Abstract
The treatment of Hodgkin lymphoma has evolved continuously since the introduction of extended-field radiotherapy in the 1960s to involved-field and then involved-node radiotherapy, multiagent chemotherapy, combined chemoradiotherapy, risk-adapted and response-adapted modulation, and, most recently, introduction of antibody-drug conjugates and immune checkpoint-blocking antibodies. These changes have translated into progressively increasing cure rates, so that 10-year survival figures now exceed 80%, compared with <50% 40 years ago. The challenge now is how to improve upon success while maintaining, or if possible improving, the quality of life for survivors. Steering between undertreatment, with the risk of avoidable recurrences, and overtreatment, with the risk of unnecessary toxicity, remains complex because control of the lymphoma and the probability of survival are no longer closely linked. This requires trials with long follow-up and continuous reappraisal of the interaction between the illness; the method used to define risk, and the type of treatment involved. One important factor in this is age: outcomes in older patients have not improved at the same rate as those in the population under 60 years of age, reflecting the need for different approaches. Recently, treatment has moved from being primarily risk-based, using baseline characteristics such as anatomical stage and severity of the illness, to a more dynamic approach that takes account of the response to therapy, using functional imaging to make an early appraisal, with the option to modulate subsequent treatment. The results of several trials indicate that this has advantages, but a combination of risk- and response-adaptation is probably ideal.
Introduction
Hodgkin lymphoma (HL) is a malignancy of the germinal center B cell, as evidenced by the presence of clonal immunoglobulin gene rearrangements and somatic hypermutation.1,2 In contrast to other B-cell tumors, the Reed-Sternberg cell tends to lack classical B-cell antigens (eg, CD20, B-cell receptor) but consistently expresses CD30. A further distinguishing feature of HL is that the microenvironment usually comprises a diverse infiltrate of immune effector cells, with relatively few tumor cells. Worldwide, an estimated 70 000 new cases of HL are diagnosed annually.3 The age at presentation of these patients is bimodal, peaking between 20 to 24 years, and 75 to 79 years.3 This presents 2 distinct treatment challenges: in the young, more intensive regimens used to secure high cure rates need to be balanced against the risks of long-term toxicity. In seeking to optimize therapy in younger patients, the strategies that have been adopted include risk adaptation by presenting features, intensification of chemotherapy, the use of consolidation radiotherapy, response-adapted treatment, and, most recently, antibody-drug conjugates. In the older group, more effective but tolerable regimens are required, with late toxicity less of a concern than disease control. It is notable that in recent trials of treatment of patients under the age of 50 years, causes of death other than lymphoma predominate,4-6 but in those over 60 years of age the majority of patients still die of the disease.7 In this review, we will discuss the current data on front-line treatment of patients with advanced-stage HL, defined here as stage IIB to IV.
Options for initial therapy in younger patients
Risk-adapted therapy
Conventional predictors such as the International Prognostic Score (IPS) have been used to stratify patients according to clinical features reflecting the extent and severity of the lymphoma, and the general condition of the patient.8 However, with the increasing efficacy of modern treatment, this score loses some discriminative power, and an updated analysis of patients of all ages treated with the doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) regimen (Table 1), or similar, in Canada between 1980 and 2010 demonstrated that the worst 7% of patients still showed a 62% freedom from progression at 5 years.9 Similarly, use of the more intensive bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone (BEACOPP) regimens (Table 1) showed the IPS to lose prognostic significance.10
Schedules and doses used in regimens for younger patients
| Trial . | Regimen . | n . | Mean/median age, y (range) . | Stage III/IV, % . | IPS ≥4, % . | CR/Cru, % . | PFS, % (y) . | OS, % (y) . | TRM, % . | Secondary malignancies, % . |
|---|---|---|---|---|---|---|---|---|---|---|
| HD200036 | ABVD ×6 | 99 | 32 (NA) | 67 | 30* | 84 | 69 (10) | 85 (10) | 0 | 1 |
| escBEACOPP ×4, then BEACOPP ×2 | 98 | 29 (NA) | 69 | 43* | 91 | 75 (10) | 84 (10) | 2 | 6 | |
| COPPEBVCAD ×6 | 98 | 33 (NA) | 71 | 44* | 83 | 76 (10) | 86 (10) | 0 | 6 | |
| GHSG HD1235 | escBEACOPP ×8 +RT | 392 | 36 (16-65) | 83 | 15 | 93 | 89 (5) | 92 (5) | 2 | 3.6 |
| escBEACOPP ×8 −RT | 395 | 35 (16-65) | 84 | 18 | 93 | 87 (5) | 91 (5) | 2.8 | 2.3 | |
| escBEACOPP ×4, then BEACOPP ×4 +RT | 393 | 36 (16-65) | 83 | 19 | 92 | 87 (5) | 91 (5) | 4.3 | 2.5 | |
| escBEACOPP ×4, then BEACOPP ×4 −RT | 394 | 35 (16-65) | 85 | 15 | 90 | 84 (5) | 90 (5) | 2.5 | 0.8 | |
| Viviani et al37 | ABVD ×6-8, then re-induction and HDT/ASCT if less than CR or PD | 168 | NR | NR | 53* | 76 | 73 (7) | 84 (7) | 1 | 1 |
| escBEACOPP ×4, then BEACOPP ×4 then re-induction and HDT/ASCT if less than CR or PD | 163 | NR | NR | 53* | 81 | 85 (7) | 89 (7) | 2 | 1 | |
| GHSG HD154 | escBEACOPP ×8 | 705 | 33 (18-60) | 83 | 15 | 90 | 86 (5) | 92 (5) | 2.1 | 4.7 |
| escBEACOPP ×6 | 711 | 34 (18-60) | 85 | 15 | 94 | 90 (5) | 95 (5) | 0.8 | 2.4 | |
| BEACOPP-14 ×8 | 710 | 33 (18-60) | 85 | 17 | 92 | 86 (5) | 95 (5) | 0.8 | 3.1 | |
| GHSG HD 1810 | escBEACOPP ×2, if PET-2+, randomization escBEACOPP ×4-6 | 219 | 30 (18-60) | 78 | 13 | 97 | 91 (3) | 97 (3) | <1 | 3 |
| escBEACOPP ×2, if PET-2+, randomization escBEACOPP+R ×4-6 | 220 | 29 (18-60) | 75 | 22 | 93 | 93 (3) | 94 (3) | 1 | 1 | |
| EORTC 20012 Intergroup trial5 | ABVD ×8 | 275 | 35 (16-67) | 100 | 100* | 83 | 73 (4) | 87 (4) | 3.3 | 3 |
| escBEACOPP ×4, then BEACOPP ×4 | 274 | 35 (16-61) | 100 | 100* | 83 (4) | 90 (4) | 2.2 | 4 | ||
| RATHL6 | ABVD ×2, if PET-2−, randomization to ABVD ×4 | 470 | 32 (18-79) | 59 | 16 | 100 | 86 (3) | 97 (3) | 0.9 | 2.8 |
| ABVD ×2, if PET-2−, randomization to ABVD ×4 | 465 | 33 (18-76) | 58 | 14 | 100 | 84 (3) | 98 (3) | 0 | 2.4 | |
| ABVD ×2, if PET-2+, for escBEACOPP ×4 or BEACOPP-14 ×6 | 172 | 32 (18-70) | 58 | 30 | 74.4 | 66 (3) | 88 (3) | 2.3 | 1.7 | |
| US Intergroup SWOG trial SO81649 | ABVD ×2, if PET-2−, for ABVD ×4 | 370 | 32 (18-60) | 100 | 51 | 96 | 82 (2) | NA | <1 | 1 |
| ABVD ×2, if PET-2+, for escBEACOPP ×6 | 55 | 55 | 64 (2) | NA | 4 | 6.1 | ||||
| AHL2011 Lysa study52 | escBEACOPP ×2, if PET−, for ABVD ×4 | 319 | 30 (16-60) | 88 | 58* | NA | 88 (2) | NA | <1 | NA |
| escBEACOPP ×2, if PET+, for escBEACOPP ×4 | 49 | NA | NA | 8 | NA | |||||
| escBEACOPP ×6 (no PET adaptation) | 401 | NA | 92 (2) | NA | NA |
| Trial . | Regimen . | n . | Mean/median age, y (range) . | Stage III/IV, % . | IPS ≥4, % . | CR/Cru, % . | PFS, % (y) . | OS, % (y) . | TRM, % . | Secondary malignancies, % . |
|---|---|---|---|---|---|---|---|---|---|---|
| HD200036 | ABVD ×6 | 99 | 32 (NA) | 67 | 30* | 84 | 69 (10) | 85 (10) | 0 | 1 |
| escBEACOPP ×4, then BEACOPP ×2 | 98 | 29 (NA) | 69 | 43* | 91 | 75 (10) | 84 (10) | 2 | 6 | |
| COPPEBVCAD ×6 | 98 | 33 (NA) | 71 | 44* | 83 | 76 (10) | 86 (10) | 0 | 6 | |
| GHSG HD1235 | escBEACOPP ×8 +RT | 392 | 36 (16-65) | 83 | 15 | 93 | 89 (5) | 92 (5) | 2 | 3.6 |
| escBEACOPP ×8 −RT | 395 | 35 (16-65) | 84 | 18 | 93 | 87 (5) | 91 (5) | 2.8 | 2.3 | |
| escBEACOPP ×4, then BEACOPP ×4 +RT | 393 | 36 (16-65) | 83 | 19 | 92 | 87 (5) | 91 (5) | 4.3 | 2.5 | |
| escBEACOPP ×4, then BEACOPP ×4 −RT | 394 | 35 (16-65) | 85 | 15 | 90 | 84 (5) | 90 (5) | 2.5 | 0.8 | |
| Viviani et al37 | ABVD ×6-8, then re-induction and HDT/ASCT if less than CR or PD | 168 | NR | NR | 53* | 76 | 73 (7) | 84 (7) | 1 | 1 |
| escBEACOPP ×4, then BEACOPP ×4 then re-induction and HDT/ASCT if less than CR or PD | 163 | NR | NR | 53* | 81 | 85 (7) | 89 (7) | 2 | 1 | |
| GHSG HD154 | escBEACOPP ×8 | 705 | 33 (18-60) | 83 | 15 | 90 | 86 (5) | 92 (5) | 2.1 | 4.7 |
| escBEACOPP ×6 | 711 | 34 (18-60) | 85 | 15 | 94 | 90 (5) | 95 (5) | 0.8 | 2.4 | |
| BEACOPP-14 ×8 | 710 | 33 (18-60) | 85 | 17 | 92 | 86 (5) | 95 (5) | 0.8 | 3.1 | |
| GHSG HD 1810 | escBEACOPP ×2, if PET-2+, randomization escBEACOPP ×4-6 | 219 | 30 (18-60) | 78 | 13 | 97 | 91 (3) | 97 (3) | <1 | 3 |
| escBEACOPP ×2, if PET-2+, randomization escBEACOPP+R ×4-6 | 220 | 29 (18-60) | 75 | 22 | 93 | 93 (3) | 94 (3) | 1 | 1 | |
| EORTC 20012 Intergroup trial5 | ABVD ×8 | 275 | 35 (16-67) | 100 | 100* | 83 | 73 (4) | 87 (4) | 3.3 | 3 |
| escBEACOPP ×4, then BEACOPP ×4 | 274 | 35 (16-61) | 100 | 100* | 83 (4) | 90 (4) | 2.2 | 4 | ||
| RATHL6 | ABVD ×2, if PET-2−, randomization to ABVD ×4 | 470 | 32 (18-79) | 59 | 16 | 100 | 86 (3) | 97 (3) | 0.9 | 2.8 |
| ABVD ×2, if PET-2−, randomization to ABVD ×4 | 465 | 33 (18-76) | 58 | 14 | 100 | 84 (3) | 98 (3) | 0 | 2.4 | |
| ABVD ×2, if PET-2+, for escBEACOPP ×4 or BEACOPP-14 ×6 | 172 | 32 (18-70) | 58 | 30 | 74.4 | 66 (3) | 88 (3) | 2.3 | 1.7 | |
| US Intergroup SWOG trial SO81649 | ABVD ×2, if PET-2−, for ABVD ×4 | 370 | 32 (18-60) | 100 | 51 | 96 | 82 (2) | NA | <1 | 1 |
| ABVD ×2, if PET-2+, for escBEACOPP ×6 | 55 | 55 | 64 (2) | NA | 4 | 6.1 | ||||
| AHL2011 Lysa study52 | escBEACOPP ×2, if PET−, for ABVD ×4 | 319 | 30 (16-60) | 88 | 58* | NA | 88 (2) | NA | <1 | NA |
| escBEACOPP ×2, if PET+, for escBEACOPP ×4 | 49 | NA | NA | 8 | NA | |||||
| escBEACOPP ×6 (no PET adaptation) | 401 | NA | 92 (2) | NA | NA |
ABVD, doxorubicin, bleomycin, vinblastine, and dacarbazine; ASCT, autologous stem cell transplantation; BEACOPP, bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone; COPPEBVCAD, cyclophosphamide, lomustine, vindesine, melphalan, prednisone, epidoxorubicin, vincristine, procarbazine, vinblastine, bleomycin; CR, complete response; Cru, unconfirmed complete response; EORTC, European Organisation for Research and Treatment of Cancer; escBEACOPP, escalated BEACOPP; GHSG, German Hodgkin Study Group; HDT, high-dose therapy; IPS, International Prognostic Score; NA, not available; NR, not reached; OS, overall survival; PD, progressive disease; PET, positron emission tomography; PFS, progression-free survival; RATHL, Response-Adjusted Therapy for Hodgkin Lymphoma; RT, radiotherapy; TRM, treatment-related mortality.
IPS ≥ 3.
Other approaches have been taken to explore heterogeneity and differential risk according to the presenting features of the illness. These have included biological parameters such as immunohistochemical features,11-13 cytokine and chemokine levels,14-18 or gene expression profiles.11 For example, Scott et al generated a 23-gene expression model from formalin-fixed paraffin-embedded tissue, which appeared to show better discrimination for groups of differing overall survival (OS) (but not progression-free survival [PFS]) than the IPS in patients treated with ABVD and Stanford V regimens.19 However, the predictive value was not confirmed when applied to other studies20,21 and this has been a feature of most such tissue analyses: none has proven sufficiently robust and discriminative to enter routine use. Another study assessed the combined predictive value of interim [18F]fluorodeoxyglucose (FDG)–positron emission tomography (PET) with tissue biomarkers.13 In this retrospective study, they observed that interim FDG-PET was the single most effective predictor of outcome in the multivariate analysis. Further, inclusion of CD68 expression and programmed death-1 (PD-1) staining pattern on tumor-infiltrating cells, and STAT expression on Reed-Sternberg cells together, uncovered a subset of patients with interim FDG-PET− scans who had poorer outcomes (3-year PFS 95% vs 63% for low risk vs high risk, respectively). These highly relevant observations will require validation in a prospective study.
The recent widespread use of FDG uptake as measured by computed tomography (CT)/PET scans for staging lymphoma has allowed more sophisticated analysis of the extent and metabolic characteristics of HL at presentation. The calculation of parameters such as total lesion glycolysis (TLG) and metabolic tumor volume (MTV) has generated apparently useful prognostic information, and in future this may form the basis for new risk models. Baseline TLG, using a standardized uptake value of 2.5 to determine the volume of disease, was observed to be a strong, independent predictor of outcome in a large international trial,22 retaining significance in multivariable analysis of progression, along with age and B symptoms. Patients with a high baseline TLG were twice as likely to suffer treatment failure or recurrence as those with low values, a finding that also pertained to those who subsequently showed complete metabolic response on interim FDG-PET scans. Other groups have similarly found baseline MTV values to be an independent prognostic factor for outcomes after salvage therapy.23 At present these are preliminary observations, and there is a clear need for comparative studies of methodology and validation of the various cutoff points currently being reported.
Intensification of conventional chemotherapy
Since the original observation of cures using 4-drug regimens based on alkylating agents,24 and the subsequent development of the ABVD regimen,25 a variety of approaches have been taken to try and improve the results. Initial multidrug regimens testing alternating,26 hybrid,27,28 and dose-dense schedules29,30 did not demonstrate superior outcomes over ABVD. It is important to note that in the administration of ABVD, dose intensity is critical and needs to be maintained irrespective of neutropenia.31,32 In the early 2000s, the German Hodgkin Study Group (GHSG) presented data on the escalated BEACOPP (escBEACOPP) regimen (Tables 1 and 2), which does offer better disease control.33,34 This, however, is at the expense of greater short- and long-term toxicity, and a number of studies have been performed to try and optimize its use. In the GHSG HD12 trial, 8 cycles of escBEACOPP were compared against 4 cycles of escBEACOPP followed by 4 cycles of standard BEACOPP, with similar PFS and OS.35 The GHSG HD15 trial compared 8 cycles of escBEACOPP to 6 cycles of escBEACOPP or 8 cycles of BEACOPP-14. Here, 6 cycles of escBEACOPP provided the best disease control and paradoxically improved OS over 8 cycles.4
Selected front-line trials in younger patients
| Regimen . | Drug . | Dose, mg/m2 . | Administration . | Length of cycle, d . | |
|---|---|---|---|---|---|
| Route . | Days of . | ||||
| ABVD | Doxorubicin | 25 | IV | 1 and 15 | 28 |
| Bleomycin | 10 | IV | 1 and 15 | ||
| Vinblastine* | 6 | IV | 1 and 15 | ||
| Dacarbazine | 375 | IV | 1 and 15 | ||
| stdBEACOPP/BEACOPP-14 | Bleomycin | 10 | IV | 8 | 14-21 |
| Etoposide | 100 | IV | 1-3 | ||
| Doxorubicin | 25 | IV | 1 | ||
| Cyclophosphamide | 650 | IV | 1 | ||
| Vincristine† | 1.4 | IV | 8 | ||
| Procarbazine | 100 | PO | 1-7 | ||
| Prednisone‡ | 40-80 | PO | 1-14 or 1-7 | ||
| GCSF | 9-13 | ||||
| escBEACOPP | Bleomycin | 10 | IV | 8 | 21 |
| Etoposide | 200 | IV | 1-3 | ||
| Doxorubicin | 35 | IV | 1 | ||
| Cyclophosphamide | 1200 | IV | 1 | ||
| Vincristine† | 1.4 | IV | 8 | ||
| Procarbazine | 100 | PO | 1-7 | ||
| Prednisone | 40 | PO | 1-14 | ||
| GCSF | Day 9 until recovery | ||||
| Regimen . | Drug . | Dose, mg/m2 . | Administration . | Length of cycle, d . | |
|---|---|---|---|---|---|
| Route . | Days of . | ||||
| ABVD | Doxorubicin | 25 | IV | 1 and 15 | 28 |
| Bleomycin | 10 | IV | 1 and 15 | ||
| Vinblastine* | 6 | IV | 1 and 15 | ||
| Dacarbazine | 375 | IV | 1 and 15 | ||
| stdBEACOPP/BEACOPP-14 | Bleomycin | 10 | IV | 8 | 14-21 |
| Etoposide | 100 | IV | 1-3 | ||
| Doxorubicin | 25 | IV | 1 | ||
| Cyclophosphamide | 650 | IV | 1 | ||
| Vincristine† | 1.4 | IV | 8 | ||
| Procarbazine | 100 | PO | 1-7 | ||
| Prednisone‡ | 40-80 | PO | 1-14 or 1-7 | ||
| GCSF | 9-13 | ||||
| escBEACOPP | Bleomycin | 10 | IV | 8 | 21 |
| Etoposide | 200 | IV | 1-3 | ||
| Doxorubicin | 35 | IV | 1 | ||
| Cyclophosphamide | 1200 | IV | 1 | ||
| Vincristine† | 1.4 | IV | 8 | ||
| Procarbazine | 100 | PO | 1-7 | ||
| Prednisone | 40 | PO | 1-14 | ||
| GCSF | Day 9 until recovery | ||||
GCSF, granulocyte colony-stimulating factor; PO, oral administration; stdBEACOPP, standard BEACOPP. Other abbreviations are explained in Table 1.
Vinblastine is capped at 10 mg per administration.
Vincristine is capped at 2 mg per administration.
Prednisone is given as 40 mg from days 1 to 14 in stdBEACOPP and 80 mg from days 1 to 7 in BEACOPP-14.
A number of trials have compared ABVD with BEACOPP regimens directly (Table 2).5,36-39 The Fondazione Italiana Linfomi compared 6 cycles of ABVD against 4 cycles of escBEACOPP and 2 standard BEACOPP (stdBEACOPP) in 197 patients in the HD2000 study.36 After 5 years’ follow-up, the BEACOPP arm demonstrated better PFS, but not OS against ABVD (PFS, 68% vs 81%; and OS, 84% vs 92%), with similar findings after 10 years, when the OS was 84% vs 85%.39 The lack of survival advantage was due in part to the development of secondary malignancies in BEACOPP survivors. The cumulative risk of developing second malignancies at 10 years was estimated at 0.9% vs 6.6% for ABVD and BEACOPP, respectively. This 6% to 7% 10-year risk of second malignancy for BEACOPP is comparable to that reported in the BEACOPP arms of the GHSG HD9 trial.34 Another important factor that prevents improved disease control translating into better survival is that recurrences after ABVD are more readily salvaged by further chemotherapy and high-dose therapy/autologous stem cell transplantation. This was demonstrated in a further study from the Italian group, which compared ABVD (6-8 cycles) against BEACOPP (4× escBEACOPP followed by 4× standard BEACOPP).37 Patients with incomplete response assessed by CT scan or subsequent disease progression proceeded to re-induction chemotherapy followed by high-dose therapy and autologous stem cell rescue. In this study, 65 of 331 patients underwent salvage treatment (45 after ABVD, 20 after BEACOPP). A higher proportion of patients previously treated with BEACOPP had poor responses to salvage therapy (26 of 45 vs 14 of 20 patients), indicative of inherent chemoresistance in these cases. At 7 years’ follow-up, freedom from first progression was higher in the BEACOPP arm (73% vs 85%) but freedom from second progression was equivalent (82% vs 88%), and again a similar OS was observed (84% vs 89%). The results from a more recent European Organisation for Research and Treatment of Cancer (EORTC) study in 549 patients with the highest-risk stage III/IV disease were similar, comparing 8 cycles of ABVD to 4 escBEACOPP plus 4 baseline BEACOPP.5 At 4 years, the event-free survival was 63.7% for ABVD vs 69.3% for BEACOPP (P = .312) and OS was 86.7% vs 90.3% (P = .208). A meta-analysis of the results of treatment comparing ABVD to escBEACOPP suggested a small survival advantage for the more intensive treatment of about 7% at 5 years,40 but this included several older studies of ABVD with less favorable outcomes, and at best the results indicate the need to treat at least 13 patients who would have survived after ABVD with a more intensive and toxic regimen, in order to prevent 1 death. This highlights the need for a better means of selecting those most in need of more intensive treatment, for which response-adapted approaches may be preferred.
Radiotherapy to consolidate the response to chemotherapy
Historically, radiotherapy was administered using relatively high doses and extended fields, resulting in the development of substantial late morbidity. However, progress in radiation therapy technology has improved targeting and decreased exposure to adjacent normal tissues without impairment of disease control.41 Nonetheless, the high cure rates observed in younger patients still call into question the necessity of radiotherapy in this setting. In the UK Lymphoma Group LY09 study, comparing ABVD with different multidrug regimens in 702 patients with advanced HL, the use of radiotherapy was advised but not mandated along these lines.42 Although more patients received radiotherapy following a partial response or having presented with bulky disease, radiotherapy conferred an improvement in 5-year event-free survival of 15% and OS of 5% overall, with no substantial difference in hazard ratios according to the stage at presentation or other prognostic features. In contrast, a randomized trial by the EORTC showed that, in patients with a complete response (CR) on CT scans at the completion of hybrid chemotherapy, there was no advantage to the use of consolidation radiotherapy.43 More recently, the GHSG has performed a similar trial, HD15, but using FDG-PET imaging to determine whether to use radiotherapy on small (<2.5 cm) residual masses, with those in complete metabolic remission showing a similar PFS (92% at 4 years) to those with complete anatomical responses on CT despite the omission of radiotherapy, a strategy that reduced the proportion of patients receiving consolidation treatment from 71% in HD9 to 11% in HD15.4 Further data on the use of FDG-PET in guiding radiotherapy come from the international Response Adapted Therapy for Hodgkin Lymphoma (RATHL) trial, where consolidation radiotherapy was not recommended if the interim FDG-PET was negative.6 Consequently, only a small proportion (6.5%) of patients received consolidation radiotherapy, and there was no indication that the omission of radiotherapy compromised the 5-year PFS, even in patients who had bulky disease or residual non–FDG-avid masses.44
Collectively, these studies indicate a similar conclusion to that drawn from the evidence on chemotherapy intensification: the approach of treating all patients with advanced disease in the same way, even when stratified by baseline prognostic features, risks overtreatment for a substantial majority. The more dynamic assessment of response as a guide to subsequent therapy holds the promise of more individualized strategies, with more therapy for those who need it, but reduced exposure to toxicity for those in whom it can safely be avoided.
A response-adapted approach using interim FDG-PET
The evidence supporting interim FDG-PET as a means of predicting outcome for HL initially came from retrospective series of patients receiving ABVD, among whom those with a metabolic remission appeared to have a substantially better prognosis than those with persistently FDG-avid disease after 2 cycles of treatment.45 This appeared superior to conventional anatomic assessment by CT, and to supersede the impact of baseline prognostic features, such that even those with the highest-risk disease had a PFS of around 95% if the interim FDG-PET was negative.46 The introduction of a standardized 5-point reporting score for interim FDG-PET has greatly improved reproducibility and reliability, with scores 1-3 usually regarded as negative and 4-5 (uptake greater than normal liver) as positive.47 This has allowed the conduct of a number of trials testing the strategy of treatment escalation for patients with a positive interim FDG-PET and deescalation for those with early metabolic remission.
Although ABVD is better tolerated than escBEACOPP, there is still a significant risk of pulmonary toxicity from its bleomycin component.48 This prompted the design of the RATHL trial.6 Here, patients with advanced HL received 2 cycles of ABVD and then underwent an interim FDG-PET. Patients with negative interim scans (score 1-3) were randomized to continue with 4 cycles of ABVD or AVD (omitting bleomycin). In interim FDG-PET+ cases (score 4-5), treatment was escalated to 6 cycles of BEACOPP-14 or 4 cycles of escBEACOPP. Using this approach, 937 of 1119 (84%) of patients had a negative interim FDG-PET. Omission of bleomycin for these patients did not compromise outcomes, as indicated by similar 3-year PFS and OS rates of 86% and 97%, and 84% and 98% in the ABVD and AVD arms, respectively. The withdrawal of bleomycin reduced the relative risk of pulmonary adverse events by 0.67. In patients treated with BEACOPP following a positive interim FDG-PET, the 3-year PFS rate was 67.5%, results very similar to those seen in the US Intergroup Trial, SO816, which used a similar approach for patients with stage III/IV disease, giving a 2-year PFS of 64% for FDG-PET+ patients who received 6 cycles of escBEACOPP.49 Although not confirmed in a randomization, these findings compare favorably to earlier reports with 2- to 3-year PFS figures of 13% to 28% for patients continuing ABVD after a positive interim FDG-PET.46,50 The Italian HD0801 trial took a more intensive approach still, in which patients with an interim FDG-PET score of 3 or more proceeded to ifosfamide-based salvage therapy followed by autologous or allogeneic transplantation.51 This resulted in a 2-year PFS of 75%, very similar to the 81% seen in the interim FDG-PET− group, although the inclusion of patients with a score of 3 in the group for escalation may have influenced this.
Although these trial results are encouraging, it is also clear that a negative interim FDG-PET scan after 2 cycles of ABVD remains an imperfect predictor of progression-free status, with around 15% of patients experiencing recurrence despite their early metabolic remission, in contrast to the reported 95% PFS figures in the previous retrospective series. This appears to be in part determined by the baseline features, such that those with stage IV disease had only 80% 3-year PFS after a negative interim FDG-PET, compared with 90% for those presenting at stage II in the RATHL study.6
The predictive utility of interim FDG-PET assessment is also affected by the antecedent therapy. After initial treatment with 2 cycles of escBEACOPP, the GHSG HD18 study found a slightly higher rate of PET+ patients (23% using cutoff score 4) than the RATHL study, but the outcomes did not differ between PET+ and PET− groups. For patients with interim FDG-PET score 4-5, 3-year PFS was 90.7%, compared with 93.8% for those with score 1-3 (P = 0·30).10 Interestingly, these results are better than those seen in the previous HD15 study, where the overall PFS was below 90%, suggesting improved patient selection for this more intensive approach.
These data argue for a combination approach to treatment optimization, incorporating both the baseline risk, whether determined by stage, IPS or baseline PET parameters, and the interim FDG-PET result. For those patients at low risk, initial treatment with ABVD allows low toxicity and good predictive power from a negative interim FDG-PET, whereas for those at higher risk, initial therapy with escBEACOPP appears to improve the disease control and the negative predictive power of the interim FDG-PET. In support of this, the French AHL2011 study52 used 2 cycles of initial escBEACOPP, with FDG-PET− cases de-escalated to 4 cycles of ABVD, whereas FDG-PET+ cases continued 4 more cycles of escBEACOPP. For the interim 2-year PFS rates for non–PET-driven and PET-driven, both arms were equivalent (91.6% compared with 88.3%, respectively, P > .05), suggesting that this may be an effective approach for high-risk patients who are less well served by initial ABVD.
For the future, response-adapted approaches may be improved by other techniques such as the detection and monitoring of circulating tumor DNA (ctDNA) through peripheral blood sampling. As a proof of principle that this might be applicable in HL, Bruscaggin et al demonstrated in a small cohort of 14 HL patients that the reduction of ctDNA correlated well to the risk of progression.53
Addition of targeted agents to initial therapy
A number of antibody-based therapies have now demonstrated good clinical activity on their own and/or in combination with chemotherapy. Although some of these directly target the tumor cell (eg, anti-CD30, brentuximab vedotin [BV]), others target the tumor microenvironment (eg, anti-PD-1, nivolumab, or pembrolizumab).
BV, an anti-CD30 monoclonal antibody conjugated to a microtubule-disrupting agent via a cleavable linker, was combined with ABVD or AVD in newly diagnosed HL patients in a phase 1, dose-escalation study.54 Dangerous pulmonary toxicity was observed in the BV-ABVD group, but if bleomycin was omitted, the maximum tolerated dose was not reached with a 1.2 mg/kg, fortnightly dosing in the BV-AVD group. The 5-year failure-free survival and OS rates were reported as 92% and 100% for BV-AVD,55 and this combination has now been tested in a randomized comparison in the phase 3 ECHELON 1 study. The trial compared standard ABVD treatment to the BV-AVD combination for patients with stage III-IV disease.56 Six cycles of treatment were planned, and an interim FDG-PET scan was performed after cycle 2, but with the option to switch therapy reserved for patients with an interim PET score of 5. A modified PFS endpoint was used, to include as an event the administration of additional anticancer therapy for patients with a PET score of 3 or more at the end of therapy. With median follow-up of 2 years, among 1334 randomized patients, the modified 2-year PFS for those receiving BV-AVD was 82.1% vs 77.2% for ABVD (P = .04). There was an excess of progressions in the ABVD arm (102 of 670 vs 90 of 664) but a substantial contribution to the difference in modified PFS came from the events recorded for subsequent treatment in patients with incomplete response (22 of 670 vs 9 of 664), an endpoint potentially prone to bias as the study was unblinded. OS was not significantly different between the 2 arms (2 year OS for BV-AVD was 96.6% vs 94.2% for ABVD) and among patients receiving BV-AVD there was an excess of peripheral neuropathy (67% vs 43%, with 11% vs 2% of grade III/IV) and febrile neutropenia, which could be mitigated by prophylaxis with granulocyte colony-stimulating factor (G-CSF). Conversely, the ABVD arm showed a small excess of pulmonary toxicity (3% vs 1% grade III/IV).56 These results suggest that the addition of BV may be another useful means to increase the efficacy of initial therapy, particularly among those patients with the highest risk disease, although longer follow-up will be important to define its full potential.
Molecular genetic studies have demonstrated that the majority of HL cases carry an alteration of the programmed death-ligand 1 (PD-L1) and PD-L2 loci.57,58 Specifically, 9p24.1 amplification was observed in advanced HL and this correlated to increased PD-L1 and PD-L2 expression on the tumor cells. PD-L1 and PD-L2 are ligands for the inhibitory T-cell receptor PD-1. Physiologically, PD-L2 is expressed on many cell types but PD-L1 is only expressed on activated T cells and antigen-presenting cells, its primary function being to raise the threshold for T-cell activation. Disruption of the PD-L1/2-PD-1 axis is postulated to reinvigorate exhausted tumor-specific T cells in solid tumors,59,60 although in HL it may alternatively disrupt growth signaling between the PD-L1+ malignant B cells and the T-cell infiltrate. A number of monoclonal antibodies are being use to target this pathway, with nivolumab and pembrolizumab most advanced in clinical development. Both are human immunoglobulin G4 (IgG4) monoclonal antibodies directed at PD-1 and block ligand binding.61,62 Nivolumab given to patients with relapsed or refractory HL demonstrated an overall response rate (ORR) of 90% in 23 patients.63 In a further single-arm phase 2 trial of nivolumab in patients with HL who have specifically relapsed post–autograft and brentuximab, 53 of 80 cases (66.3%) responded with a median duration of response of 7.8 months.64 Similar results were observed in a phase 2 study of pembrolizumab, where an ORR of 69% was achieved.65 The mechanism of action for these agents remains to be determined, as does the potential for combining them with either conventional chemotherapy or BV, both of which are under investigation. The responses seen in relapsed and refractory disease have not proven durable in the majority of patients, but they offer an alternative approach for patients earlier in the course of their treatment, and in particular for those with positive interim FDG-PET scans in whom new approaches are evidently required.
Initial treatment of older patients
A third of all patients with advanced HL present at 60 years of age or older.3 There is no standard front-line regimen in this group and a relative paucity of trial data, although overall the outcomes are worse, with more treatment failure and more toxicity. The most commonly used regimens are ABVD; vinblastine, cyclophosphamide, procarbazine, prednisolone, etoposide, mitoxantrone, and bleomycin (VEPEMB); prednisone, vinblastine, doxorubicin, gemcitabine (PVAG); and chlorambucil, vinblastine, procarbazine, and prednisolone (ChlVPP)/etoposide, vincristine, doxorubicin (Adriamycin) (EVA); and ChlVPP (Table 3). The main difficulties with ABVD in this population are cardiotoxicity with doxorubicin and pulmonary toxicity with bleomycin.66,67 The GHSG analyzed patients aged 60 to 75 years within the HD10 and HD11 trials, where 4 cycles of ABVD were administered to early-stage HL cases.66 Compared with the younger cohort, there was over double the amount of grade 4 toxicity, a higher proportion of patients had reduced dosing and longer delays in treatment. Treatment-related mortality of 5% was observed, compared with <1% in younger patients. The 5-year PFS was 75% vs 90%, and 5-year OS 81% compared with 97% in the younger cohort.
Schedules and doses used in regimens for older patients
| Regimen . | Drug . | Dose, mg/m2 . | Administration . | Length of cycle, d . | |
|---|---|---|---|---|---|
| Route . | Days of . | ||||
| ChlVPP/EVA | Chlorambucil* | 6 | PO | 1-7 | 28 |
| Vinblastine† | 6 | IV | 8 | ||
| Procarbazine | 90 | PO | 1-7 | ||
| Prednisone | 50 | PO | 1-7 | ||
| Etoposide | 75 | PO | 1-5 | ||
| Vincristine‡ | 1.4 | IV | 1 | ||
| Doxorubicin | 50 | IV | 8 | ||
| ChlVPP | Chlorambucil* | 6 | PO | 1-14 | 28 |
| Vinblastine† | 6 | IV | 1 and 8 | ||
| Procarbazine | 100 | PO | 1-14 | ||
| Prednisone | 40 | PO | 1-14 | ||
| VEPEMB | Vinblastine† | 6 | IV | 1 | 28 |
| Cyclophosphamide | 500 | IV | 1 | ||
| Procarbazine | 100 | PO | 1-5 | ||
| Prednisone | 30 | PO | 1-5 | ||
| Etoposide | 60 | PO | 15-19 | ||
| Mitoxantrone | 6 | IV | 15 | ||
| Bleomycin | 10 | IV | 15 | ||
| PVAG | Prednisone | 40 | PO | 1-5 | 22 |
| Vinblastine† | 6 | IV | 1 | ||
| Doxorubicin | 50 | IV | 1 | ||
| Gemcitabine | 1000 | IV | 1 | ||
| Regimen . | Drug . | Dose, mg/m2 . | Administration . | Length of cycle, d . | |
|---|---|---|---|---|---|
| Route . | Days of . | ||||
| ChlVPP/EVA | Chlorambucil* | 6 | PO | 1-7 | 28 |
| Vinblastine† | 6 | IV | 8 | ||
| Procarbazine | 90 | PO | 1-7 | ||
| Prednisone | 50 | PO | 1-7 | ||
| Etoposide | 75 | PO | 1-5 | ||
| Vincristine‡ | 1.4 | IV | 1 | ||
| Doxorubicin | 50 | IV | 8 | ||
| ChlVPP | Chlorambucil* | 6 | PO | 1-14 | 28 |
| Vinblastine† | 6 | IV | 1 and 8 | ||
| Procarbazine | 100 | PO | 1-14 | ||
| Prednisone | 40 | PO | 1-14 | ||
| VEPEMB | Vinblastine† | 6 | IV | 1 | 28 |
| Cyclophosphamide | 500 | IV | 1 | ||
| Procarbazine | 100 | PO | 1-5 | ||
| Prednisone | 30 | PO | 1-5 | ||
| Etoposide | 60 | PO | 15-19 | ||
| Mitoxantrone | 6 | IV | 15 | ||
| Bleomycin | 10 | IV | 15 | ||
| PVAG | Prednisone | 40 | PO | 1-5 | 22 |
| Vinblastine† | 6 | IV | 1 | ||
| Doxorubicin | 50 | IV | 1 | ||
| Gemcitabine | 1000 | IV | 1 | ||
ChlVPP, chlorambucil, vinblastine, procarbazine, and prednisolone; EVA, etoposide, vincristine, doxorubicin (Adriamycin); PVAG, prednisone, vinblastine, doxorubicin, gemcitabine; VEPEMB, vinblastine, cyclophosphamide, procarbazine, prednisolone, etoposide, mitoxantrone, and bleomycin. Other abbreviations are explained in Table 2.
Chlorambucil is capped at 10 mg per administration.
Vinblastine is capped at 10 mg per administration.
Vincristine is capped at 2 mg per administration.
VEPEMB has half the total dose of bleomycin of ABVD, and the regimens were compared in a small phase 3 study, and the 5-year PFS and OS rates were 15% to 20% lower than seen with ABVD.68 However, the regimen did have less toxicity compared with ABVD, where cases of grade 4 cardiac and lung toxicity were documented. In older patients where cardiac toxicity is less of a concern, PVAG is an alternative.69 A small phase 2 study demonstrated CR, 3-year PFS and OS rates (78%, 58% and 66%, respectively) comparable to VEPEMB. The grade 3/4 infection rate was, however, double that reported for 4 cycles of ABVD,66 suggesting that it might be more appropriate for “fitter” older patients.
Another option is a hybrid regimen, ChlVPP/EVA.28,70 Although it is more toxic in terms of infection, neuropathy, and mucositis, it does not contain bleomycin and thus is potentially another option for the older but fitter patients where lung toxicity is a particular concern. For the less fit older patients, ChlVPP has been shown in several studies to have moderate activity,71-73 with 5-year and 10-year OS of around 60% and 40%, with the caveat that the numbers of patients who were 60 years of age and older in these studies were small.
Potentially, the most promising initial treatment of older patients might include BV. BV as a single agent had a good ORR, but responses were transient with a median PFS of 10 months and increased toxicity, notably peripheral neuropathy, and fatigue in older patients.74,75 However, when administered in a sequential manner with AVD, where 2 doses of BV were followed by 6 cycles of AVD and a further 4 cycles of BV, durable and high initial response rates were observed (ORR, 81%; CR, 77%; and 22-month PFS, 85%).76
Finally, BV combined with bendamustine is also an alternative approach. Bendamustine monotherapy produced an ORR of 53% in a small cohort of patients with relapsed and refractory HL, albeit with median duration of response only 5 months.77 A subsequent phase 1/2 study examined a combination of escalating doses of BV (1.2 mg/kg to 1.8 mg/kg and bendamustine (70 mg/m2, 80 mg/m2, and 90 mg/m2).78 In an interim analysis, 36 heavily pretreated patients were evaluated. The combination was well tolerated and maximum tolerated dose was not reached. An ORR of 67% was observed with 19% of cases in CR. Clearly, these are preliminary data and the final results are awaited, but this may be a potentially useful combination for older patients.
Conclusions
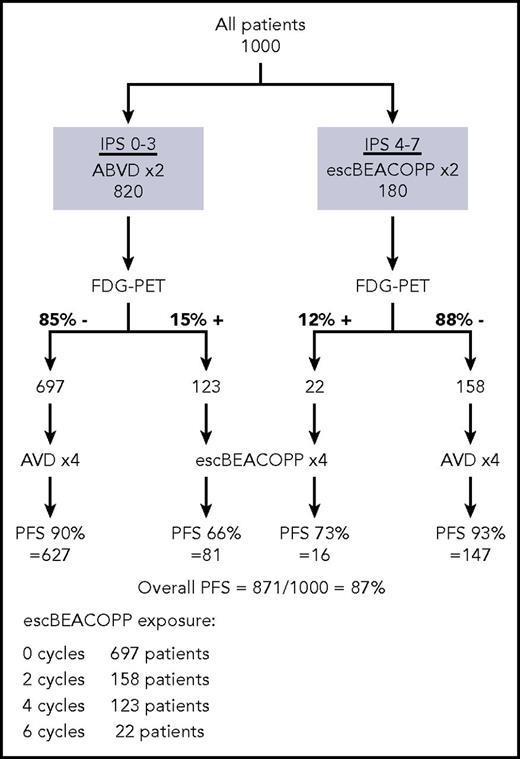
The strategies for optimizing treatment in advanced HL continue to evolve, as new types of treatment and new means of stratification come into practice. For the present, in fit under-60-year- olds with low-risk, advanced-stage HL, we give initial treatment with 2 cycles of ABVD, followed by an interim FDG-PET scan, and if this is negative, bleomycin can be safely omitted. For patients with high-risk disease (stage IV disease or IPS ≥ 4), there is a 20% chance of recurrence within 3 years with this approach, despite a negative interim FDG-PET, whereas initial treatment with escBEACOPP, or perhaps BV-AVD, may be preferable, with the option of deescalation to AVD if the interim FDG-PET is negative. Using this approach, the PFS of all patients might be anticipated to improve, while simultaneously reducing the need for intensive chemotherapy (Figure 1). A negative interim FDG-PET indicates that consolidation radiotherapy may be unnecessary, even for those with initially bulky disease. In patients who have a positive interim FDG-PET after ABVD, outcomes appear better if treatment is escalated using BEACOPP, but new approaches are needed in this group. These options might include the replacement of procarbazine with dacarbazine, which demonstrated less toxicity but equivalent efficacy in a pediatric setting,79 or the incorporation of BV or anti–PD-1 antibodies into initial therapy, but these await definition.
Merging of different risk-adapted approaches. The flowchart shows the hypothetical numbers of patients who would receive escBEACOPP if the RATHL and AHL2011 approaches are merged. The estimated figures are derived from the results of both trials, with extrapolation from the results of RATHL assuming that, after a negative interim PET, AVD is equivalent to ABVD.
Merging of different risk-adapted approaches. The flowchart shows the hypothetical numbers of patients who would receive escBEACOPP if the RATHL and AHL2011 approaches are merged. The estimated figures are derived from the results of both trials, with extrapolation from the results of RATHL assuming that, after a negative interim PET, AVD is equivalent to ABVD.
For patients over 60 years of age, at the time of writing, there is clearly no established front-line standard of care and the choice of therapy remains dependent on individual comorbid risk factors as well as institutional experience.
Acknowledgments
S.H.L. was supported by a Cancer Research UK Clinician Scientist Fellowship (C30010/A15269).
Authorship
Contribution: S.H.L. and P.W.M.J. wrote the manuscript and generated all of the tables and figures.
Conflict-of-interest disclosure: P.W.M.J. has received honoraria from Takeda, Bristol-Myers Squibb, and Novartis. S.H.L. declares no competing financial interests.
Correspondence: Peter W. M. Johnson, Cancer Research UK Centre, Somers Cancer Research Building, Southampton General Hospital, Southampton SO16 6YD, United Kingdom; e-mail: johnsonp@soton.ac.uk.