Abstract
With defined chemotherapy and radiotherapy (RT) and risk-adapted treatment, early-stage classical Hodgkin lymphoma (HL) has become curable in a majority of patients. Hence, a major current goal is to reduce treatment-related toxicity while maintaining long-term disease control. Patients with early-stage favorable disease (ie, limited stage without risk factors [RFs]) are frequently treated with 2 cycles of doxorubicin, bleomycin, vinblastine, and dacarbazine (2×ABVD) followed by 20-Gy involved-field or involved-site RT (IF/ISRT). In patients with early-stage unfavorable disease (ie, limited stage with RFs), 4 cycles of chemotherapy are usually consolidated with 30-Gy IF/ISRT. Compared with 4×ABVD, 2 cycles of bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone (2×BEACOPPescalated) followed by 2×ABVD improved 5-year progression-free survival (PFS), with similar 5-year overall survival. Recently, treatment strategies based on [18F]fluorodeoxyglucose positron emission tomography (PET) response were evaluated. In early-stage unfavorable HL, a majority of patients achieved a negative interim PET after 2×ABVD and an excellent outcome after 4×ABVD, whereas in those with a positive interim PET, 2×BEACOPPescalated improved 5-year PFS. Furthermore, a PET-guided RT approach was evaluated to decrease long-term toxicity. Although both the RAPID and H10 trials reported poorer disease control without RT, PET-guided omission of RT can constitute a valid therapeutic option in patients with an increased risk of RT-associated toxicity (eg, because of sex, age, or disease localization). Implementation of drugs such as the anti-CD30 antibody-drug conjugate brentuximab vedotin or the anti–programmed death 1 antibodies nivolumab or pembrolizumab might allow further reduction of overall mortality and improve quality of life in affected patients.
Introduction
Classical Hodgkin lymphoma (HL) has become 1 of the most curable malignancies today as a result of improved risk-adapted combined modality treatment (CMT) and more recent positron emission tomography (PET)–guided approaches. This is particularly true for limited disease (ie, early-stage disease), which is diagnosed in >50% of patients.1,2 On the basis of clinical risk factors (RFs), patients with early-stage HL are grouped into early-stage favorable or unfavorable disease categories (Table 1).3
Definition of early-stage unfavorable disease by different study groups
| RF . | GHSG . | EORTC . | NCCN . | NCIC . |
|---|---|---|---|---|
| ESR and BSs* | >50 mm/h w/o BSs | >50 mm/h w/o BSs | >50 mm/h or any BSs | >50 mm/h or any BSs |
| >30 mm/h w/BSs | >30 mm/h w/BSs | |||
| LMM (>one-third of thoracic diameter) | Yes† | Yes | Yes | Yes |
| Nodal involvement | >2 nodal areas | >3 nodal areas | >3 nodal sites | >3 nodal sites |
| Bulky disease | — | — | >10 cm | >10 cm |
| Age | — | ≥50 y | — | ≥40 y |
| EN disease | Any EN† disease | — | — | — |
| Histology | — | — | — | MC or LD |
| RF . | GHSG . | EORTC . | NCCN . | NCIC . |
|---|---|---|---|---|
| ESR and BSs* | >50 mm/h w/o BSs | >50 mm/h w/o BSs | >50 mm/h or any BSs | >50 mm/h or any BSs |
| >30 mm/h w/BSs | >30 mm/h w/BSs | |||
| LMM (>one-third of thoracic diameter) | Yes† | Yes | Yes | Yes |
| Nodal involvement | >2 nodal areas | >3 nodal areas | >3 nodal sites | >3 nodal sites |
| Bulky disease | — | — | >10 cm | >10 cm |
| Age | — | ≥50 y | — | ≥40 y |
| EN disease | Any EN† disease | — | — | — |
| Histology | — | — | — | MC or LD |
Early-stage unfavorable disease defined as clinical stage I or II with ≥1 RF.
BS, B symptom; EN, extranodal; EORTC, European Organisation for Research and Treatment of Cancer; ESR, erythrocyte sedimentation rate; GHSG, German Hodgkin Study Group; LD, lymphocyte depletion; LMM, large mediastinal mass; MC, mixed cellularity; NCCN, National Comprehensive Cancer Network; NCIC: National Cancer Institute of Canada; w/, with; w/o, without.
For example, night sweats, weight loss, fever.
Stage IIB with the respective RF considered advanced-stage disease by the GHSG.
The improved tumor control in early-stage HL has resulted in a significant reduction of HL-associated mortality and reduced the need for second-line therapy. However, treatment-associated toxicity significantly contributes to long-term morbidity and mortality.4,5 To reduce treatment-associated morbidity, more recent trials have evaluated response-adapted strategies and/or the implementation of modern drugs to reduce intensity of conventional chemotherapy and/or radiotherapy (RT).6
In this review, we discuss currently available data on early-stage HL, including risks and benefits of different treatment strategies. Current issues of therapeutic principles as well as ongoing trials incorporating targeted drugs are discussed.
Pretreatment risk stratification
Exact initial staging and risk stratification are indispensable for a correct risk group allocation and treatment decision in HL.7 The application of [18F]fluorodeoxyglucose ([18F]FDG)–PET in addition to a contrast-enhanced computed tomography (CT) scan revealed a higher disease stage in up to 25% of patients in retrospective analyses.8,9 The higher sensitivity and specificity of PET/CT also allows omission of initial bone marrow biopsy in patients with inconspicuous bone marrow [18F]FDG uptake.10 Robust, ideally prospective and randomized, data evaluating the effect of initial PET/CT on long-term disease control or feasibility of interim PET analyses are lacking. Nevertheless, initial PET/CT is recommended if available in the 2014 Lugano classification11 and the more recent international Response Evaluation Criteria in Lymphoma.12 This is supported by the current NCCN13 and European Society of Medical Oncology (ESMO)14 HL guidelines.
In addition to stage, clinical RFs are commonly used to differentiate favorable and unfavorable risk groups. Although this dichotomous categorization is based on historical data, an analysis of patients treated with 4 cycles of doxorubicin, bleomycin, vinblastine, and dacarbazine (4×ABVD) in the GHSG HD10 or HD11 trial confirmed sensitivity of the 3 mainly applied risk stratifications with contemporary treatment (Table 1).3
CMT
Large-field RT was the first treatment to achieve sustained disease control in HL. On the basis of large prospective randomized trials such as the GHSG HD7 trial and the EORTC H7/8 trials, the combination of chemotherapy and RT became accepted as standard of care in early-stage HL because of significantly improved tumor control.15-17 Over time, several randomized trials showed that RT field size could be safely reduced from subtotal nodal irradiation (STNI) to involved-field RT (IFRT) without loss of efficacy after ABVD or ABVD-like treatment.16,18
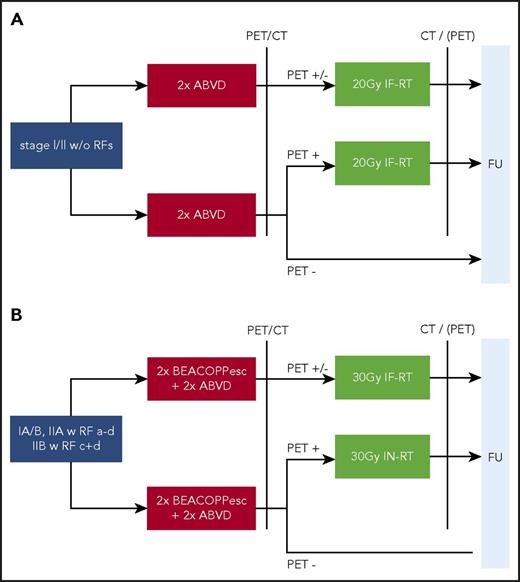
In the quest to reduce RT-associated second neoplasia (SN) and organ toxicity, new RT techniques with reduced RT field volume, such as involved-site RT (ISRT) or involved-node RT (INRT), were developed. Herein, RT field size is restricted to the individual pretherapeutic volume, in contrast to IFRT, where involved lymphoid regions irrespective of individual HL extent are irradiated. The RT field volume of ISRT is slightly larger and includes safety margins of 1.5 cm in addition to the INRT target volume.19-21 INRT was applied in the EORTC H10 trial and evaluated in comparison with IFRT in the GHSG HD17 trial, with results pending (Figure 1). On the basis of the recommendations of the International Lymphoma Radiation Oncology Group, ISRT is widely preferred to INRT and regarded as standard in daily routine, despite a paucity of clinical data, because it does not require initial imaging in RT treatment position.19 The role of RT in the treatment of early-stage HL has frequently been discussed and is an ongoing controversy.
Trial design of the GHSG HD16 and HD17 studies in early-stage HL. (A) HD16 trial in early-stage favorable HL with 1150 patients randomly assigned. (B) HD17 trial in early-stage unfavorable HL with 1009 patients randomly assigned. RFs were as follows: a, large mediastinal mass; b, extranodal disease; c, elevated erythrocyte sedimentation rate (with B symptoms, >30mm/h; without B symptoms, >50 mm/h); and d, ≥3 nodal areas. BEACOPP, bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone; FU, follow-up.
Trial design of the GHSG HD16 and HD17 studies in early-stage HL. (A) HD16 trial in early-stage favorable HL with 1150 patients randomly assigned. (B) HD17 trial in early-stage unfavorable HL with 1009 patients randomly assigned. RFs were as follows: a, large mediastinal mass; b, extranodal disease; c, elevated erythrocyte sedimentation rate (with B symptoms, >30mm/h; without B symptoms, >50 mm/h); and d, ≥3 nodal areas. BEACOPP, bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone; FU, follow-up.
In a pooled retrospective analysis, patients treated with ABVD alone in the National Cancer Institute of Canada/Eastern Cooperative Oncology Group H.6 trial were compared with patients with a comparable risk profile treated with CMT in either GHSG HD10 or HD11. Patients treated with CMT had a significantly longer time to disease progression (8-year time to progression, 93% vs 87%; hazard ratio [HR], 0.44; 95% confidence interval [CI], 0.24-0.78), but no overall survival (OS) difference was detected. Subgroup analyses indicated that those patients with early-stage favorable disease achieving a complete response by CT scan after 2×ABVD might not need consolidating IFRT.22 Also evaluating omission of RT without PET stratification, a large retrospective US National Cancer Database analysis and a recent Cochrane meta-analysis compared sole chemotherapy with CMT, showing poorer progression-free survival (PFS) without RT.23,24 The results are limited by the short follow-up, potentially missing reports on RT-associated morbidity and mortality. In addition, heterogeneous chemotherapeutic regimens and patients treated in different decades make the generalizability of the results questionable, especially in the contemporary era of PET-based RT omission. Results of PET-guided omission of RT are shown in Tables 2 and 3 and discussed in the respective sections on early-stage favorable and unfavorable HL.
Results of randomized phase 3 trials in early-stage favorable HL
| . | N/arm . | Clinical characteristic, % . | Treatment . | PFS, % (95% CI) . | OS, % (95% CI) . | Reason for death (%) . | SN, % (95% CI) . | SN SIR (95% CI) . | Reference . |
|---|---|---|---|---|---|---|---|---|---|
| Non–PET-guided therapy | |||||||||
| HD7 | 311 | CS I, 45 CS II, 55 BSs, 6 Bulk/LMM, 0 EN, 0 >60 y, 10 | 30-Gy EFRT + 10-Gy IFRT | 15 y, 52.2 (44.9-59.5) | 15 y, 77.1 (71.2-83.0) | HL (2), SN (5), CVasc (3), respiratory (2) | 15 y, 16 (11-22) | 15 y, 2.7 (1.9-3.6) | 5 |
| 316 | 2×ABVD + 30-Gy EFRT + 10-Gy IFRT | 72.8 (65.6-80.0) | 79.7 (73.9-85.6) | HL (2), SN (6), CVasc (3), respiratory (1) | 14 (9-19) | 3.0 (2.2-4.0) | |||
| H7F | 168 | CS I, NR CS II, NR BSs, 5 Bulk/LMM, 2 EN, NR >60 y, NR | 6×EBVP + 36-Gy IFRT | 10-y EFS, 88 (82-92) | 10 y, 92 (84-95) | HL (1), SN (1) | 2.9 (0.9-9.0) | NR | 17 |
| 165 | 40-Gy STNI | EFS, 78 (70-83) | 92 (85-95) | HL (3), SN (1) | 2.3 (0.7-7.4) | NR | |||
| HD10 | 298 | CS I, 32 CS II, 68 BSs, 8 Bulk/LMM, 0 EN, 0 >60 y, 12 | 4×ABVD + 30-Gy IFRT | 10 y, 87.4 (82.9-91.9) | 10 y, 93.6 (90.5-96.7) | HL (1), SN (2), CVasc (1) | 10 y, 8 (5-12) | 10 y, 2.1 (1.4-3.1) | 5,25 |
| 298 | 4×ABVD + 20-Gy IFRT | NR | NR | HL (1), SN (1) | 6 (3-10) | 1.5 (0.9-2.3) | |||
| 295 | 2×ABVD + 30-Gy IFRT | NR | NR | SN (2), HL (1) | 8 (4-11) | 1.6 (1.0-2.5) | |||
| 299 | 2×ABVD + 20-Gy IFRT | 87.2 [82.9-91.5] | 10 y, 94.1 (91.1-97.1) | HL (1), SN (1), CVasc (1) | 9 (5-13) | 2.1 (1.4-3.2) | |||
| HD13 | 566 | CS I, 32 CS II, 68 BSs, 9 Bulk/LMM, 0 EN, 0 >60 y, 12 | 2×ABVD + 30-Gy IFRT | 5 y, 93.5 (91.1-95.9) | 5 y, 97.6 (96.1-99.1) | SN (1), HL (<1) | NR | NR | 1 |
| 167 | 2×AV + 30-Gy IFRT | 78.9 (72.5-85.3) | 98.1 (96.0-100) | SN (4), HL (1) | NR | NR | |||
| 198 | 2×ABVD + 30-Gy IFRT | 5 y, 82.1 (76.6-87.7) | 94.1 (90.8-97.5) | HL (3), SN (2) | NR | NR | |||
| 571 | 2×AVD + 30-Gy IFRT | 89.6 (86.7-92.5) | 97.6 (96.2-99.0) | SN (1), HL (< 1) | NR | NR | |||
| PET-guided therapy | |||||||||
| RAPID | PET-3−, 209 | CS I, 33; CS II, 67 Favorable, 67.5 Unfavorable, 32.5 BSs, 0 Bulk w/o LMM, 1.2 EN, NR >60 y, NR | 3×ABVD + 30-Gy IFRT | 3 y, 94.6 (91.5-97.7) | 3 y, 97.1 (94.8-99.4) | HL (1.4) | NR | NR | 27 |
| PET-3−, 211 | 3×ABVD + 30-Gy IFRT | 90.8 (86.9-94.8) | 99.0 (97.6-100) | HL (0.9) | NR | NR | |||
| PET-3+, 145 | 4×ABVD + 30-Gy IFRT | 87.6 (NR) | 95.5 (NR) | HL (3.4) | NR | NR | |||
| H10F | PET-2−, 227 | CS I, 27 CS II, 73 BSs, 10.3 Bulk/LMM, 0 EN, NR >60 y, NR | 3×ABVD + 30(+6)-Gy INRT | 5 y, 99.0 (95.9-99.7) | 5 y, 100 (NA) | SN (<1) | 1.3 (NR) | NR | 6,26 |
| PET-2−, 238 | 4×ABVD | 87.1 (82.1-90.8) | 99.6 (97.0-99.9) | Toxicity of second line (<1) | 2.9 (NR) | NR | |||
| 185* | 3×ABVD + 30(+6)-Gy INRT | 3 y, 98.9 (95.6-99.7) | 3 y, 100 (NA) | NA | 0.5 (NR) | NR | |||
| PET-2+, 97 | 3×ABVD or 2×ABVD + 2×BEACOPPesc + 30(+6)-Gy INRT | NR separately; refer to PET-guided therapy for cumulative results of PET+ patients | |||||||
| . | N/arm . | Clinical characteristic, % . | Treatment . | PFS, % (95% CI) . | OS, % (95% CI) . | Reason for death (%) . | SN, % (95% CI) . | SN SIR (95% CI) . | Reference . |
|---|---|---|---|---|---|---|---|---|---|
| Non–PET-guided therapy | |||||||||
| HD7 | 311 | CS I, 45 CS II, 55 BSs, 6 Bulk/LMM, 0 EN, 0 >60 y, 10 | 30-Gy EFRT + 10-Gy IFRT | 15 y, 52.2 (44.9-59.5) | 15 y, 77.1 (71.2-83.0) | HL (2), SN (5), CVasc (3), respiratory (2) | 15 y, 16 (11-22) | 15 y, 2.7 (1.9-3.6) | 5 |
| 316 | 2×ABVD + 30-Gy EFRT + 10-Gy IFRT | 72.8 (65.6-80.0) | 79.7 (73.9-85.6) | HL (2), SN (6), CVasc (3), respiratory (1) | 14 (9-19) | 3.0 (2.2-4.0) | |||
| H7F | 168 | CS I, NR CS II, NR BSs, 5 Bulk/LMM, 2 EN, NR >60 y, NR | 6×EBVP + 36-Gy IFRT | 10-y EFS, 88 (82-92) | 10 y, 92 (84-95) | HL (1), SN (1) | 2.9 (0.9-9.0) | NR | 17 |
| 165 | 40-Gy STNI | EFS, 78 (70-83) | 92 (85-95) | HL (3), SN (1) | 2.3 (0.7-7.4) | NR | |||
| HD10 | 298 | CS I, 32 CS II, 68 BSs, 8 Bulk/LMM, 0 EN, 0 >60 y, 12 | 4×ABVD + 30-Gy IFRT | 10 y, 87.4 (82.9-91.9) | 10 y, 93.6 (90.5-96.7) | HL (1), SN (2), CVasc (1) | 10 y, 8 (5-12) | 10 y, 2.1 (1.4-3.1) | 5,25 |
| 298 | 4×ABVD + 20-Gy IFRT | NR | NR | HL (1), SN (1) | 6 (3-10) | 1.5 (0.9-2.3) | |||
| 295 | 2×ABVD + 30-Gy IFRT | NR | NR | SN (2), HL (1) | 8 (4-11) | 1.6 (1.0-2.5) | |||
| 299 | 2×ABVD + 20-Gy IFRT | 87.2 [82.9-91.5] | 10 y, 94.1 (91.1-97.1) | HL (1), SN (1), CVasc (1) | 9 (5-13) | 2.1 (1.4-3.2) | |||
| HD13 | 566 | CS I, 32 CS II, 68 BSs, 9 Bulk/LMM, 0 EN, 0 >60 y, 12 | 2×ABVD + 30-Gy IFRT | 5 y, 93.5 (91.1-95.9) | 5 y, 97.6 (96.1-99.1) | SN (1), HL (<1) | NR | NR | 1 |
| 167 | 2×AV + 30-Gy IFRT | 78.9 (72.5-85.3) | 98.1 (96.0-100) | SN (4), HL (1) | NR | NR | |||
| 198 | 2×ABVD + 30-Gy IFRT | 5 y, 82.1 (76.6-87.7) | 94.1 (90.8-97.5) | HL (3), SN (2) | NR | NR | |||
| 571 | 2×AVD + 30-Gy IFRT | 89.6 (86.7-92.5) | 97.6 (96.2-99.0) | SN (1), HL (< 1) | NR | NR | |||
| PET-guided therapy | |||||||||
| RAPID | PET-3−, 209 | CS I, 33; CS II, 67 Favorable, 67.5 Unfavorable, 32.5 BSs, 0 Bulk w/o LMM, 1.2 EN, NR >60 y, NR | 3×ABVD + 30-Gy IFRT | 3 y, 94.6 (91.5-97.7) | 3 y, 97.1 (94.8-99.4) | HL (1.4) | NR | NR | 27 |
| PET-3−, 211 | 3×ABVD + 30-Gy IFRT | 90.8 (86.9-94.8) | 99.0 (97.6-100) | HL (0.9) | NR | NR | |||
| PET-3+, 145 | 4×ABVD + 30-Gy IFRT | 87.6 (NR) | 95.5 (NR) | HL (3.4) | NR | NR | |||
| H10F | PET-2−, 227 | CS I, 27 CS II, 73 BSs, 10.3 Bulk/LMM, 0 EN, NR >60 y, NR | 3×ABVD + 30(+6)-Gy INRT | 5 y, 99.0 (95.9-99.7) | 5 y, 100 (NA) | SN (<1) | 1.3 (NR) | NR | 6,26 |
| PET-2−, 238 | 4×ABVD | 87.1 (82.1-90.8) | 99.6 (97.0-99.9) | Toxicity of second line (<1) | 2.9 (NR) | NR | |||
| 185* | 3×ABVD + 30(+6)-Gy INRT | 3 y, 98.9 (95.6-99.7) | 3 y, 100 (NA) | NA | 0.5 (NR) | NR | |||
| PET-2+, 97 | 3×ABVD or 2×ABVD + 2×BEACOPPesc + 30(+6)-Gy INRT | NR separately; refer to PET-guided therapy for cumulative results of PET+ patients | |||||||
BS, B symptom; CS, clinical stage; CVasc, cardiovascular; EBVP, epirubicin, bleomycin, vinblastine, prednisone; EFRT, extended-field RT; EFS, event-free survival; EN, extranodal; esc, escalated; NA, not applicable; NR, not reported; SIR, standardized incidence ratio.
Treated without PET stratification after safety amendment of the H10 trial.
Results of randomized phase 3 trials in early-stage unfavorable HL
| . | N/arm . | Clinical characteristic, % . | Treatment . | PFS, % (95% CI) . | OS, % (95% CI) . | Reason for death, % . | SN, %(95% CI) . | SN SIR (95% CI) . | Reference . |
|---|---|---|---|---|---|---|---|---|---|
| Non–PET-guided therapy | |||||||||
| HD.6 | 196 | CS I, 33 CS II, 67 BSs, 0 Bulk/LMM, 0 EN, NR | 4×-6×ABVD | 12-y FFDP, 87 | 12 y, 94 | HL (3), SN (2), CVasc (1) | 5.1 (NR) | NR | 32 |
| 64 | 35-Gy STNI | 87 | 98 | SN (<1) | 11.3 (NR) | NR | |||
| 139 | 2×ABVD + 35-Gy STNI | 94 | 81 | SN (6.4), HL (3), CVasc (1), other (5.7) | NR | ||||
| HD8 | 532 | CS I, 8 CS II, 90 CS III, 2 BSs, 26 Bulk/LMM, 18 EN, 7 >60 y, 8 | 2×COPP/ABVD + 30-Gy EFRT | 15 y, 72.7 (8.2-77.2) | 15 y, 80.5 (76.7-84.3) | SN (6), HL (3), CVasc (2) | 17 (13-21) | 3.6 (2.9-4.5) | 5 |
| 532 | 2×COPP/ABVD + 30-Gy IFRT | 73.8 (69.2-78.3) | 82.4 (78.7-86.0) | SN (5), HL (3), CVasc (3) | 14 (10-18) | 2.6 (2.0-3.3) | |||
| HD11 | 356 | CS I, 6 CS II, 94 BSs, 29 Bulk/LMM, 20 EN, 10 >60 y, 7 | 4×ABVD + 30-Gy IFRT | 10 y, 83.3 (78.9-87.6) | 10 y, 90.8 (87.5-94.2) | HL (3), SN (1) | 6 (3-9) | 1.4 (0.8-2.3) | 5 |
| 347 | 4×BVD + 20-Gy IFRT | 74.6 (69.3-79.9) | 89.5 (86.1-93.0) | HL (3), SN (2) | 7 (4-9) | 2.4 (1.5-3.7) | |||
| 341 | 4×BEACOPPb + 30-Gy IFRT | 82.9 (78.4-87.3) | 90.5 (87.2-93.9) | HL (3), SN (2) | 7 (4-10) | 2.2 (1.4-3.3) | |||
| 351 | 4×BEACOPPb + 30-Gy IFRT | 81.9 (77.3-86.5) | 91.4 (88.2-94.7) | HL (3), SN (2) | 5 (3-8) | 1.7 (1-2.6) | |||
| HD14 | 765 | CS I, 4.8 CS II, 95.2 BSs, 29.9 Bulk/LMM, 18.7 EN, 8.1 >50 y, 9.3 >60 y, 0 | 4×ABVD + 30-Gy IFRT | 5 y, 89.1 (86.3-91.9) | 5 y, 96.8 (95.2-98.4) | HL, SN, toxicity second line (<1 each) | 2.2 (NR) | NR | 2 |
| 763 | 2×BEACOPPesc + 2×ABVD + 30 Gy IFRT | 95.4 (93.7-97.1) | 97.2 (95.8-98.6) | HL, SN (<1 each) | 2.0 (NR) | NR | |||
| E2496 | 135 | CS I, 11 CS II, 89 BSs, 52 Bulk/LMM, 100 EN, 12.5 >60 y, NR | 6×-8×ABVD + 36-Gy IFRT | 5-y FFS, 85 (NR) | 5 y, 96 (NR) | Respiratory, HL (<1 each) | NR | NR | 37 |
| 129 | 6×-8×Stanford V + 36-Gy IFRT | 79 (NR) | 92 (NR) | Toxicity of second line (3), HL (1.5) | NR | NR | |||
| PET-guided therapy | |||||||||
| H10U | PET-2−, 292 | CS I, 14 CS II, 86 BSs, 40.3 Bulk/LMM, 42.2 EN, NR >60 y, NR | 4×ABVD + 30(+6)-Gy INRT | 5 y, 92.1 (88.0-94.8) | 5 y, 96.7 (93.7-98.3) | HL, SN, CVasc (<1 each) | 3.4 (NR) | NR | 6,26 |
| PET-2−, 302 | 6×ABVD | 89.6 (85.5-92.6) | 98.3 (96.0-99.3) | HL, SN (<1 each) | 2.9 (NR) | NR | |||
| 320* | 4×ABVD + 30(+6)-Gy INRT | 3 y, 95.5 (92.5-97.3) | 3 y, 99.7 (97.7-100) | HL, unknown (<1 each) | 1.2 (NR) | NR | |||
| PET-2+, 192† | 4×ABVD + 30(+6)-Gy INRT | 77.4 (70-4-82.9) | 89.3 (83.4-93.2) | HL, SN (<1 each) | 2.1 (NR) | NR | |||
| PET-2+, 169† | 2×ABVD + 2×BEACOPPesc + 30(+6)-Gy INRT | 90.6 (84.7-94.3) | 96.0 (91.1-98.2) | HL (6), SN (<1), toxicity of second line (<1) | 2.4 (NR) | NR | |||
| PET-3−, 211 | 3×ABVD + 30-Gy IFRT | 90.8 (86.9-94.8) | 99.0 (97.6-100) | HL (0.9) | NR | NR | |||
| PET-3+, 145 | 4×ABVD + 30-Gy IFRT | 87.6 (NR) | 95.5 (NR) | HL (3.4) | NR | NR | |||
| . | N/arm . | Clinical characteristic, % . | Treatment . | PFS, % (95% CI) . | OS, % (95% CI) . | Reason for death, % . | SN, %(95% CI) . | SN SIR (95% CI) . | Reference . |
|---|---|---|---|---|---|---|---|---|---|
| Non–PET-guided therapy | |||||||||
| HD.6 | 196 | CS I, 33 CS II, 67 BSs, 0 Bulk/LMM, 0 EN, NR | 4×-6×ABVD | 12-y FFDP, 87 | 12 y, 94 | HL (3), SN (2), CVasc (1) | 5.1 (NR) | NR | 32 |
| 64 | 35-Gy STNI | 87 | 98 | SN (<1) | 11.3 (NR) | NR | |||
| 139 | 2×ABVD + 35-Gy STNI | 94 | 81 | SN (6.4), HL (3), CVasc (1), other (5.7) | NR | ||||
| HD8 | 532 | CS I, 8 CS II, 90 CS III, 2 BSs, 26 Bulk/LMM, 18 EN, 7 >60 y, 8 | 2×COPP/ABVD + 30-Gy EFRT | 15 y, 72.7 (8.2-77.2) | 15 y, 80.5 (76.7-84.3) | SN (6), HL (3), CVasc (2) | 17 (13-21) | 3.6 (2.9-4.5) | 5 |
| 532 | 2×COPP/ABVD + 30-Gy IFRT | 73.8 (69.2-78.3) | 82.4 (78.7-86.0) | SN (5), HL (3), CVasc (3) | 14 (10-18) | 2.6 (2.0-3.3) | |||
| HD11 | 356 | CS I, 6 CS II, 94 BSs, 29 Bulk/LMM, 20 EN, 10 >60 y, 7 | 4×ABVD + 30-Gy IFRT | 10 y, 83.3 (78.9-87.6) | 10 y, 90.8 (87.5-94.2) | HL (3), SN (1) | 6 (3-9) | 1.4 (0.8-2.3) | 5 |
| 347 | 4×BVD + 20-Gy IFRT | 74.6 (69.3-79.9) | 89.5 (86.1-93.0) | HL (3), SN (2) | 7 (4-9) | 2.4 (1.5-3.7) | |||
| 341 | 4×BEACOPPb + 30-Gy IFRT | 82.9 (78.4-87.3) | 90.5 (87.2-93.9) | HL (3), SN (2) | 7 (4-10) | 2.2 (1.4-3.3) | |||
| 351 | 4×BEACOPPb + 30-Gy IFRT | 81.9 (77.3-86.5) | 91.4 (88.2-94.7) | HL (3), SN (2) | 5 (3-8) | 1.7 (1-2.6) | |||
| HD14 | 765 | CS I, 4.8 CS II, 95.2 BSs, 29.9 Bulk/LMM, 18.7 EN, 8.1 >50 y, 9.3 >60 y, 0 | 4×ABVD + 30-Gy IFRT | 5 y, 89.1 (86.3-91.9) | 5 y, 96.8 (95.2-98.4) | HL, SN, toxicity second line (<1 each) | 2.2 (NR) | NR | 2 |
| 763 | 2×BEACOPPesc + 2×ABVD + 30 Gy IFRT | 95.4 (93.7-97.1) | 97.2 (95.8-98.6) | HL, SN (<1 each) | 2.0 (NR) | NR | |||
| E2496 | 135 | CS I, 11 CS II, 89 BSs, 52 Bulk/LMM, 100 EN, 12.5 >60 y, NR | 6×-8×ABVD + 36-Gy IFRT | 5-y FFS, 85 (NR) | 5 y, 96 (NR) | Respiratory, HL (<1 each) | NR | NR | 37 |
| 129 | 6×-8×Stanford V + 36-Gy IFRT | 79 (NR) | 92 (NR) | Toxicity of second line (3), HL (1.5) | NR | NR | |||
| PET-guided therapy | |||||||||
| H10U | PET-2−, 292 | CS I, 14 CS II, 86 BSs, 40.3 Bulk/LMM, 42.2 EN, NR >60 y, NR | 4×ABVD + 30(+6)-Gy INRT | 5 y, 92.1 (88.0-94.8) | 5 y, 96.7 (93.7-98.3) | HL, SN, CVasc (<1 each) | 3.4 (NR) | NR | 6,26 |
| PET-2−, 302 | 6×ABVD | 89.6 (85.5-92.6) | 98.3 (96.0-99.3) | HL, SN (<1 each) | 2.9 (NR) | NR | |||
| 320* | 4×ABVD + 30(+6)-Gy INRT | 3 y, 95.5 (92.5-97.3) | 3 y, 99.7 (97.7-100) | HL, unknown (<1 each) | 1.2 (NR) | NR | |||
| PET-2+, 192† | 4×ABVD + 30(+6)-Gy INRT | 77.4 (70-4-82.9) | 89.3 (83.4-93.2) | HL, SN (<1 each) | 2.1 (NR) | NR | |||
| PET-2+, 169† | 2×ABVD + 2×BEACOPPesc + 30(+6)-Gy INRT | 90.6 (84.7-94.3) | 96.0 (91.1-98.2) | HL (6), SN (<1), toxicity of second line (<1) | 2.4 (NR) | NR | |||
| PET-3−, 211 | 3×ABVD + 30-Gy IFRT | 90.8 (86.9-94.8) | 99.0 (97.6-100) | HL (0.9) | NR | NR | |||
| PET-3+, 145 | 4×ABVD + 30-Gy IFRT | 87.6 (NR) | 95.5 (NR) | HL (3.4) | NR | NR | |||
Lower RT dose administered in case of complete or partial response after systemic therapy.
b, baseline; CVasc, cardiovascular; esc, escalated; FFDP, freedom from disease progression; NR, not reported; SIR, standardized incidence ratio.
Treated without PET stratification after safety amendment of the H10 trial.
Including 97 patients with early-stage favorable disease.
Early-stage favorable HL
Non–PET-adapted strategies
In a recent updated analysis of the GHSG HD7 trial, the superiority of CMT compared with extended-field RT alone was confirmed in patients with early-stage favorable disease with 15-year PFS estimates of 73% vs 52%, respectively. However, OS did not differ significantly between the trial arms. Of note, only a minority of deaths were related to HL. Instead, potentially treatment-related SN and cardiovascular and pulmonary disease accounted for most deaths (Table 2).5
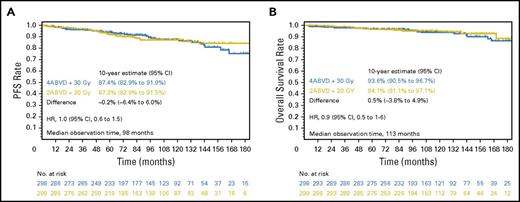
To reduce treatment-related toxicity, the GHSG HD10 trial compared 4× vs 2×ABVD as well as 20-Gy or 30-Gy IFRT. In the initial analysis, 2×ABVD plus 20-Gy IFRT was noninferior to the other treatment arms with regard to freedom from treatment failure (FFTF; 5-year FFTF, 91%) as well as 5-year OS (97%).25 The recent follow-up analysis confirmed the excellent outcomes with 2×ABVD plus 20-Gy IFRT (Table 2; Figure 2). With a standardized incidence ratio of 2.1 in the least and the most intensive treatment arms, no differences in terms of SN have been observed so far.5 Further reduction of chemotherapy intensity by omission of bleomycin and/or dacarbazine in the GHSG HD13 trial resulted in a significant reduction of tumor control. Compared with ABVD, 5-year FFTF differences of −11.5% (with ABV), −15.2% (AV), and −3.9% (AVD), exceeding the predefined noninferiority margin of −6% within the 95% CI of −7.7% to −0.15 (Table 2), were documented.1
PFS and OS rates of the most and least intensive treatment arms in the HD10 trial at 10-year follow-up. PFS (A) and OS (B) in patients treated in the most (blue) and least (yellow) intensive arms in the HD10 trial. Reprinted from Sasse et al5 with permission.
PFS and OS rates of the most and least intensive treatment arms in the HD10 trial at 10-year follow-up. PFS (A) and OS (B) in patients treated in the most (blue) and least (yellow) intensive arms in the HD10 trial. Reprinted from Sasse et al5 with permission.
PET-adapted strategies
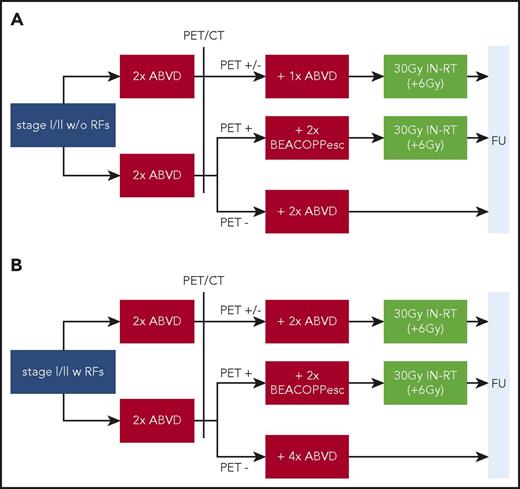
Following the idea of individualized, response-adapted therapy, [18F]FDG-PET–guided RT in early-stage HL has been evaluated in more recent large randomized trials to further reduce treatment-associated long-term toxicity. The results of the H10F+U6,26 (Figure 3), the British RAPID,27 and the US Intergroup 50604 (Cancer and Leukemia Group B/Alliance) trials28 have already been published; the GHSG HD16 (early-stage favorable) and HD17 (early-stage unfavorable) trials (Figure 1) have finished recruitment, but results are pending. During the quest to omit RT, the relevant chemotherapy-associated morbidity29,30 and late relapses of HL occurring especially in patients with early-stage disease should be acknowledged.31
Trial design of the EORTC/Lymphoma Study Association/Fondazione Italiana Linfomi H10F/U study in early-stage HL. (A) H10F trial in early-stage favorable HL with 754 patients randomly assigned. (B) H10U trial in early-stage unfavorable HL with 1196 patients randomly assigned. RFs were as follows: age ≥50 years, LMM, elevated erythrocyte sedimentation rate (with B symptoms, >30 mm/h; without B symptoms, >50 mm/h), ≥3 nodal areas. esc, escalated; FU, follow-up.
Trial design of the EORTC/Lymphoma Study Association/Fondazione Italiana Linfomi H10F/U study in early-stage HL. (A) H10F trial in early-stage favorable HL with 754 patients randomly assigned. (B) H10U trial in early-stage unfavorable HL with 1196 patients randomly assigned. RFs were as follows: age ≥50 years, LMM, elevated erythrocyte sedimentation rate (with B symptoms, >30 mm/h; without B symptoms, >50 mm/h), ≥3 nodal areas. esc, escalated; FU, follow-up.
In the 50604 phase 2 trial, patients with nonbulky stage I/II disease with a negative interim PET/CT after 2×ABVD (Deauville score, 1-3; 131 of 144 evaluable patients) were treated with an additional 2×ABVD without consolidative RT, whereas patients with a positive interim PET/CT (12 of 144 patients) received 2×BEACOPPescalated plus 30-Gy IFRT. Estimated 3-year PFS rates of 92% and 66%, respectively, for the PET− and PET+ cohorts were reported in an interim analysis. These data support the favorable prognosis with a negative PET/CT after 2×ABVD; however, PFS estimates in the PET+ arm were imprecise because of the low patient numbers.28
In the British RAPID trial, 602 patients with stage I/IIA HL and no mediastinal bulk received 3×ABVD followed by a PET/CT. Patients with a negative PET/CT (Deauville score, 1-2) were randomly assigned to receive 30-Gy IFRT or no further treatment. PET+ patients received an additional cycle of ABVD plus 30-Gy IFRT. Approximately two-thirds of patients enrolled had a favorable risk profile according to GHSG or EORTC risk classification. PET− patients in the intent-to-treat and per-protocol cohorts had PFS differences of 3.8% (95% CI, −8.8-1.3; 3-year PFS, 94.6% vs 90.8%) and 6.3% (95% CI, −11.0%-1.6%; 3-year PFS, 97.1% vs 90.8%) favoring consolidative IFRT, respectively. In both analysis sets, the upper CI limit exceeded the predefined noninferiority margin of 7%.27
In a similar fashion, the EORTC H10F trial evaluated a response-adapted RT approach based on PET after 2×ABVD (Figure 3). Patients in the standard arm received 30-Gy INRT after 1 additional cycle of ABVD. In the experimental arm, PET− patients were treated with 2 additional cycles of ABVD without consolidative RT. In the preplanned interim analysis of PET− patients, futility of the trial was declared by the independent data monitoring committee because of an increased number of HL-related events in the non-RT arms (25 vs 8 events).26 With omission of RT in PET− patients, noninferiority could not be demonstrated in the recent final analysis according to the predefined noninferiority margin of 10%, despite a 50% increase in chemotherapy compared with standard 2×ABVD plus 20-Gy IFRT. The 5-year PFS rates with and without RT were 99.0% and 87.1%, respectively.6
In summary, both the RAPID and H10 trials showed a statistically significant loss in tumor control in patients with early-stage favorable disease who were PET− after 3× to 4×ABVD, with differences in 3- and 5-year PFS of 3.8% and 11.9%, respectively. Thus, a PET-guided RT approach cannot be generally recommended as yet if maximum disease control is the main goal of therapy. Nevertheless, a PET-based approach with consolidative ABVD instead of RT according to the H10 or RAPID trial constitutes a feasible option for defined patients based on their individual characteristics or treatment goals, because overall prognosis remains good. Examples include a young woman who receiving RT of axillary and mediastinal lymph nodes and a patient with preexisting cardiovascular conditions receiving a relevant cardiac RT dose. In these patients, the individual statistical risk of relapse and the risk of RT-associated SN or cardiac events should be carefully weighed and primary goal of therapy discussed with the patient.
The latest NCCN13 and ESMO14 guidelines both recommend combined modality treatment with 2×ABVD and consolidative 20-Gy ISRT as treatment in early-stage favorable HL. Consistent with the RAPID trial, 3×ABVD followed by an interim PET and potential omission of RT is alternatively recommended by the NCCN. For PET+ patients after 2×ABVD, the ESMO guidelines recommend 2×BEACOPPescalated plus 30-Gy ISRT. Of note, the NCCN recommendations are restricted to stage IA to IIA disease, whereas the ESMO guidelines also include stage IIB disease without RFs.
Early-stage unfavorable HL
Non–PET-adapted strategies
In the pre-PET era, the National Cancer Institute of Canada/Eastern Cooperative Oncology Group HD.6 trial was the largest randomized trial comparing ABVD alone with RT-containing therapy in stage I/IIA nonbulky HL and predominantly enrolled patients with an unfavorable risk profile (69%). Patients were randomly assigned to either 4× to 6×ABVD alone or STNI alone (favorable risk profile) or 2×ABVD followed by STNI (unfavorable risk profile). Although a lower freedom from disease progression (87% vs 92%; P = .05) was reported with ABVD alone, the documented 12-year OS was significantly lower in the CMT arm (94% vs 87%).32 These results must be interpreted with care, because deaths in the CMT group were frequently associated with reasons other than HL or potentially RT-related toxicities. Since then, large-field RT has become obsolete because, based on the results of the EORTC/GELA H8U and the GHSG HD8 trial, 4 cycles of chemotherapy plus 30-Gy IFRT were defined as standard of care in patients with early-stage unfavorable HL.16,33 In HD8, long-term noninferiority of IFRT compared with extended-field RT was demonstrated.5 With regard to treatment-associated long-term toxicity, a nonsignificant trend toward less SN with IFRT was observed in the recent follow-up analysis (15-year cumulative, 14% vs 17%; P = .3).5 Because of the long latency of solid SN, prolonged follow-up is crucial to finally assess the risk of SN with more limited RT fields.
In terms of systemic therapy, ABVD is widely regarded as a standard regimen in early-stage unfavorable HL. Nevertheless, the long-term PFS of 83% at 10 years achieved with 4×ABVD plus 30-Gy IFRT leaves room for improvement.5,34 To reduce the relapse rate and potentially enable a reduction of the RT dose, a moderate chemotherapy intensification was implemented in the GHSG HD11 trial; patients were randomly assigned to receive either 4×ABVD or 4×BEACOPPbaseline plus either 30- or 20-Gy IFRT.34 The most recent follow-up showed no PFS improvement in the intensified chemotherapy arms (10-year PFS estimate [+20-Gy IFRT], 82% vs 75%; HR, 0.8; 95% CI, 0.6-1.1; 10-year PFS estimate [+30-Gy IFRT], 83% vs 83%; HR, 1.1; 95% CI, 0.7-1.5). With regard to RT dose, noninferiority of 20-Gy IFRT could only be shown if applied after 4×BEACOPPbaseline (HR, 1.0; 95% CI, 0.7-1.5; Table 3).5 Thus, a consolidative RT dose of 30 Gy is required after 4×ABVD, but reduction might be feasible after more intensive systemic therapy.
A further intensification of chemotherapy in early-stage unfavorable HL was evaluated in the GHSG HD14 trial, where 2×BEACOPPescalated plus 2×ABVD (ie, 2 + 2) resulted in a significant PFS advantage compared with 4×ABVD, with a 5-year PFS difference of 6.2% (95.4% vs 89.1%; HR, 0.45; 95% CI, 0.3-0.69; Table 3). The 2 + 2 approach is associated with more acute, predominantly hematologic, toxicity, but no difference in long-term toxicity or OS has been documented so far.2 Again, longer follow-up is needed to assess potential long-term benefits and risks with upfront intensive therapy in patients with early-stage unfavorable disease.
Among the patients with limited-stage disease but RFs present, those with bulky mediastinal disease or a LMM, which is present in ∼20%, are of concern.35 In the HD14 trial, an LMM and elevated erythrocyte sedimentation rate were independent RFs for PFS in multivariate analyses. Although not powered to detect differences between treatment arms or risk groups, the analysis suggests that particularly patients with 1 of these risk factors, accounting for ∼80% of all included patients, might benefit from intensified strategies such as the 2 + 2 regimen.2,36 A preplanned subset analysis of the US Intergroup E2496 trial in patients with stage I/II HL and mediastinal bulky disease reported 5-year PFS rates of 85% and 79% after treatment with 6× to 8×ABVD or Stanford V for 12 weeks plus 30-Gy IFRT, respectively, without significant difference between these treatment arms.37 In summary, the currently available data on patients with an LMM indicate that these patients might benefit from intensified treatments. The observation that 72.9% of patients with an LMM in H10U achieved PET− status on an interim scan after 2×ABVD (PET-2)6 suggests that early response by PET might help identify the subset of patients with an LMM in need of intensified therapy, thereby enabling more individualized treatment.
PET-adapted strategies
To address the need for intensified therapy in some patients with early-stage unfavorable HL, the EORTC/Lymphoma Study Association/Fondazione Italiana Linfomi H10U trial evaluated a response-adapted chemotherapy approach based on the result of PET-2.
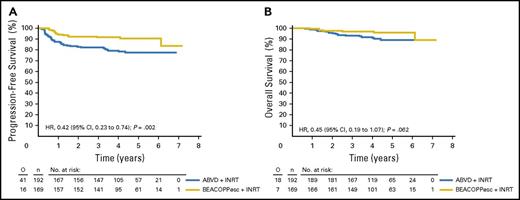
In the standard arm of the randomized European phase 3 H10U trial, patients received 4×ABVD plus 30-Gy INRT irrespective of their PET-2 status. In the experimental arm, PET-2+ patients (ie, Deauville score ≥3) were switched to an intensified treatment with 2×BEACOPPescalated plus 30-Gy INRT. PET-2− patients received an additional 4×ABVD without consolidative RT (Figure 3). PET-2− patients in the standard arm (n = 292) had a 5-year PFS of 92.1%, whereas PET-2+ patients in the experimental arm (n = 264) had a similar 5-year PFS of 90.6% after intensified treatment with BEACOPPescalated plus 30-Gy INRT. Compared with the 5-year PFS of 77.4% in the standard arm, a significant benefit (HR, 0.42; 95% CI, 0.2-0.7) and a nonsignificant 5-year OS difference favoring the intensified treatment arm were documented (Table 3; Figure 4).6 Thus, the H10U trial shows that an intensified treatment approach with 2×ABVD plus 2×BEACOPPescalated plus 30-Gy INRT compares favorably to standard 4×ABVD plus 30-Gy INRT in PET-2+ patients. Importantly, a majority of patients in H10 (ie, the 77.8% of patients who were PET-2−) seemed to be sufficiently treated with 4×ABVD.
PFS and OS of PET-2+patients in the H10 trial. PFS (A) and OS (B) in patients PET+ after 2×ABVD randomly assigned to either standard treatment with ABVD plus INRT or treatment with BEACOPPescalated plus INRT. esc, escalated. Reprinted from André et al6 with permission.
PFS and OS of PET-2+patients in the H10 trial. PFS (A) and OS (B) in patients PET+ after 2×ABVD randomly assigned to either standard treatment with ABVD plus INRT or treatment with BEACOPPescalated plus INRT. esc, escalated. Reprinted from André et al6 with permission.
Although there are obvious limitations when comparing data across trials conducted in different patient and health care settings or time periods, robust efficacy end points might allow an orientating estimate of the relative efficacy of different strategies. In the H10U trial, 5-year PFS and OS were 92.1% and 96.7% in PET-2− and 90.6% and 96.0% in PET-2+ patients, respectively. The data for the total early-stage unfavorable cohort have not yet been reported, and approximately one-third of patients had early-stage favorable disease. In the HD14 trial, conducted some years earlier than H10U and without PET stratification, 5-year PFS and OS were 95.4% and 97%, respectively, the highest rates reported in early-stage unfavorable disease so far. Treatment with initial 2×ABVD, as in H10U, reduces toxicity in a majority of patients likely by avoiding BEACOPPescalated. However, the therapeutic strategy is chosen without knowing PET-2 status after 2×ABVD, and intensification by 2×BEACOPPescalated might be too late in a subset of patients requiring intensive upfront therapy. If maximum tumor control is the major goal of therapy, 2 + 2 is a justified therapeutic option. PET-guided chemotherapy after initial 2×ABVD provides an effective therapy in patients for whom reduction of treatment-associated toxicity (eg, for preservation of fertility) is a major goal in addition to efficacy. 4×ABVD is an option in frailer patients who are not suitable candidates for intensified chemotherapy with BEACOPPescalated or in settings where adequate supportive care facilities are not available.
As outlined, the H10U trial evaluated response-adapted RT in addition to interim PET/CT-based chemotherapy (Figures 3 and 4). Patients in the standard arm received 30-Gy INRT after additional 2×ABVD, whereas in the experimental arm, PET-2− patients were treated with additional 4×ABVD but without RT. As in the H10F cohort, noninferiority of RT omission could not be shown in H10U, despite a total of 6×ABVD. In contrast to the early-stage favorable cohort, differences in 5-year PFS were much smaller, at 92.1% vs 89.6% (−2.5%),6 underlining the relation between intensity of systemic therapy and required consolidative RT in patients with early-stage unfavorable disease also observed in HD11. Because, at least with IFRT, RT fields are usually large in these patients as a result of the frequent presence of ≥3 involved areas, omission of RT might actually spare them relevant toxicity and hence be suitable in selected patients (ie, axillary/mediastinal RT, preexisting cardiovascular or pulmonary disease, young age).
Four cycles of polychemotherapy followed by 30-Gy IFRT or ISRT are recommended by both the current NCCN and ESMO guidelines for patients with early-stage unfavorable HL. Both guidelines list 4×ABVD or 2 + 2 as valid strategies, and the NCCN guideline13 additionally includes Stanford V over 12 weeks for patients with bulky disease >10 cm. A PET-guided approach consistent with H10U is alternatively recommended in the ESMO guidelines.14
Patients >60 years of age
The development of evidence-based treatment recommendations in elderly patients with HL is hampered by the scarcity of prospective trial data and by the fact that the available data do not result from preplanned subgroup analyses. In addition, only a minority of this growing patient population has been included in clinical trials, although currently, a number of early-phase trials are evaluating novel treatment approaches in patients >60 years of age.38,39
The most commonly used chemotherapy in patients with HL age >60 years is ABVD; the application of more intensive treatment approaches such as BEACOPP variants cannot be recommended because of excessive therapy-related mortality in this age group.40 On the basis of subgroup data from larger phase 3 trials, 2×ABVD plus 20-Gy IF/ISRT constitutes a feasible and effective treatment in elderly patients with early-stage favorable HL.41 Analyses of the HD10, HD11, and HD13 trials revealed that in patients age >60 years, 4×ABVD is associated with a relevant rate of severe adverse events, particularly hematotoxicity and bleomycin-associated lung toxicity, resulting in an increased treatment-associated mortality compared with those receiving only 2 cycles of ABVD.30 In early-stage unfavorable HL, 2 cycles of ABVD followed by 2 cycles AVD and 30-Gy IF/ISRT constitute a safer therapeutic strategy, with the limitation that this deescalating approach has not been formally evaluated yet. To improve the outcome of elderly patients with early-stage disease, innovative new clinical trials are urgently needed.
Future perspectives
Despite reductions in cumulative chemotherapy and RT dose and field size, treatment-associated morbidity remains relevant, and future research in limited-stage HL thus aims at reducing toxicity while maintaining or improving long-term cure rates.
Although PET-guided approaches might help to individualize chemotherapy and RT exposure, implementation of novel agents such as brentuximab vedotin (BV) or the anti–programmed death 1 antibodies nivolumab or pembrolizumab, approved for the treatment of relapsed or refractory HL, might further optimize therapy.42-44 By exploiting potential synergies (eg, in combination with chemotherapy, RT, or other targeted agents) and the distinct HL biology with its dependence on a protective immunosuppressive microenvironment,45 these strategies might also improve outcomes for elderly patients or those with relevant comorbidities such as HIV. Fueled by the exciting data in relapsed or refractory HL, a number of phase 1/2 trials of first-line therapy for HL are planned or actively enrolling patients to investigate combinations of ABVD variants with BV (eg, registered at www.clinicaltrials.gov as NCT02505269, NCT01868451), nivolumab (NCT03004833, NCT03033914), or pembrolizumab (NCT03226249) as well as BV consolidation after chemotherapy (NCT02298283). In addition, trials in elderly patients are studying BV monotherapy (NCT02191930) or a combination of BV and nivolumab (NCT01716806, NCT02758717) as well as AVD chemotherapy combined with BV and nivolumab (NCT03233347). Preliminary results of a multicohort exploratory phase 2 trial with 4×BV-AVD plus either 30- or 20-Gy ISRT in early-stage unfavorable HL were recently reported. Across both cohorts, ≥93% of patients were PET− after systemic therapy, and complete remission was achieved in all patients after CMT. Although results of more limited RT field, PET-guided RT, and longer follow-up for PFS/OS are pending, the combination of BV-AVD seems to be safe and effective, warranting future research.46 This active trial landscape reflects the quest to optimally balance risks and benefits associated with therapy for limited-stage HL. However, only randomized phase 3 trials can change standards of care in this highly curable patient group.
In early-stage favorable HL, 2×ABVD plus 20-Gy IFRT is still associated with a roughly two-fold increased standardized incidence ratio of SN in a recent analysis. Implementation of novel agents in this group of patients is thus warranted if conventional chemotherapy and/or RT are further decreased and high cure rates are at least maintained. In patients with early-stage unfavorable disease, more intensive chemotherapy and a higher RT dose are required to achieve acceptable long-term cure rates. In these patients, the investigation of novel drugs is warranted to reduce upfront treatment intensity, achieve PET− disease status after systemic therapy in a vast majority of patients to potentially enable omission of RT, and overall achieve a PFS superior to that with 4×ABVD plus 30-Gy IFRT.
To enable further individualization of treatment intensity, pretherapeutic risk stratification should be optimized. Promising approaches to identify a truly unfavorable subset are new PET imaging techniques such as metabolic tumor volume or total lesion glycolysis,47 histological characteristics such as PD-L1 or major histocompatibility complex class 2 expression,48 or characteristics of circulating tumor DNA.49 When interpreting upcoming data from early-phase trials and further developing the therapeutic roadmap in early-stage HL, it is important to also keep in mind the excellent long-term outcomes achieved with conventional, often far less costly, and well-established therapies.
Summary
With risk-adapted CMT, early-stage HL has become curable in a vast majority of patients. To reduce treatment-associated toxicity, RT fields have been decreased, and response-adapted strategies have been evaluated. In patients with a favorable risk profile, 2×ABVD plus 20-Gy IFRT results in excellent long-term survival rates, and IFRT is frequently replaced by the more limited ISRT. Treatment in the unfavorable risk group usually consists of 4 cycles of multiagent chemotherapy plus 30-Gy limited-field RT. 2×BEACOPPescalated followed by 2×ABVD (2 + 2) is preferred to 4×ABVD if optimal tumor control is the primary treatment goal. A PET-guided chemotherapy strategy with 2×BEACOPPescalated administered only in PET+ patients after 2 initial cycles of ABVD is an effective and less toxic alternative to 2 + 2 if reduction of treatment-associated toxicity is of high priority. Consolidative RT improves disease control in early-stage HL, but omission might be feasible in selected patients with PET− disease, especially for patients with early-stage unfavorable disease. The implementation of modern drugs such as BV, nivolumab, or pembrolizumab might help in reshaping the therapeutic landscape to diminish overall mortality and improve long-term quality of life.
Authorship
Contribution: All authors analyzed the available data and prepared the manuscript.
Conflict-of-interest disclosure: P.J.B. receives research funding, honoraria, and travel grants from Bristol-Myers Squibb and Takeda. A.E. receives research funding from Affimed Therapeutics, Bristol-Myers Squibb, and Takeda and is a consultant for Bristol-Myers Squibb and Takeda. S.S. declares no competing financial interests.
Correspondence: Andreas Engert, University Hospital of Cologne, Kerpener Strasse 62, 50937 Cologne, Germany; e-mail: a.engert@uni-koeln.de.
References
Author notes
P.J.B. and S.S. contributed equally to this work.