acute lymphoblastic leukemia cells38 and acting synergistically with therapy-induced ROS in mediating apoptosis in CLL.39 In contrast, we document that SYK activation does not affect CLL cell death induced by H2O2. High H2O2 concentration in our experimental setting may overcome a possible contribution of SYK. However, further investigations are needed to address the mechanistic role of SYK hyperactivation in CLL cell death and clinical behavior.
Differential responsiveness of BCR proteins evoked by H2O2 in the absence of BCR engagement is associated with divergent expression levels of catalase and GPX across patients with CLL. Lower levels of catalase and GPX expression are more frequently detected in patients with favorable prognostic parameters (M-CLL) and a slower progression of the disease. In contrast, high levels of catalase are detected in CLL with a more aggressive disease. Consistently, increased catalase expression has been observed in some cancers40-42 and in HL-60 cancer cells, where it accounts for low sensitivity to H2O2 exposure.43,44 However, catalase downregulation also has been detected in cancer and has been associated with proliferation, migration, and invasion in cancer cells.45,46 Interestingly, a dual role in carcinogenesis has been also reported for GPX.47 However, only catalase inhibition increases the phosphorylation response of leukemic cells to H2O2. Moreover, exogenous catalase completely reverses protein phosphorylation induced by H2O2. Taken together, these results point to catalase as a major element of the antioxidant machinery responsible for regulating redox signaling sensitivity in CLL cells. Theoretically, lower catalase activity may account for an escalated accumulation of H2O2 within leukemic cells, with the consequent greater inhibition of phosphatases and signaling increase. In contrast, higher catalase activity can be responsible for a greater H2O2 conversion and a lower, if any, effect on signaling. Differential catalase expression in CLL supports the existence of 2 main disease subtypes characterized by a disparity in clinical outcome, probably as a consequence of differences not only in underlying genetic lesions, epigenetic changes, activated signaling pathways, and interactions with the microenvironment, but also in the redox machinery.
Although some studies are shedding light on the regulation of catalase at both transcriptional45,48 and activity levels, such as the p53–catalase direct interaction,49 the precise molecular mechanisms controlling its expression and activity are still poorly understood. Future studies aimed at deciphering the signaling pathways and the molecular mechanisms that regulate catalase expression and activity in CLL could be of crucial relevance for the development of therapies targeting redox pathways.
In the subset of patients with CLL characterized by redox hypersensitivity and a lesser aggressive disease, in addition to detecting low catalase and GPX levels, we also document an elevated accumulation of cellular ROS. Consistently, downregulation of antioxidant enzymes in cancer is mainly associated with a high production of H2O2.50-52 Moreover, our data are in accordance with a recent study showing that higher levels of ROS in CLL cells are associated with a slower disease progression.24 One possibility is that catalase downregulation induces escalated levels of ROS that promote antitumor signals such as cell death or susceptibility to apoptosis in CLL, thus accounting for less aggressive behavior of cancer cells. Accordingly, Linley et al identified a significant inverse correlation between ROS levels and the proportion of viable cells.24
In addition to decreased expression of antioxidant enzymes, other mechanisms may be responsible for the generation of an increased amount of ROS in malignant cells, including an enhanced mitochondrial respiration and NOX enzymes.15,51 A recent study showed that CLL cells accumulate higher levels of ROS than normal B cells through an elevated respiratory capacity linked to an increased mitochondrial mass.28 Thus, differences in mitochondrial amount with a consequent increase in oxidative phosphorylation may also be responsible for variation in ROS levels among individual CLL samples. In agreement, we show higher amounts of mitochondria in redox hypersensitive CLL cells as compared with redox low-sensitivity cells. However, this increase is not associated with augmented mitochondrial superoxide ions. One possible explanation is that in redox hypersensitive CLL cells, superoxide ions may be rapidly converted to cytosolic H2O2, whose levels may accumulate in CLL with low catalase. However, the role of mitochondria in generating different ROS levels in CLL subsets is not clear and deserves to be further investigated.
In conclusion, this study shows that differential redox profiles in CLL are associated with divergent clinical behaviors and advances our understanding of the redox and signaling heterogeneity of CLL. Future challenges are to design therapeutic strategies targeting redox pathways that could implement the effectiveness of current therapies and overcome drug resistance in CLL.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank all patients who have donated samples for this study.
This work was supported by grants from Fondazione Cassa di Risparmio di Verona, Vicenza, Belluno e Ancona and Associazione Italiana Ricerca sul Cancro (grant 6599) (M.T.S.); Associazione Italiana Ricerca sul Cancro (grant IG16797) (C.L.); Fondazione Cassa di Risparmio di Verona, Vicenza, Belluno e Ancona (grant 2015.0872) (M.T.S.); and Basic Research Program 2015, University of Verona (RATs 2015) (R.C.). I.D. is a fellow of Fondazione Umberto Veronesi.
Authorship
Contribution: C.C. designed and performed experiments, analyzed data, and contributed to writing the manuscript; R.C. performed modeling analyses and interpreted data; I.D. performed and analyzed reverse transcription polymerase chain reaction experiments; O.P. and O.L. performed flow cytometry experiments; E.M. managed clinical data; C.L., G.P., and M.D. contributed to the study design; M.T.S. designed and coordinated the study, interpreted data, and wrote the manuscript; and all authors reviewed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Maria Teresa Scupoli, Laboratorio Universitario di Ricerca Medica, Policlinico G.B. Rossi, P.le L. A. Scuro, 10, 37134 Verona, Italy; e-mail: mariateresa.scupoli@univr.it.

![Figure 3. Association of redox sensitivity with biological and clinical characteristics and clinical progression. (A) Responses of BCR signaling proteins, measured as the log2-fold change in phosphorylation between H2O2-modulated and unmodulated conditions, were associated with biological and clinical parameters (ie, IGHV status [n = 42], CD38 expression [n = 42], ZAP70 expression [n = 42], cytogenetic mutations [n = 41], Binet stage [n = 42], and requirement of treatment during follow-up [n = 42]). Differences in sample sizes depend on availability of biological and clinical information. Lines indicate mean values. Student t test and 1-way ANOVA test were used for comparisons, as appropriate. *P ≤ .05; **P ≤ .01. (B) Kaplan-Meier curves of TTFT for subgroups of patients with CLL defined by pSYK (n = 41), pERK1/2 (n = 41), or the sum of pSYK, pERK1/2, and pp38 (n = 41) responses to H2O2 (each measured as log2-fold change). High and low pSYK and pERK1/2 values (log2-fold change) were defined using threshold values of the probability density function of log2-fold phosphoprotein data (see supplemental Methods and supplemental Figure 4). P values are from the logrank test. High and low summing value was defined using the median value of the distribution. The dot plot shows pSYK, pERK1/2, and pp38 responses to H2O2 for each individual patient, as described in Figure 1C. High and low values used to compute the Kaplan-Meier curves are highlighted in red and blue, respectively. Lines indicate median values.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/131/17/10.1182_blood-2017-08-800466/4/m_blood800466f3.jpeg?Expires=1769168648&Signature=YfcPU518Z3-MQC3FiQKHdpulWCDTQgrD0pcOw8CHsSiVvXgIhQ1gtBsDnDXDJdB0s4ZmkLWB~kbpGeL0fhGBi6ik8qJdwhNKE007zpgXz~XTFfdU4aQlo9SsQaRaXvc~WZiASarGGifLKAjSDuPLodwb0xaJvNwcwHphtqXuthZORiPk4rNdA6mZXDB0H2MQ1JunpLF0YLX4J~yWO7pC5xEiByl~OJwsrcfP48w2Jl0ULkC9uLZLD-rViaVCpZUIa4gYKcoNWVo7NdpveQXR-RLmzpTHS-STheCLXixwa8uzM35iLQxxNmuO9P0V27DQ6N1vM5u~XzdnD~Lj1tgeQg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal