Key Points
SCD patients with a recent VOC episode have lower frequencies and numbers of HO-1hi patrolling monocytes.
Heme-driven SCD vaso-occlusion is exacerbated in mice lacking patrolling monocytes and reversed following patrolling monocyte transfer.
Abstract
Patients with sickle cell disease (SCD) suffer from intravascular hemolysis associated with vascular injury and dysfunction in mouse models, and painful vaso-occlusive crisis (VOC) involving increased attachment of sickle erythrocytes and activated leukocytes to damaged vascular endothelium. Patrolling monocytes, which normally scavenge damaged cells and debris from the vasculature, express higher levels of anti-inflammatory heme oxygenase 1 (HO-1), a heme degrading enzyme. Here, we show that HO-1–expressing patrolling monocytes protect SCD vasculature from ongoing hemolytic insult and vaso-occlusion. We found that a mean 37% of patrolling monocytes from SCD patients express very high levels of HO-1 (HO-1hi) vs 6% in healthy controls and demonstrated that HO-1hi expression was dependent on uptake of heme-exposed endothelium. SCD patients with a recent VOC episode had lower numbers of HO-1hi patrolling monocytes. Heme-mediated vaso-occlusion by mouse SCD red blood cells was exacerbated in mice lacking patrolling monocytes, and reversed following transfer of patrolling monocytes. Altogether, these data indicate that SCD patrolling monocytes remove hemolysis-damaged endothelial cells, resulting in HO-1 upregulation and dampening of VOC, and that perturbation in patrolling monocyte numbers resulting in lower numbers of HO-1hi patrolling monocyte may predispose SCD patients to VOC. These data suggest that HO-1hi patrolling monocytes are key players in VOC pathophysiology and have potential as therapeutic targets for VOC.
Introduction
Sickle cell disease (SCD) results from a mutation encoding a single amino acid substitution within the β-globin chain, causing hemoglobin to polymerize when deoxygenated to form rigid polymers within red blood cells (RBCs). SCD patients suffer from a range of complications, including vaso-occlusive crisis (VOC), marked by severe pain, resulting from vascular occlusion involving attachment of rigid sickle RBCs, activated leukocytes, and possibly platelets to the underlying activated, damaged vascular endothelium.1,2 SCD patients also suffer from intravascular hemolysis in which extracellular hemoglobin and its product, heme, are released into the circulation, causing oxidative damage and an inflammatory cascade.3-5 Haptoglobin and hemopexin, which normally remove free hemoglobin and heme, respectively, from the circulation, are depleted in SCD patients.6,7 Excessive free heme activates the underlying endothelium, increasing expression of endothelial adhesion molecules and apoptotic markers, which in turn promote attachment of activated leukocytes and RBCs to the vessel wall.4,8-10 Data from mouse models of SCD have demonstrated that administration of free heme induces vascular stasis.9,11
Heme oxygenase 1 (HO-1) plays an important role in heme detoxification by degrading heme into iron, carbon monoxide, and biliverdin, and further conferring cytoprotective, anti-inflammatory, and antioxidant effects through its breakdown products.12 Induction of HO-1 plays an important role in protection of endothelium and tissues against hemolysis and oxidative stress.13-15 We and others have shown that circulating monocytes in humans express a higher level of HO-1 than other blood leukocytes,16,17 with the highest expression in the so-called nonclassical CD14lowCD16+ monocytes. This subset, also referred to as endothelial patrolling monocytes (PMos), scavenge particles that attach to endothelium and phagocytose cellular debris derived from the damaged endothelial cells (ECs).18 PMos play important roles in some diseases such as in atherosclerosis19 and Alzheimer disease,20 where absence of endothelial protection by PMos renders the endothelium more susceptible to atherosclerotic plaque growth and amyloid β deposition in Alzheimer disease, respectively.
We recently showed that SCD patients express even higher levels of HO-1 in CD14lowCD16+ monocytes than healthy individuals and that the higher HO-1 level in this subset is associated with a T-cell anti-inflammatory profile, which may lower the risk of alloimmunization.16 However, the underlying mechanism responsible for heightened HO-1 levels in SCD was not investigated. Furthermore, the endothelial scavenging role of PMos in SCD has not been previously examined. We hypothesized that the monocyte subsets expressing high levels of HO-1 protect the vasculature in SCD.
Methods
Detailed experimental procedures are outlined in the supplemental Data, available on the Blood Web site. The SCD cohort (n = 52, age range 12-48) was on a chronic RBC transfusion exchange therapy using leukodepleted CEK phenotype-matched units. Some patients (n = 15, age range 14-48) had a recent VOC episode (within 1 month of blood sampling) or a history of recurrent VOCs. To control for transfusions, phenotypic characterization of HO-1–expressing cells was performed on a separate SCD cohort with no recent (minimum 3 months) transfusion (n = 15, age range 9-20). For in vitro studies, human umbilical vein endothelial cells (HUVECs) labeled with or without carboxyfluorscein diacetate succinimidyl ester (CFSE) were pretreated with exogenous hemin and cocultured with purified monocyte fractions before analysis by flow cytometry and ImageStream. For in vivo studies, PKH26-labeled RBCs (1.5 × 109) were injected first and followed by hemin (30 μmol/kg) into mice lacking PMos (Nr4a1−/−) or wild-type C57BL/6 (WT) mice. For adoptive transfers, 5 × 105 sorted PMos or classical monocytes (CMos) from Nr4a1-GFP+/− mice were injected into Nr4a1−/− recipients. Quantification of PKH26-labeled RBCs or ICAM-1 expression on endothelium was performed by whole mount imaging using confocal microscopy. Data, analyzed by GraphPad Prism, are represented as mean ± standard error of the mean (SEM). Statistical significance of the differences between groups was determined by 2-tailed Student t test. P values < .05 were considered statistically significant.
Results
Monocyte HO-1 expression in SCD
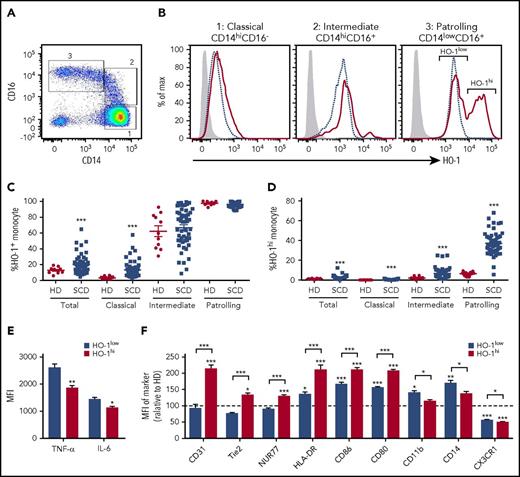
As previously reported,16 HO-1 expression levels differ in the circulating monocyte subsets from low levels in CMos to highest in the PMos. As compared with race-matched healthy donors (HDs), SCD patients expressed much higher HO-1 levels in all monocytes subsets (Figure 1A-C). Strikingly, analysis of PMos from SCD patients revealed 2 distinct peaks of HO-1 expression, HO-1hi and HO-1low. A mean 37% of circulating PMos in transfused SCD patients expressed HO-1hi as compared with 6% in HD (Figure 1D; P < .001). The frequency and numbers of HO-1hi PMos correlated with hemolytic markers (supplemental Figure 1A-B) and SCD patients with similar markers of hemolysis as those on chronic transfusions who had not received transfusions for at least the prior 3 months also had comparably high levels of HO-1hi PMos (supplemental Figure 1C). Furthermore, HO-1hi PMo frequencies were comparable regardless of alloimmunization state of the patients (data not shown). We next examined the effector functions of HO-1hi vs HO-1low expressing SCD PMos. Because negligible steady state cytokine production was observed ex vivo, purified PMos from SCD were stimulated with lipopolysaccharide (LPS). We found lower levels of proinflammatory tumor necrosis factor-α (TNF-α) and to a lesser extent interleukin-6 (IL-6) cytokines expressed by HO-1hi cells following stimulation (Figure 1E; supplemental Figure 1D; P < .001). Despite comparable levels of many monocyte-specific markers, including Fc receptors (CD16, CD32, CD64), heme and hemoglobin scavenging receptors (CD91 and CD163, respectively), integrins (CD11a, CD18, CD49b), and PMo-specific markers CD43 and Slan (data not shown), comparison of HO-1hi vs HO-1low populations revealed several differences in expression levels of other markers. We found higher levels of activation markers (CD80, CD86, and HLA-DR), angiogenetic markers CD31 and Tie2, and PMo-specific transcription factor Nur77/Nr4a1, but lower levels of CD11b integrin, CX3CR1 chemokine receptor, and CD14 (Figure 1F; supplemental Figure 1D). Collectively, these data indicate that SCD patients have an expanded subpopulation of circulating PMos expressing high levels of HO-1.
HO-1hiPMo characterization. (A) Representative dot plot of CD14 and CD16 expression in circulating monocytes of SCD patients showing 3 subsets: classical CD14hiCD16− (gate 1), intermediate CD14hiCD16+ (gate 2), and patrolling CD14lowCD16+ (PMo) (gate 3). (B) Representative histograms comparing HO-1 expression in the 3 monocyte subsets from SCD patients (red solid line) and race-matched healthy controls (blue dashed line). Isotype control is shown as gray-filled histogram. Gating for HO-1+ population was set according to the corresponding isotype background. Two peaks of HO-1 expression, indicated as HO-1hi and HO-1low, can be clearly discerned in PMos from SCD patients. (C) Frequencies of total HO-1+–expressing circulating monocyte populations (relative to isotype control) in race-matched healthy controls (n = 10) and SCD patients (n = 52). (D) Frequencies of HO-1hi–expressing monocytes (based on the gating strategy in PMos as shown in panel B) in the same individuals as in panel C: 10 race-matched healthy controls and 52 SCD patients. (E) PMos from SCD patients (n = 8) were purified, and following 4 hours LPS (200 ng/mL) stimulation, intracellular TNF-α and IL-6 expression in HO-1hi vs HO-1low subpopulations of PMos was measured by flow cytometry. (F) Expression of monocyte markers in HO-1hi and HO-1low PMos from SCD patients (n = 21) relative to levels expression by HD HO-1low PMo. Data represent mean ± SEM; means in panels C and D were compared using 2-tailed Student t test. Means in panels E and F were compared using 2-tailed paired Student t test. *P < .05; **P < .01; ***P < .001. MFI, mean fluorescence intensity.
HO-1hiPMo characterization. (A) Representative dot plot of CD14 and CD16 expression in circulating monocytes of SCD patients showing 3 subsets: classical CD14hiCD16− (gate 1), intermediate CD14hiCD16+ (gate 2), and patrolling CD14lowCD16+ (PMo) (gate 3). (B) Representative histograms comparing HO-1 expression in the 3 monocyte subsets from SCD patients (red solid line) and race-matched healthy controls (blue dashed line). Isotype control is shown as gray-filled histogram. Gating for HO-1+ population was set according to the corresponding isotype background. Two peaks of HO-1 expression, indicated as HO-1hi and HO-1low, can be clearly discerned in PMos from SCD patients. (C) Frequencies of total HO-1+–expressing circulating monocyte populations (relative to isotype control) in race-matched healthy controls (n = 10) and SCD patients (n = 52). (D) Frequencies of HO-1hi–expressing monocytes (based on the gating strategy in PMos as shown in panel B) in the same individuals as in panel C: 10 race-matched healthy controls and 52 SCD patients. (E) PMos from SCD patients (n = 8) were purified, and following 4 hours LPS (200 ng/mL) stimulation, intracellular TNF-α and IL-6 expression in HO-1hi vs HO-1low subpopulations of PMos was measured by flow cytometry. (F) Expression of monocyte markers in HO-1hi and HO-1low PMos from SCD patients (n = 21) relative to levels expression by HD HO-1low PMo. Data represent mean ± SEM; means in panels C and D were compared using 2-tailed Student t test. Means in panels E and F were compared using 2-tailed paired Student t test. *P < .05; **P < .01; ***P < .001. MFI, mean fluorescence intensity.
Heme-treated HUVECs induce high level of HO-1 expression in PMo
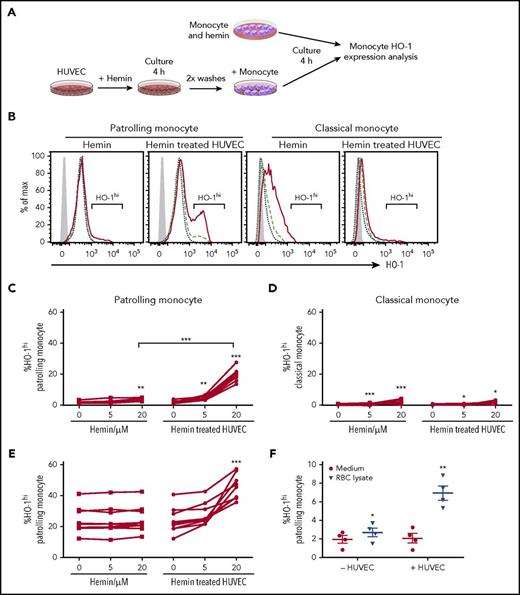
We hypothesized that uptake of free hemoglobin/heme was responsible for expansion of the HO-1hi subpopulation of PMos in SCD. We therefore tested whether exposure of HD monocytes to cell-free heme, which is the major substrate for HO-1, can induce HO-1hi expression in PMos. Surprisingly, treatment of purified monocytes with different doses of hemin (oxidized heme) in a whole serum culture system resulted in only a small albeit significant increase in the frequency of HO-1hi–expressing monocytes (Figure 2A-D). Because CD14lowCD16+ monocytes are considered PMos, which scavenge the endothelium, we reasoned that interaction with ECs was important for monocyte response to stimuli, including free heme. Indeed, coculture with hemin-exposed HUVECs induced a 10-fold increase in the frequency of HO-1hi PMos from HDs (Figure 2C; 2.1% ± 0.3% to 19.8% ± 1.7%, P < .001). Because HUVEC is large vessel–cell derived, we also tested HO-1hi PMo frequency in hemin-treated human microvascular endothelial cell cocultures and found a comparable increase (1.9 ± 0.4 to 17.2 ± 2.5; P < .001). Exposure to heme-treated HUVECs resulted in further increase in the HO-1hi PMo population from SCD patients (Figure 2E; 24% ± 3% to 45% ± 3%; P < .001), whereas hemin by itself had no effect (Figure 2E). Addition of heme-binding protein, hemopexin, to HUVECs during heme exposure blocked expansion of the HO-1hi PMos in the cocultures, confirming that HO-1hi induction is indeed mediated through heme (supplemental Figure 2E). Although direct addition of exogenous hemin increased overall HO-1 expression in CMos from HD or SCD patients, it had minimal effects on HO-1hi expression (Figure 2D; supplemental Figure 2A-D). Similarly, hemin-exposed HUVECs had minimal effect on HO-1hi CMos from HD (Figure 2D; 1.7% ± 0.4% vs 2.5% ± 0.5%) or SCD patients (3.7% ± 0.5% vs 5.9% ± 1%). Similar to the effects seen with exogenous hemin, hemolyzed RBCs induced expansion of HO-1hi–expressing PMos only when in contact with HUVECs (Figure 2F). Altogether, these data suggest that interaction of cell-free heme with ECs is a prerequisite for optimal induction of HO-1hi expression in PMo.
Hemin-treated HUVECs induce high-level HO-1 expression in PMo. (A) Schematic representation of experimental design. Purified total monocytes were cultured directly with hemin or with HUVECs pretreated with hemin for 4 hours before monocyte HO-1 expression analysis. (B) Representative histograms comparing HO-1 expression in monocyte subsets from the cultures without or with HUVECs. No hemin (blue short dashed line), hemin 5 µM (green long dashed line), and hemin 20 µM (red solid line). Isotype control is shown as gray-filled histogram. Frequencies of HO-1hi–expressing (C) PMos and (D) CMos from HDs (n = 7) following 4 hours of exposure of purified monocytes to heme alone (5 and 20 µM) or HUVECs pretreated with hemin (5 and 20 µM). (E) Frequencies of HO-1hi–expressing PMos from SCD patients (n = 9) using the same experimental protocol as for HDs. (F) Frequencies of HO-1hi–expressing PMos from HDs (n = 4) exposed to RBC lysate (120 µM total heme level) alone or HUVECs pretreated with RBC lysate. Data represent mean ± SEM; means were compared using 2-tailed paired Student t test. *P < .05; **P < .01; ***P < .001.
Hemin-treated HUVECs induce high-level HO-1 expression in PMo. (A) Schematic representation of experimental design. Purified total monocytes were cultured directly with hemin or with HUVECs pretreated with hemin for 4 hours before monocyte HO-1 expression analysis. (B) Representative histograms comparing HO-1 expression in monocyte subsets from the cultures without or with HUVECs. No hemin (blue short dashed line), hemin 5 µM (green long dashed line), and hemin 20 µM (red solid line). Isotype control is shown as gray-filled histogram. Frequencies of HO-1hi–expressing (C) PMos and (D) CMos from HDs (n = 7) following 4 hours of exposure of purified monocytes to heme alone (5 and 20 µM) or HUVECs pretreated with hemin (5 and 20 µM). (E) Frequencies of HO-1hi–expressing PMos from SCD patients (n = 9) using the same experimental protocol as for HDs. (F) Frequencies of HO-1hi–expressing PMos from HDs (n = 4) exposed to RBC lysate (120 µM total heme level) alone or HUVECs pretreated with RBC lysate. Data represent mean ± SEM; means were compared using 2-tailed paired Student t test. *P < .05; **P < .01; ***P < .001.
PMos uptake hemin-damaged HUVECs
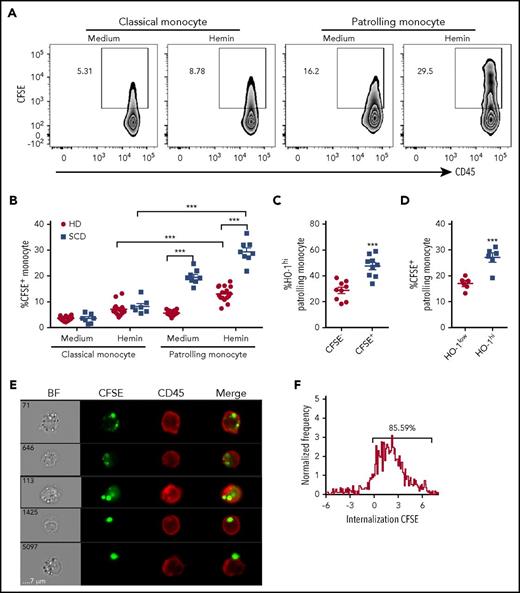
The above studies demonstrated that HO-1hi expression is induced in PMos following coculture with hemin-treated ECs. Exposure to heme is known to potentiate injury to ECs.4,8 Because PMos scavenge and uptake damaged vascular endothelium,18 we hypothesized that HO-1hi expression is the result of engulfment of heme-exposed HUVECs by PMos. To test this, purified monocytes from HDs or SCD patients were cocultured with CFSE-labeled HUVECs that had been pretreated without or with hemin, and the frequency of CFSE+ material (which is exclusively HUVEC derived) associated with PMos vs CMos was quantified by flow cytometry. Exposure to hemin-treated HUVECs resulted in higher frequencies of CFSE+ PMos from HDs (Figure 3A-B; 6% ± 1% vs 13% ± 3%; p < .001) and SCD patients (Figure 3B; 20% ± 3% vs 29% ± 4%; P < .001). In contrast, frequency of CFSE+ CMos from either HD or SCD patients was minimally affected by hemin (Figure 3B). Interestingly, CFSE+ PMos from SCD patients were at least twofold higher compared with HDs before and after hemin treatment, suggesting that SCD PMos have greater capacity for uptake of cellular material (Figure 3B). We also found twofold higher frequency of HO-1hi expression in CFSE+ as compared with CFSE− PMos from HD following coculture with hemin-treated HUVECs (Figure 3C), suggesting that expansion of HO-1hi–expressing PMos is associated with increased uptake of heme-exposed endothelium. Comparison of levels of CFSE+ material in PMos from SCD patients indicated that HO-1hi–expressing cells had greater levels of endothelial-derived material than HO-1low cells (Figure 3D). To discriminate between adherent and internalized materials in monocyte, Image Stream flow cytometry was performed. Approximately 85% of CFSE+ PMos from SCD patients had intracellularly localized CFSE after coculture with hemin-treated HUVECs (Figure 3E-F), indicating that heme-exposed ECs are indeed taken up by PMos. Taken together, these results indicate that PMos uptake heme-exposed EC-derived material, resulting in HO-1hi expression.
PMos uptake hemin-exposed HUVECs. CFSE-labeled HUVECs were pretreated without (“medium”) or with hemin (20 µM) before coculturing with purified monocytes from HDs (n = 18, filled circles) or SCD patients (n = 8, filled squares) for 4 hours. (A) Representative dot plots comparing CFSE+ classical and PMos. (B) Frequencies of CFSE+ classical and PMos are shown. (C) Frequencies of HO-1hi–expressing cells in PMos from HDs (n = 9) comparing CFSE− (no attachment/uptake of CFSE+ cellular material) and CFSE+ (representing cells that have adhered to/taken up CFSE+ cellular material) subpopulations following coculture with labeled hemin-treated HUVECs. (D) Comparison of frequencies of CFSE+ PMos from SCD patients (n = 6) in HO-1low vs HO-1hi subpopulations following coculture with HUVECs. (E) Representative flow cytometry images acquired simultaneously and gated on CFSE+ patrolling CD14lowCD16+ monocytes from SCD patients (from panel A). Right to left: single-channel bright field (BF), CFSE (representing HUVEC material in green), CD45 (representing PMos in red), and merged images showing CFSE+ materials within CD45+ cells in the first 4 rows, but not in the last row where CFSE+ material is attached to the external surface of PMo. (F) Histogram depicting degree of CFSE+ material internalized within PMos with internalization score: <0 represents CFSE+ material attached to the surface of PMos; >0 represents CFSE+ material internalized by PMo. Percentage of cells with internalization score of >0 is indicated. Data represent mean ± SEM; means were compared using 2-tailed paired Student t test (medium vs hemin treatment as well as monocyte subset vs other monocyte subset in the same donor/patient) and unpaired Student t tests (HD vs SCD patients). ***P < .001.
PMos uptake hemin-exposed HUVECs. CFSE-labeled HUVECs were pretreated without (“medium”) or with hemin (20 µM) before coculturing with purified monocytes from HDs (n = 18, filled circles) or SCD patients (n = 8, filled squares) for 4 hours. (A) Representative dot plots comparing CFSE+ classical and PMos. (B) Frequencies of CFSE+ classical and PMos are shown. (C) Frequencies of HO-1hi–expressing cells in PMos from HDs (n = 9) comparing CFSE− (no attachment/uptake of CFSE+ cellular material) and CFSE+ (representing cells that have adhered to/taken up CFSE+ cellular material) subpopulations following coculture with labeled hemin-treated HUVECs. (D) Comparison of frequencies of CFSE+ PMos from SCD patients (n = 6) in HO-1low vs HO-1hi subpopulations following coculture with HUVECs. (E) Representative flow cytometry images acquired simultaneously and gated on CFSE+ patrolling CD14lowCD16+ monocytes from SCD patients (from panel A). Right to left: single-channel bright field (BF), CFSE (representing HUVEC material in green), CD45 (representing PMos in red), and merged images showing CFSE+ materials within CD45+ cells in the first 4 rows, but not in the last row where CFSE+ material is attached to the external surface of PMo. (F) Histogram depicting degree of CFSE+ material internalized within PMos with internalization score: <0 represents CFSE+ material attached to the surface of PMos; >0 represents CFSE+ material internalized by PMo. Percentage of cells with internalization score of >0 is indicated. Data represent mean ± SEM; means were compared using 2-tailed paired Student t test (medium vs hemin treatment as well as monocyte subset vs other monocyte subset in the same donor/patient) and unpaired Student t tests (HD vs SCD patients). ***P < .001.
Mechanism of HO-1hi induction by heme-damaged HUVECs in PMo
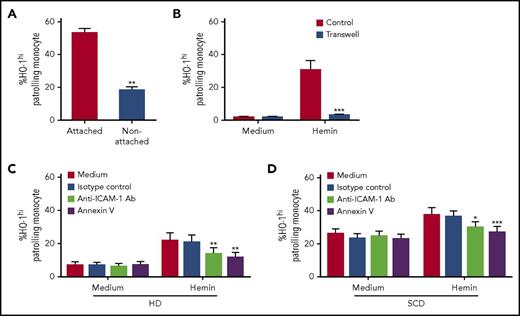
We next examined the molecular basis of HUVEC-monocyte interactions responsible for HO-1hi induction. HO-1 expression profiles were first analyzed in adherent and nonadherent monocytes in monocyte-HUVEC cocultures. We found a significantly higher frequency of HO-1hi PMos in the fraction firmly attached to HUVECs as compared with the nonadherent monocytes (Figure 4A; 53% ± 3% vs 18% ± 2%; P < .01). To test whether HO-1hi induction requires cell-cell contact between PMos and HUVECs, we also performed transwell studies. HO-1hi was induced only when heme-exposed HUVECs and PMos were in direct contact (Figure 4B; 30% ± 6% vs 2.9% ± 0.2%; P < .001), which further demonstrates the requirement for cell-cell interaction for HO-1hi expression. We next assessed the phenotype of heme-exposed HUVECs. We found roughly a two- to fivefold increase in expression of phosphatidylserine (PS, annexin V+), selectins, and ICAM-1 and VCAM-1 on heme-treated HUVECs (supplemental Figure 3A-E), consistent with previous reports showing activation and apoptosis of endothelium by heme.8,21 Because the endothelial scavenging activity of PMos is exclusively dependent on ICAM-1/2 expression on ECs, and because PMos uptake dead cell material,18 we next investigated the role of ICAM-1 and PS moieties on heme-exposed ECs in induction of PMo HO-1hi. Pretreatment of heme-exposed HUVECs with blocking antibodies against ICAM-1, but not VCAM-1 (data not shown) as well as pretreatment with annexin V, inhibited HO-1 induction in PMos from HD and SCD patients (Figure 4C-D; P < .05), thus identifying PS moieties as well as ICAM-1 as key molecules involved in monocyte-HUVEC interactions that mediate HO-1hi induction. Altogether, these data suggest HO-1hi induction in PMos requires direct attachment to and engulfment of activated, apoptotic ECs damaged by heme.
Mechanism of HO-1hiinduction by heme-damaged HUVECs in PMos. (A) Following culturing of purified monocytes from HDs (n = 4) with hemin-pretreated HUVECs (20 µM) for 4 hours, frequency of HO-1hi in nonadherent vs adherent monocytes was analyzed. (B) Frequencies of HO-1hi–expressing PMos from HDs (n = 5) in the transwell culture system (blue bars) in which HUVECs exposed to hemin (20 μM) or not (“medium”) were placed in the bottom well and separated from purified monocytes on the top well. At the same time, as a control (“Control”), monocytes were also cocultured directly with HUVECs exposed to hemin (20 µM) or not (“medium”). Hemin (20 µM) exposed HUVEC was preincubated with annexin V or blocking antibodies anti-ICAM-1, VCAM-1, or isotype control for 30 minutes before addition of purified monocyte from (C) HDs (n = 5) and (D) SCD patients (n = 8). Frequencies of HO-1hi–expressing PMos were then analyzed. Data represent mean ± SEM; means were compared using 2-tailed paired Student t test. *P < .05; **P < .01; ***P < .001. Ab, antibody.
Mechanism of HO-1hiinduction by heme-damaged HUVECs in PMos. (A) Following culturing of purified monocytes from HDs (n = 4) with hemin-pretreated HUVECs (20 µM) for 4 hours, frequency of HO-1hi in nonadherent vs adherent monocytes was analyzed. (B) Frequencies of HO-1hi–expressing PMos from HDs (n = 5) in the transwell culture system (blue bars) in which HUVECs exposed to hemin (20 μM) or not (“medium”) were placed in the bottom well and separated from purified monocytes on the top well. At the same time, as a control (“Control”), monocytes were also cocultured directly with HUVECs exposed to hemin (20 µM) or not (“medium”). Hemin (20 µM) exposed HUVEC was preincubated with annexin V or blocking antibodies anti-ICAM-1, VCAM-1, or isotype control for 30 minutes before addition of purified monocyte from (C) HDs (n = 5) and (D) SCD patients (n = 8). Frequencies of HO-1hi–expressing PMos were then analyzed. Data represent mean ± SEM; means were compared using 2-tailed paired Student t test. *P < .05; **P < .01; ***P < .001. Ab, antibody.
Because Toll-like receptor-4 (TLR-4) is a receptor for heme,22 we next tested whether induction of HO-1hi PMos by heme-exposed HUVECs is mediated by TLR-4. Preincubation with the TLR-4 inhibitor TAK-242 had no effect on HO-1hi PMos expansion, suggesting that TLR-4 is not involved in HO-1hi induction in our system (supplemental Figure 3F-G). Furthermore, there was a lack of expression of TLR-4–associated cytokine TNF-α in PMos cocultured with heme-treated HUVECs (supplemental Figure 3H). These data suggest that HO-1hi induction in PMos by heme-exposed HUVECs is TLR-4 independent, which may also explain the lack of proinflammatory TNF cytokine expression in these PMos, given that TLR-4 engagement normally induces a proinflammatory response.
SCD patients with VOC have lower levels of PMos than non-VOC SCD patients
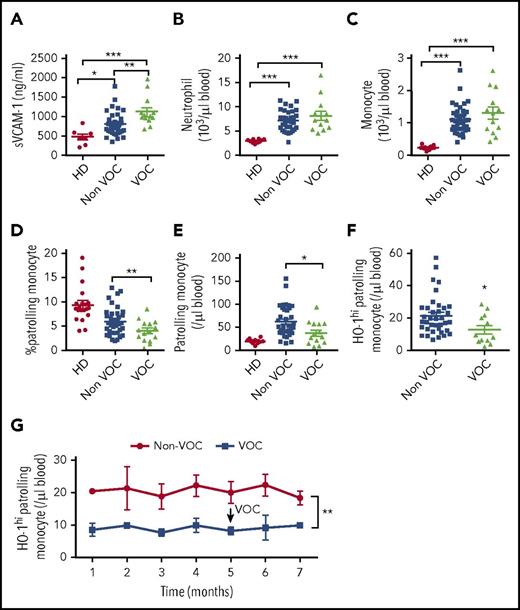
SCD patients suffer from VOC, resulting from increased attachment of SCD RBCs and neutrophils to activated, damaged endothelium.23 We hypothesized that inadequate numbers of PMos will predispose SCD patients to episodes of VOC because of decreased removal of activated, damaged ECs by PMos. Among SCD patients receiving chronic transfusions, patients with a recent VOC episode or those with a history of recurrent VOCs (grouped as “VOC”) had significantly higher levels of circulating sVCAM-1 (Figure 5A; 1122 ± 104 ng/mL vs 807 ± 52 ng/mL; P < .01), which is consistent with previous reports supporting an association between VOC and endothelial activation.23,24 The VOC group had significantly lower hemoglobin levels (8.6 ± 1.3 g/L vs 9.5 ± 1.3 g/L; P = .02) and higher levels of total plasma heme (142 ± 70 μM vs 98 ± 46 μM; P = .05), but no difference in haptoglobin levels (data not shown). All SCD patients had higher neutrophil and monocyte counts than race-matched HDs, but the numbers were comparable in patients with or without VOC (Figure 5B-C). In contrast, frequency of PMos was 30% lower in the VOC group (Figure 5D; P < .05). Strikingly, the numbers of PMos and the HO-1hi–expressing subset were decreased by almost half in the VOC group (Figure 5E-F; HO-1hi PMo: 21.3 ± 1.9 cells per microliter vs 12.6 ± 2.4 cells per microliter; P = .02). In a subgroup of patients, HO-1hi PMos measurements were available for a few months before and after the vaso-occlusive event, and the numbers remained lower than in non-VOC patients longitudinally sampled over a similar period of monthly intervals (Figure 5G; P < .01). We did not find significant differences in HO-1hi PMos numbers in patients on hydroxyurea alone (25 ± 9 cells per microliter vs 21 ± 6 cells per microliter; P = .8) or on hydroxyurea and regular transfusions (15 ± 6 cells per microliter vs 21 ± 3 cells per microliter; P = .4). Taken together, these data demonstrate for the first time that the numbers of PMos and specifically the HO-1hi subpopulation correlate negatively with VOC in SCD.
Reduced HO-1hiPMos in SCD patients at risk of VOC. (A) Soluble VCAM-1 levels in platelet-free plasma from race-matched HDs (n = 10) and SCD patients grouped as “Non-VOC” (n = 38) and “VOC” (n = 15, see “Methods” for patient characteristics) were tested by enzyme-linked immunosorbent assay. Absolute (B) neutrophil and (C) monocyte counts in peripheral blood were determined by Advia Hematology Analyzer. (D) Frequency of PMos within total circulating monocyte population. Absolute numbers of (E) PMos and (F) HO-1hi PMos were calculated based on monocyte counts and monocyte subset frequency. (G) Absolute numbers of HO-1hi PMos at monthly intervals in the VOC (n = 6) and non-VOC (n = 11) groups. The arrow indicates the timing of the vaso-occlusive event in the VOC group. Data represent mean ± SEM; means in panels A-F were compared using 2-tailed Student t test and means in panel G were compared using 2-way analysis of variance. *P < .05; **P < .01; ***P < .001.
Reduced HO-1hiPMos in SCD patients at risk of VOC. (A) Soluble VCAM-1 levels in platelet-free plasma from race-matched HDs (n = 10) and SCD patients grouped as “Non-VOC” (n = 38) and “VOC” (n = 15, see “Methods” for patient characteristics) were tested by enzyme-linked immunosorbent assay. Absolute (B) neutrophil and (C) monocyte counts in peripheral blood were determined by Advia Hematology Analyzer. (D) Frequency of PMos within total circulating monocyte population. Absolute numbers of (E) PMos and (F) HO-1hi PMos were calculated based on monocyte counts and monocyte subset frequency. (G) Absolute numbers of HO-1hi PMos at monthly intervals in the VOC (n = 6) and non-VOC (n = 11) groups. The arrow indicates the timing of the vaso-occlusive event in the VOC group. Data represent mean ± SEM; means in panels A-F were compared using 2-tailed Student t test and means in panel G were compared using 2-way analysis of variance. *P < .05; **P < .01; ***P < .001.
PMos inhibit hemin-induced vaso-occlusion in vivo
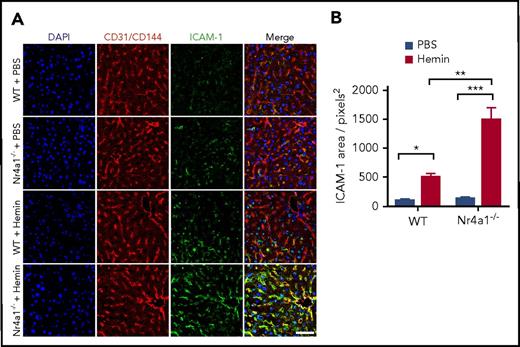
To formally test the role of PMos under hemolytic stress in vivo, we first examined HO-1 expression in circulating leukocytes of the Townes SCD mouse model (SS) as compared with those of litter-matched non-SCD control (AA) and heterozygous mice. Similar to what we observed in humans,16 HO-1 expression levels were low in neutrophils and lymphocytes, whereas monocytes had higher expression levels, especially in PMos (Ly6C−) with those from SS mice expressing the highest HO-1 expression (43% ± 6%; supplemental Figure 4A-B). We next tested the effects of free heme on vascular activation in mice lacking PMos by comparing the effects of injected hemin into Nr4a1-knockout (Nr4a1−/−) mice, which have a selective loss of PMos,25 and WT mice. We found significantly higher (threefold) upregulation of ICAM-1 expression on blood vessels of Nr4a1−/− mice as compared with WT mice following hemin treatment (Figure 6A-B; P < .05). As expected, PMos remained absent in Nr4a1−/− mice treated with hemin (data not shown), whereas HO-1 levels were upregulated in circulating Ly6C− PMos of hemin-treated WT mice (supplemental Figure 4C). These data suggest that loss of PMos in vivo impacts heme-mediated activation of vascular ECs.
Hemin induces higher vascular ICAM-1 expression in Nr4a1−/−mice. WT mice and Nr4a1−/− mice were injected with hemin (30 μmol/kg mouse) or phosphate-buffered saline (PBS). (A) Whole mount immunofluorescence analysis of livers 24 hours postinjection showing ICAM-1 (green), CD31/CD144 (red), and 4′,6-diamidino-2-phenylindole (DAPI) (blue). Scale bar = 50 µm. Yellow in “Merge” images indicates colocalization of ICAM-1 (green) expression on CD31/CD144+ ECs (red). A pronounced increase in ICAM1 expression was evident due to hemin injection in Nr4a1−/− mice. (B) Quantification of the area of blood vessels expressing ICAM-1 in the Nr4a1−/− and control groups using Image J software. Data represent mean ± SEM; means were compared using 2-tailed Student t test. *P < .05; **P < .01; ***P < .001 (n = 6-8 mice per group).
Hemin induces higher vascular ICAM-1 expression in Nr4a1−/−mice. WT mice and Nr4a1−/− mice were injected with hemin (30 μmol/kg mouse) or phosphate-buffered saline (PBS). (A) Whole mount immunofluorescence analysis of livers 24 hours postinjection showing ICAM-1 (green), CD31/CD144 (red), and 4′,6-diamidino-2-phenylindole (DAPI) (blue). Scale bar = 50 µm. Yellow in “Merge” images indicates colocalization of ICAM-1 (green) expression on CD31/CD144+ ECs (red). A pronounced increase in ICAM1 expression was evident due to hemin injection in Nr4a1−/− mice. (B) Quantification of the area of blood vessels expressing ICAM-1 in the Nr4a1−/− and control groups using Image J software. Data represent mean ± SEM; means were compared using 2-tailed Student t test. *P < .05; **P < .01; ***P < .001 (n = 6-8 mice per group).
We next examined the role of PMos in heme-induced VOC by sickle RBCs using Nr4a1−/− mice. To do this, we first set up a stasis model to examine sickle RBC sequestration in WT mice treated with cell-free heme. PKH26-labeled RBCs from Townes SS or control AA mice were transfused into WT recipients and 24 hours later injected with hemin. Immunofluorescence analysis after 24 hours indicated that heme treatment resulted in higher numbers of PKH26+ cells in the livers of mice transfused with sickle RBCs than those transfused with AA RBCs (supplemental Figure 4D-F; 58 ± 4 RBCs/0.18 mm2 area vs 40 ± 4 RBCs/0.18 mm2 area; P < .01) despite no significant difference between the 2 groups in the absence of heme (supplemental Figure 4F).
Using this model, we next examined the role of PMos in SCD stasis by comparing WT and Nr4a1−/− mice transfused with PKH26-labeled SS RBCs. Immunofluorescence analysis of liver vasculature showed higher numbers of PKH26+ sickle RBCs in hemin-treated Nr4a1−/− mice than in WT mice (Figure 7A-B,D; P < .01). Examination of liver sections also revealed increased blood vessel stasis and leukocyte infiltration in Nr4a1−/− mice than in WT mice (Figure 7C). Finally, we tested whether transfer of PMos can rescue sickle RBC stasis in Nr4a1−/− mice. GFP+Ly-6C− PMos and classical (Ly6C+GFPlow) monocytes from Nr4a1-GFP mice were adoptively transferred into Nr4a1−/− mice prior to hemin injection. Transfer of PMos but not CMos significantly lowered the number of PKH26-labeled sickle RBCs in liver vasculature (Figure 7E-H; 104.3 ± 13.3 RBCs/0.18 mm2 area vs 72.8 ± 7.6 RBCs/0.18 mm2 area; P < .01). Altogether, these data demonstrate that PMos protect against heme-driven endothelial activation and that they can inhibit hemolysis-driven SCD VOC.
Increased sickle RBC stasis in Nr4a1−/−mice and reversal by adoptively transferred PMo. (A) Experimental schedule for induction of sickle RBC stasis and analysis. WT mice and Nr4a1−/− mice were transfused with PKH26-labeled mouse sickle RBCs (1.5 × 109 RBCs per mouse) followed by injection of hemin (30 μmol/kg mouse) followed by analysis at the indicated times. As a control, some of the transfused mice received PBS instead of hemin. (B) Whole mount immunofluorescence of perfused livers from WT or Nr4a1−/− mice showing CD31/CD144 (endothelial markers, green) and PKH26 (red). Scale bar = 50 µm. (C) Representative hematoxylin and eosin (H&E)–stained liver sections of sickle RBC transfused mice (scale bars = 200 µm [first 2 rows] and 50 µm [last row]). Black arrows indicate RBC stasis within blood vessels. Red arrows depict leukocyte infiltration. (D) Enumeration of PKH26+, representing sickle RBCs (“SS RBC”), per image in perfused livers in panel B as quantified using Image J software, indicating increased stasis in hemin-treated Nr4a1−/− mice. (E) Gating strategy for sorting GFP+ Ly6C− PMos and GFPlowLy-C6+ CMo populations from spleen and blood of Nr4a1-GFP reporter mice. (F) Experimental schedule for adoptive transfer of sorted monocyte subsets into sickle RBC stasis model and analysis. PBS or purified PMos or CMos were adoptively transferred (5 × 105 monocytes per mouse) into Nr4a1−/− mice that had received first PKH26-labeled sickle RBCs (1.5 × 109 RBCs per mouse) followed by injection of hemin (30 μmol/kg mouse) and analysis at the indicated times. (G) Representative confocal images in liver blocks of perfused mice comparing control PBS mice (no adoptive transfer) and PMos or CMos adoptively transferred mice. Scale bar = 50 μm. (H) Enumeration of sickle RBCs per image in perfused livers as quantified using Image J software. Data represent mean ± SEM; means were compared using 2-tailed Student t test. **P < .01; ***P < .001 (n = 6-8 mice per group).
Increased sickle RBC stasis in Nr4a1−/−mice and reversal by adoptively transferred PMo. (A) Experimental schedule for induction of sickle RBC stasis and analysis. WT mice and Nr4a1−/− mice were transfused with PKH26-labeled mouse sickle RBCs (1.5 × 109 RBCs per mouse) followed by injection of hemin (30 μmol/kg mouse) followed by analysis at the indicated times. As a control, some of the transfused mice received PBS instead of hemin. (B) Whole mount immunofluorescence of perfused livers from WT or Nr4a1−/− mice showing CD31/CD144 (endothelial markers, green) and PKH26 (red). Scale bar = 50 µm. (C) Representative hematoxylin and eosin (H&E)–stained liver sections of sickle RBC transfused mice (scale bars = 200 µm [first 2 rows] and 50 µm [last row]). Black arrows indicate RBC stasis within blood vessels. Red arrows depict leukocyte infiltration. (D) Enumeration of PKH26+, representing sickle RBCs (“SS RBC”), per image in perfused livers in panel B as quantified using Image J software, indicating increased stasis in hemin-treated Nr4a1−/− mice. (E) Gating strategy for sorting GFP+ Ly6C− PMos and GFPlowLy-C6+ CMo populations from spleen and blood of Nr4a1-GFP reporter mice. (F) Experimental schedule for adoptive transfer of sorted monocyte subsets into sickle RBC stasis model and analysis. PBS or purified PMos or CMos were adoptively transferred (5 × 105 monocytes per mouse) into Nr4a1−/− mice that had received first PKH26-labeled sickle RBCs (1.5 × 109 RBCs per mouse) followed by injection of hemin (30 μmol/kg mouse) and analysis at the indicated times. (G) Representative confocal images in liver blocks of perfused mice comparing control PBS mice (no adoptive transfer) and PMos or CMos adoptively transferred mice. Scale bar = 50 μm. (H) Enumeration of sickle RBCs per image in perfused livers as quantified using Image J software. Data represent mean ± SEM; means were compared using 2-tailed Student t test. **P < .01; ***P < .001 (n = 6-8 mice per group).
Discussion
SCD patients suffer from ongoing intravascular hemolysis, which results in the release of free hemoglobin and heme into the circulation, causing activation and apoptosis of the underlying vasculature. Furthermore, painful VOC, a clinical hallmark of SCD, resulting from obstruction of vasculature by sickle RBCs is associated with microvascular damage. PMos scavenge the vasculature and remove damaged cells and debris, thus clearing the vascular beds from potentially unwanted toxic or inflammatory stimuli. In the present study, we identified circulating PMos as potentially playing a key role in protecting the endothelium against hemolysis-induced damage and vaso-occlusion in SCD. We demonstrated that these PMos engulf heme-damaged ECs, resulting in HO-1 upregulation. In SCD patients, high levels of HO-1 were detected in a subpopulation of PMos, and importantly, reduced numbers of HO-1hi PMos were associated with recent VOC episodes or history of recurrent VOCs. Mice lacking PMos were susceptible to hemolysis-induced endothelial activation and vascular stasis in the presence of sickle RBCs, and transfer of PMos partially ameliorated the stasis phenotype. Altogether, these data are consistent with a model in which SCD PMos continuously scavenge and remove heme-damaged ECs, which would otherwise attract and/or bind to adherent sickle RBCs and leukocytes. In this model, inability to clear heme-damaged endothelial layers due to inadequate numbers of circulating PMos results in recruitment and adherence of leukocytes and sickle RBCs to the damaged vessel walls, causing obstruction in the vasculature and VOC.
In the present study, we found a readily detectable novel subpopulation of circulating PMos in SCD patients expressing higher levels of the heme detoxifying enzyme HO-1, which we refer to as the HO-1hi PMo. As we and others have previously reported, direct addition of exogenous free heme to monocytes induced HO-1 expression. Surprisingly, however, free heme had minimal effect on the induction of HO-1hi subpopulation in either HD or SCD patients. Rather, optimal HO-1hi induction in circulating PMos required the presence of ECs and EC-associated PS and ICAM expression. We posit that in the sickle vasculature, ECs exposed to heme released through intravascular destruction of sickle RBCs and/or microparticles derived from sickle RBCs9 become activated and damaged. Consistent with this, numbers of circulating ECs (CECs), which are indicators of vascular damage, are five- to 10-fold higher in SCD at steady state and during crisis, respectively,26 although frequency of their apoptotic CECs were less than in HDs.27 Because levels of vascular endothelial growth factor that protect against apoptosis are high in SCD,27,28 future studies are needed to characterize CECs numbers, including apoptotic frequencies to establish the extent of endothelial damage in our patient cohort. By exclusively labeling HUVECs, we further demonstrated that heme-damaged endothelial material is preferentially taken up by PMos but not CMos, thereby inducing HO-1hi expression in PMos only. The preferential uptake of cellular material, which may include heme-damaged EC-derived microparticles, by PMos is consistent with their phagocytic function in the vasculature.29 Interestingly, PMos from SCD patients expressed higher levels of activation markers and were more effective than HD PMos in the uptake of endothelial-derived cellular material, suggesting that SCD PMos are more potent scavengers. Of note, HO-1hi SCD PMos had higher phagocytic activity than those expressing HO-1low levels. It remains to be determined whether the heightened phagocytic activity is also directed against other cells, such as other dead cells, RBC-derived microparticles,9 or sickle dense cells present within SCD vasculature.
We postulate that increased scavenging activity of SCD endothelial PMos is necessary to decrease danger signals resulting from hemolysis-associated cellular damage, thereby reducing leukocyte recruitment to the vessel wall. It follows that if the numbers and/or scavenging activity of SCD PMos are compromised, the vasculature will not be effectively cleared of danger signals, resulting in endothelial activation and inflammation in the vessel wall. In support of this, mice lacking PMos had increased endothelial activation (Figure 6) and leukocyte infiltration following heme treatment (Figure 7C). Interestingly, HO-1hi PMos from SCD patients expressed higher levels of CD31 and Tie2, 2 markers associated with promoting angiogenesis in tumors and tissue remodeling.30-33 We speculate that in conditions such as VOC when the numbers of HO-1hi PMos are reduced, this tissue repair function may also be compromised.
Previous studies have shown that heme through binding to TLR-4 in myeloid cells induces TNF-α secretion, causing an inflammatory response.22,34,35 Furthermore, PMos activated through TLR ligands can induce TNF-α secretion, resulting in inflammation and damage to the blood vessel.18,36 These data are consistent with a role for TLRs in inducing inflammation in SCD settings.11,37 Interestingly, in the setting of atherosclerosis and Alzheimer disease, PMos play a protective role and prevent endothelial damage,19 similar to our findings. In our model, we did not detect TNF-α expression in PMos cocultured with heme-treated HUVECs, and the heme effects on PMos were independent of TLR-4 (supplemental Figure 3F-G). These data suggest that under TLR-independent conditions where heme does not induce a proinflammatory response in PMos, these cells protect and prevent endothelial damage.
A key finding of our study is a potential role for HO-1hi PMos in protecting against VOC. This is based on our data showing that lower numbers of HO-1hi PMos in SCD patients are associated with VOC. Because the numbers were low months before or after VOC, we hypothesize that these patients harbor genetic defects in PMos developmental program25 and/or HO-1 signaling pathway, such as in Nrf-2 that controls HO-1 induction.38 It should be noted that our patient population was on a chronic transfusion protocol, and patients on chronic transfusions rarely develop VOC. Thus, low PMo HO-1hi numbers may be unique to high-risk populations. However, we also found lower numbers of HO-1hi PMos in nonchronically transfused SCD patients during acute pain crisis (n = 5) compared with SCD patients without VOC (6 ± 1 cells per microliter vs 21 ± 2 cells per microliter; P = .008). We propose that heightened expression of HO-1, which degrades heme into CO and biliverdin, both with anti-inflammatory anti-cytotoxic properties,39-41 is critical for the protective function of PMos against VOC. Consistent with this, inhibition of HO-1 activity exacerbated SCD vaso-occlusion,14 and myeloid-specific HO-1 deletion induced inflammation during non-SCD reperfusion injury,42 whereas HO-1 overexpression inhibited LPS-stimulated TNF-α expression in human monocytes.43 Similarly, we found lower expression of proinflammatory TNF-α and IL-6 cytokines in HO-1hi as compared with HO-1low SCD PMos following stimulation (Figure 1F). Potential mechanisms include upregulation of anti-inflammatory pathways44 and/or downregulation of CD14.45 Comparative studies of HO-1 activity in purified HO-1hi vs HO-1low subsets remain to be performed, but because HO-1 is expressed intracellularly, any cell sorting will prohibit isolation of live cells needed for activity measurements. Further confirmation for a role for HO-1 as the critical regulator of PMos functional activity in SCD will require PMos cell-type–specific deletion of HO-1.
In summary, our data support a key role for HO-1hi PMos as “housekeepers” of the SCD vasculature through their ability to scavenge and remove hemolysis-damaged endothelium. We postulate that deficiency in numbers or activity of these PMos hampers the effective clearance of heme-damaged endothelium, which in turn results in the recruitment of neutrophil and other inflammatory cells to the damaged endothelium, and in the presence of sickle RBCs precipitates a vaso-occlusive event. Altogether, these data are consistent with the cytoprotective function of HO-1hi PMos against vascular dysfunction and their role as key players in VOC pathophysiology. Furthermore, the data suggest that strategies to increase HO-1hi PMos in SCD patients, including targeting Nr4a146 to expand PMos, may represent a potent therapeutic approach against VOC.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Weili Bao (New York Blood Center [NYBC]) for mouse maintenance and suggestions regarding data analysis, Mihaela Barbu-Stevanovic and Sean D'Italia (NYBC) for cell sorting, and services provided by the Memorial Sloan Kettering Pathology Department for animal tissue sections and H&E staining.
This study was supported by National Institutes of Health, National Heart, Lung, and Blood Institute (R01HL121415 and R01HL130139) (K.Y.) and American Heart Association (K.Y.).
Authorship
Contribution: Y.L. conceived the idea and performed and analyzed all the experiments; F.J. assisted Y.L. with human sample preparation and mouse experimentation; W.Y. and A.M. assisted with whole mount and immunofluorescence studies and analysis; P.S., R.W., D.F.F., C.M., D.M., and S.T.C. were involved with all aspects of selection, recruitment, and provision of blood samples from patients and controls; K.Y. was responsible for experimental design, manuscript writing, and project supervisions; and Y.L. and K.Y. wrote the manuscript with consultation and contribution from all coauthors.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Karina Yazdanbakhsh, Laboratory of Complement Biology, New York Blood Center, 310 East 67th St, New York, NY 10065; e-mail: kyazdanbakhsh@nybc.org.

![Figure 7. Increased sickle RBC stasis in Nr4a1−/− mice and reversal by adoptively transferred PMo. (A) Experimental schedule for induction of sickle RBC stasis and analysis. WT mice and Nr4a1−/− mice were transfused with PKH26-labeled mouse sickle RBCs (1.5 × 109 RBCs per mouse) followed by injection of hemin (30 μmol/kg mouse) followed by analysis at the indicated times. As a control, some of the transfused mice received PBS instead of hemin. (B) Whole mount immunofluorescence of perfused livers from WT or Nr4a1−/− mice showing CD31/CD144 (endothelial markers, green) and PKH26 (red). Scale bar = 50 µm. (C) Representative hematoxylin and eosin (H&E)–stained liver sections of sickle RBC transfused mice (scale bars = 200 µm [first 2 rows] and 50 µm [last row]). Black arrows indicate RBC stasis within blood vessels. Red arrows depict leukocyte infiltration. (D) Enumeration of PKH26+, representing sickle RBCs (“SS RBC”), per image in perfused livers in panel B as quantified using Image J software, indicating increased stasis in hemin-treated Nr4a1−/− mice. (E) Gating strategy for sorting GFP+ Ly6C− PMos and GFPlowLy-C6+ CMo populations from spleen and blood of Nr4a1-GFP reporter mice. (F) Experimental schedule for adoptive transfer of sorted monocyte subsets into sickle RBC stasis model and analysis. PBS or purified PMos or CMos were adoptively transferred (5 × 105 monocytes per mouse) into Nr4a1−/− mice that had received first PKH26-labeled sickle RBCs (1.5 × 109 RBCs per mouse) followed by injection of hemin (30 μmol/kg mouse) and analysis at the indicated times. (G) Representative confocal images in liver blocks of perfused mice comparing control PBS mice (no adoptive transfer) and PMos or CMos adoptively transferred mice. Scale bar = 50 μm. (H) Enumeration of sickle RBCs per image in perfused livers as quantified using Image J software. Data represent mean ± SEM; means were compared using 2-tailed Student t test. **P < .01; ***P < .001 (n = 6-8 mice per group).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/131/14/10.1182_blood-2017-12-819870/4/m_blood819870f7.jpeg?Expires=1769150084&Signature=y0DB09i2L9xvHEtDYv2LxFG1MMTnLRisQYkDZrzvjPaw7AyYzHSsrxtmydIpIBExJZkAeuwutVI-La7y0sL1LLRa3-yf0gPRQL6oSNG12~TKV9GNAzz0ki0eHmOdNuxM4-3yynTqz9FGiIZM-jYiB2W2-puVD5bbfTGGLmr-FWorzJkzkVYXZxzQHXVcakV~ohhYxBsgyzj9byXR7rm03hEPRvVWvwM1mS0~mE9JqbAmSFTuTlAsm-xj6SygMjm6YOq2WbJaSN9KmDRZEiv1sgsD1O2vxYAe75CvKM8GH5SkGOLqwH6nkLLIMg5MItH0F74sDppqNRFtsuQ6kHPzuw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal