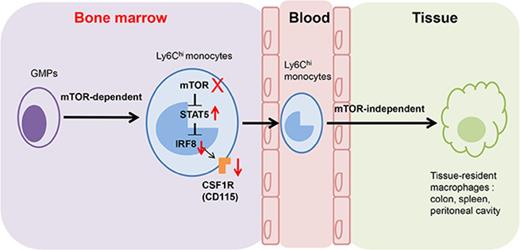
Key Points
mTOR intrinsically controls monocyte/macrophage development in the early stage.
mTOR masters monocyte development via the STAT5-IRF8-CD115 pathway.
Abstract
Monocytes and macrophages play a key role in defending pathogens, removing the dead cells or cell debris, and wound healing. The mammalian target of rapamycin (mTOR) inhibitor rapamycin (RPM) is widely used in clinics to treat patients with organ transplantation or tumors. The role of mTOR in monocyte/macrophage development remains to be clarified. Here we found that mTOR intrinsically controls monocyte/macrophage development, as evidenced by the decreased percentages and cell numbers of CD11b+F4/80+ cells resulting from mTOR inhibition in SCID mice, mTOR-deficient mice, and mixed chimera mice, and the in vitro colony formation and monocyte/macrophage induction assays. However, Lyzs-mTOR knockout mice displayed normal levels of monocytes/macrophages, indicating that mTOR is not essential for the survival and maturation of monocytes/macrophages. Further studies showed that mTOR deficiency significantly reduced macrophage colony-stimulating factor receptor CD115 expression at the transcriptional and translational levels. The molecular mechanism studies indicate that the impaired monocyte/macrophage development caused by mTOR deficiency is mainly a result of the overactivated STAT5 and subsequent downregulation of IRF8, but not the altered cell metabolism and autophagy. Therefore, our work identifies that mTOR is an intrinsic master for monocyte/macrophage development at the early stages through regulating STAT5-IRF8-dependent CD115-expressing pathway. Long-term usage of RPM may cause a defect of myeloid progenitors in bone marrow.
Introduction
Monocytes/macrophages develop in bone marrow from hematopoietic progenitors that successively generate common myeloid progenitors (CMPs) and macrophage/dendritic cell (DC) precursors (MDPs).1,2 MDPs can differentiate into common monocyte progenitors (cMoPs), from which monocytes developed. Ly6C+ and Ly6C− monocyte subsets then leave the bone marrow and circulate within the blood. Ly6C− monocytes contribute to some tissue-resident macrophages,3 whereas Ly6ChighLy6C−monocytes convert into Ly6C− monocytes to patrol in steady state and give rise to inflammatory macrophages and DCs in the inflammatory environment.3 As the technology develops, more detailed information about macrophage development has been revealed.4,5 Monocytes are key regulators in inflammation and pathogen challenge, whereas macrophages play important roles in development, wound healing, immune surveillance, and maintaining homeostasis. Thus, mastering the development and homeostasis activities of monocytes/macrophages to avoid some aberrant emergencies caused by gene mutation or undue drug treatment seems to be of importance.
The specific mammalian target of rapamycin (mTOR) inhibitors are widely used as immunosuppressive drugs in transplantation. Recent studies have demonstrated the important status of mTOR in innate immune cell homeostasis and inflammatory functions.6-12 However, the role of mTOR in monocyte/macrophage development and homeostasis needs to be explored. We employed here rapamycin (RPM)-treated severe combined immunodeficiency (SCID) mice, ER-mTOR knockout (KO) mice, and Lyzs-mTORKO mice to investigate the role of mTOR in monocyte/macrophage development in the steady state. We found that RPM treatment significantly blocked the early development of monocytes in an intrinsic manner. The inhibitory effects of RPM on monocyte development should result in caution during RPM use in clinics.
Materials and methods
Detailed information for the materials and methods is provided in supplemental Materials, available on the Blood Web site.
Mice
C57BL/6 (B6), CD45.1 mice, and SCID mice were purchased from the Beijing Laboratory Animal Research Center (Beijing, China). The myeloid-specific mTOR conditional knockout mice (LyzsCre-mTORloxp/loxp, Lyzs-mTORKO mice) and tamoxifen-induced mTOR knockout mice (ER-Cre-mTORloxp/loxp, ER-mTORKO mice) were obtained by cross-breeding. All animal experiments were performed according to the institutional guidelines and approved by the Animal Ethics Committee of the Institute of Zoology, Beijing, China.
Cell isolation and flow cytometry analysis
Bone marrow cells, splenocytes, peripheral white blood cells, and peritoneal cavity macrophages were obtained as described previously.12,13 For flow cytometric analysis of surface markers, cells were stained with antibodies in phosphate-buffered saline containing 0.1% bovine serum albumin and 0.1% NaN3.14 All the monoclonal antibodies (mAbs) were purchased from eBioscience, BioLegend, or BD Pharmingen. The stained samples were analyzed by Beckman Coulter Epics XL benchtop FCM (Beckman Coulter) with FCS software.
Mixed bone marrow chimeras
Bone marrow cells (BMCs) from the in vivo tamoxifen-treated WT or ER-mTOR KO (CD45.2+) mice were mixed with cells from competitor (CD45.1+) mice at a 1:1 ratio and transferred to the mixed cells, totaling 1 to 2 × 107 cells, into lethally irradiated congenic (CD45.1+) mice, as described previously.15
Progenitor purification and cultures
Bone marrow was prepared as described before.13
Methylcellulose colony-forming assays
Lin−sca-1−c-kit+CD34hiCD16/32+ 50 000 granulocyte-monocyte progenitors (GMPs) were sorted and placed in MethoCult M3134 supplemented with 50 ng/mL stem cell factor (SCF), 10 ng/mL interleukin 6 (IL-6), 10 ng/mL IL-3, 20 ng/mL macrophage colony-stimulating factor (M-CSF), and 10 μg/mL insulin (StemCell Technologies). Colonies were counted and analyzed after 5 to 7 days of culture, as described.16
RNA extraction and quantitative polymerase chain reaction analysis
Total RNA was isolated with TRIzol (Invitrogen, Carlsbad, CA) or E.Z.N.A. total RNA kit I (#R6834), including the contaminating DNA process through RNase-free DNase digestion. The primers used in the present study are listed in supplemental Table 1.
Protein extraction and western blotting
Next, 1 × 106 purified Lin−sca-1−c-kit+CD34hiCD16/32+ GMPs were collected and resuspended in 100 µL RIPA lysis buffer (50 mM Tris–HCl pH 7.4, 1% NP-40, 0.25% Na-deoxycholate, 150 mM NaCl, 1 mM EDTA pH 7.4) with the protease inhibitor and phosphatase inhibitor set (Sigma). The samples were used for western blot analysis, as previously reported.12
Glucose uptake assay
Glucose uptake by macrophages was determined by the glucose uptake fluorometric assay kit (MAK084, Sigma-Aldrich).
Dual-luciferase assays
293T cells were incubated for 18 hours and transfected at 70% to 80% confluence in a 24-well plate. pGL3-basic with different CD115 promoter fragments, pcDNA3.1-IRF8 and pRL-TK (30:20:1.5), were cotransfected into cells by lipofectamine 3000 Reagent (Thermo Fisher Scientific), as indicated. Luciferase activities were normalized to renilla luciferase activities (Firefly luc/Renilla luc). Each transfection was performed in duplicate.
Results
RPM significantly decreases macrophages in mice
To address the effect of RPM on macrophage development, we employed the T-/B-cell-deficient SCID mice. RPM treatment significantly decreased the levels of the tissue residence CD11b+F4/80+macrophages in the peritoneal cavity, spleen, colon, and pulmonary alveoli (P < .001; supplemental Figure 1). In contrast, the levels of CD11b+Ly6G+ neutrophils were increased in the tissues of SCID mice after RPM treatment (supplemental Figure 2). We also detected whether RPM had similar effects on macrophages in immunocompetent B6 mice. As shown in supplemental Figure 3, the percentages and cell numbers of CD11b+F4/80+ and CD11c+F4/80+ macrophages in colon and pulmonary alveoli of RPM-administrated B6 mice reduced remarkably. RPM also decreased the cell numbers of CD11b+/hiF4/80+/low macrophages in peritoneal cavity and spleen. Therefore, RPM treatment selectively decreased the physiological levels of macrophage populations in mice.
The intrinsic effects of mTOR on monocyte/macrophage development
To determine the mTOR regulation in monocyte/macrophage development, we employed the mTOR-deleted mice by crossing mTORflox/flox mice with ER-Cre mice to generate ER-Cre mTORflox/flox mice (ER-mTORKO) (supplemental Figure 4A). We treated both WT and ER-mTORKO mice with tamoxifen for 4 days and then rested the mice for 2 to 5 days. Such treatment resulted in efficient mTOR deletion in splenic macrophages, peritoneal cavity macrophages, progenitors, bone marrow-derived monocytes, and splenic neutrophils of ER-mTORKO mice (supplemental Figure 4B-C). Compared with tamoxifen-treated WT mice, tamoxifen-treated ER-mTORKO mice showed reduced levels of CD11b+/highF4/80+ macrophages in the peritoneal cavity, spleen, and colon (P < .01; Figure 1A-C). It has been demonstrated that tissue-resident macrophages can derive from yolk sac macrophages and adult bone marrow monocytes.17 Schulz et al showed that 1 macrophage subpopulation is the bone marrow-derived CD11bhighF4/80low macrophages, and another is originated from yolk sac, identified as the CD11blowF4/80bright macrophages.18 In line with this standard, 2 macrophage populations were detected in the spleen of both WT and ER-mTORKO mice, and the levels of the yolk sac-originated CD11blowF4/80high population did not show detectable alteration in spleens of ER-mTORKO mice, although significantly decreased CD11bhighF4/80lowcells were observed in ER-mTORKO mice (P < .01; Figure 1A-C). These results suggest that short-term mTOR deficiency after birth decreases the CD11bhighF4/80low macrophage development from the hemopoietic stem cells (HSCs), but had no detectable effects on the yolk sac-derived CD11blowF4/80bright macrophages. In addition, the cell numbers of other immune cells were identical in WT and mTOR-deficient mice (supplemental Figure 5). Therefore, mTOR plays an important role in maintaining macrophage development that originated from bone marrow after birth.
Loss of mTOR intrinsically disrupts macrophage development in mice. WT and ER-mTOR KO mice were injected intraperitoneally with tamoxifen for 4 days and then rested for 2 to 5 days for analysis. (A) Cells of spleen, peritoneal cavity, and colon from tamoxifen-treated WT and ER-mTOR KO mice were stained with anti-CD11b mAb and anti-F4/80 mAb and analyzed by flow cytometry. The percentages (B) and cell numbers (C) of CD11b+F4/80+ cells in peritoneal cavity, spleen, and colon from tamoxifen-treated WT and ER-mTOR KO mice were summarized. (D) Mixed chimeric mice were generated by adoptive transfer either CD45.2+ WT or ER-mTOR KO BMCs mixed with CD45.1+ WT BMCs at the ratio of 1:1 into lethally irradiated CD45.1+ recipient mice. (E) The staining patterns of CD45.1+ and CD45.2+ CD11b+F4/80+ macrophages in mixed chimeras were shown. (F) The ratios of WT CD45.1+ and CD45.2+ WT or mTOR-deficient CD11b+F4/80+ macrophages in spleens, peritoneal cavity, and colon of mixed chimeric mice were calculated. Data are 1 representative of 3 independent experiments. *P < .05; **P < .01; ***P < .001 compared with WT control.
Loss of mTOR intrinsically disrupts macrophage development in mice. WT and ER-mTOR KO mice were injected intraperitoneally with tamoxifen for 4 days and then rested for 2 to 5 days for analysis. (A) Cells of spleen, peritoneal cavity, and colon from tamoxifen-treated WT and ER-mTOR KO mice were stained with anti-CD11b mAb and anti-F4/80 mAb and analyzed by flow cytometry. The percentages (B) and cell numbers (C) of CD11b+F4/80+ cells in peritoneal cavity, spleen, and colon from tamoxifen-treated WT and ER-mTOR KO mice were summarized. (D) Mixed chimeric mice were generated by adoptive transfer either CD45.2+ WT or ER-mTOR KO BMCs mixed with CD45.1+ WT BMCs at the ratio of 1:1 into lethally irradiated CD45.1+ recipient mice. (E) The staining patterns of CD45.1+ and CD45.2+ CD11b+F4/80+ macrophages in mixed chimeras were shown. (F) The ratios of WT CD45.1+ and CD45.2+ WT or mTOR-deficient CD11b+F4/80+ macrophages in spleens, peritoneal cavity, and colon of mixed chimeric mice were calculated. Data are 1 representative of 3 independent experiments. *P < .05; **P < .01; ***P < .001 compared with WT control.
To address whether the reduction of macrophages in ER-mTORKO mice was an intrinsic defect, we generated mixed chimera mice. The recipients (CD45.1+) were lethally irradiated and reconstituted with tamoxifen-treated CD45.2+WT (CD45.2+) or ER-mTORKO BMCs (CD45.2+) along with WT BMCs (CD45.1+) at a 1:1 ratio (Figure 1D). About 3 months after reconstitution, we analyzed the contributions of CD45.2+ WT and mTORKO BMCs to various immune compartments. CD11b+F4/80+ macrophages in the spleen, peritoneal cavity, and colon derived from CD45.2+ mTOR-deficient donor cells were greatly reduced compared with the CD45.2+ WT cells (Figure 1E-F). Meanwhile, other cell populations were comparable in these mixed chimeras, regardless of whether donor BMCs were deficient of mTOR or not (supplemental Figure 6). Thus, deficiency of mTOR selectively affected monocyte/macrophage development and maintenance in a cell-autonomous manner.
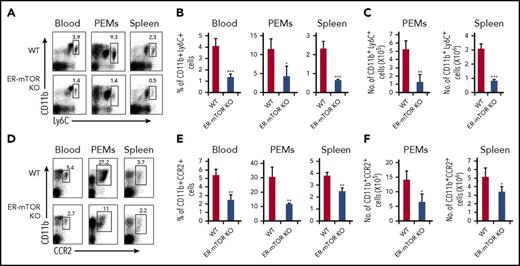
HSCs colonize the bone marrow from embryonic day 17.5 onward and produced bone marrow monocytes that seed the blood continuously throughout life.3 In a classical viewpoint, bone marrow monocytes (Ly6Chigh monocytes) are considered as classic monocytes that can be recruited to inflammatory sites and are the precursors differentiating into tissue mononuclear phagocytes.19,20 At the steady state, Ly6Chighmonocytes originated from bone marrow HSCs and required CCR2 for the exit into tissues, where they differentiate into macrophage-like cells.21 CCR2 expression on macrophages may be indicative of their monocyte origin.22-24 Ly6Chigh monocytes make a great contribution to tissue macrophage development and maintenance.25-30 Thus, we evaluated these subsets of monocytes in the peripheral blood, spleen, and peritoneal cavity of the tamoxifen-treated WT and ER-mTORKO mice. The levels of CD11b+Ly6Chigh and CD11b+CCR2+ monocytes were significantly reduced in ER-mTORKO mice (P < .01; Figure 2). Therefore, mTOR is essential to support monocyte/macrophage development and homeostasis in the steady state.
Loss of mTOR leads to significant reduction of monocytes in ER-mTOR mice. The cells of spleens, peritoneal cavity, and blood from tamoxifen-treated WT and ER-mTOR KO mice were stained with anti-CD11b mAb and anti-Ly6C mAb or anti-CCR2 mAbs. (A) Representative fluorescence-activated cell sorter analysis of spleens, peritoneal cavity, and blood monocytes for staining CD11b+Ly6Chi was shown. The percentages (B) and cell numbers (C) of CD11b+Ly6Chi cells in peritoneal cavity, spleens, and blood from tamoxifen-treated WT and ER-mTOR KO mice were compared. (D) Representative fluorescence-activated cell sorter analysis of spleens, peritoneal cavity, and blood monocytes for staining CD11b+CCR2+ was shown. The percentages (E) and cell numbers (F) of CD11b+CCR2+ cells in peritoneal cavity, spleen, and blood from tamoxifen-treated WT and ER-mTOR KO mice were shown. Data are representative of 5 independent experiments. *P < .05; **P < .01; ***P < .001 compared between WT and ER-mTOR KO mice.
Loss of mTOR leads to significant reduction of monocytes in ER-mTOR mice. The cells of spleens, peritoneal cavity, and blood from tamoxifen-treated WT and ER-mTOR KO mice were stained with anti-CD11b mAb and anti-Ly6C mAb or anti-CCR2 mAbs. (A) Representative fluorescence-activated cell sorter analysis of spleens, peritoneal cavity, and blood monocytes for staining CD11b+Ly6Chi was shown. The percentages (B) and cell numbers (C) of CD11b+Ly6Chi cells in peritoneal cavity, spleens, and blood from tamoxifen-treated WT and ER-mTOR KO mice were compared. (D) Representative fluorescence-activated cell sorter analysis of spleens, peritoneal cavity, and blood monocytes for staining CD11b+CCR2+ was shown. The percentages (E) and cell numbers (F) of CD11b+CCR2+ cells in peritoneal cavity, spleen, and blood from tamoxifen-treated WT and ER-mTOR KO mice were shown. Data are representative of 5 independent experiments. *P < .05; **P < .01; ***P < .001 compared between WT and ER-mTOR KO mice.
Normal development of macrophages in Lyzs-mTOR KO mice
Does mTOR deficiency at the late developing stage affect the survival and maturation of monocytes/macrophages? We observed monocyte/macrophage development in Lyzs-mTORKO mice in which mTOR mainly was deleted beyond the monocytic lineage stage. To confirm whether mTOR deletion mainly occurred in the late stage of monocytic lineage in Lyzs-mTORKO mice, we detected the biochemical function of mTOR in different development stages by examining the canonical targets of mTOR; namely, the phosphorylation of S6 by immunoblotting. Lyzs-mTORKO mice showed no decrease in S6 phosphorylation at the GMP stage (Figure 3A), but Ly6Chighmonocytes in bone marrow reduced the phosphorylation of S6 by about 60% in these mice, and macrophages in spleens downregulated the phosphorylation of S6 by almost 90%, indicating mTOR deletion in Lyzs-mTORKO mice indeed happened among the monocyte stage and afterward (Figure 3A). Surprisingly, the levels of macrophages in spleens and pulmonary alveoli of the Lyz-mTORKO mice were similar to those seen in WT mice, except for the slight decrease in peritoneal cavity and increase in the colon (Figure 3B-D). Furthermore, CD11b+Ly6Chigh monocytes in the peritoneal cavity, spleen, and blood in Lyzs-mTORKO mice were not significantly altered (supplemental Figure 7). CD11b+CCR2+ monocytes were also not impaired in Lyzs-mTORKO mice (data not shown). Furthermore, when the sorted CD11b+CD115+Ly6C+ monocytes and the CD11b+CD115+Ly6C− monocytes from the bone marrow of WT and Lyz-mTORKO mice were cultured in the presence of M-CSF for 3 days, the percentages and cell numbers of the CD11b+F4/80+ macrophages developed from monocytes of Lyzs-mTORKO mice were comparable to those of WT cells (supplemental Figure 8). Therefore, mTOR deletion at the late developmental stage of monocytes makes no detectable contribution to the monocyte maintenance and the differentiation potentiality to macrophages.
Lyzs-mTOR KO mice show normal percentage and cell number of macrophages. (A) GMPs, monocytes, neutrophils, and macrophages were sorted from bone marrow, spleens, and the peritoneal cavity of WT and Lyzs-mTOR mice, respectively; p-S6 expression was analyzed by western blot. (B) Representative fluorescence-activated cell sorter analysis of spleens, peritoneal cavity, and colon macrophages for staining CD11b+F4/80+ was shown. The percentages (C) and cell numbers (D) of CD11b+F4/80+ cells in peritoneal cavity, spleen, and colon from WT and Lyzs-mTOR KO mice were assayed. Data are representative of 3 independent experiments.
Lyzs-mTOR KO mice show normal percentage and cell number of macrophages. (A) GMPs, monocytes, neutrophils, and macrophages were sorted from bone marrow, spleens, and the peritoneal cavity of WT and Lyzs-mTOR mice, respectively; p-S6 expression was analyzed by western blot. (B) Representative fluorescence-activated cell sorter analysis of spleens, peritoneal cavity, and colon macrophages for staining CD11b+F4/80+ was shown. The percentages (C) and cell numbers (D) of CD11b+F4/80+ cells in peritoneal cavity, spleen, and colon from WT and Lyzs-mTOR KO mice were assayed. Data are representative of 3 independent experiments.
mTOR masters the differentiation into monocytes/macrophages of GMPs
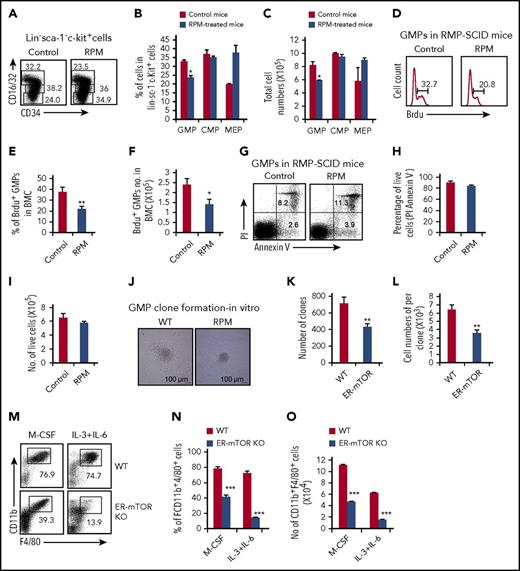
Does mTOR regulate monocyte/macrophage development by affecting the generation and differentiating capacity of their progenitors? After SCID mice were treated with RPM for 3 weeks, we detected levels of megakaryocyte-erythroid progenitors (CD34lowCD16/32low), CMPs (CD34highCD16/32low), and GMPs (CD34highCD16/32high), according to the FcγRII/III (CD16/32) and CD34 expression levels in that gated Lin−Sca-1−c-Kit+ cells by multiple flow cytometry. No significant cell percentage and cell number changes of CMPs were disclosed in the bone marrow of RPM-treated SCID mice compared with control mice, but GMPs slightly but significantly decreased in RPM-treated mice (P < .05; Figure 4A-C). The effect of mTOR deficiency on the various myeloid progenitors was also observed in the tamoxifen-treated ER-mTORKO mice. The levels of CMPs and megakaryocyte-erythroid progenitors were unaffected, even though GMPs were significantly decreased in ER-mTORKO mice after tamoxifen treatment (supplemental Figure 9). The percentages and cell numbers of the Lin−Sca-1+c-kit+ population increased and the percentage of CD150+CD48−HSCs did not change in ER-mTORKO mice with short-term mTOR deficiency, but the cell number of CD150+CD48− HSCs decreased slightly (supplemental Figure 10). Thus, mTOR inhibition predominately impaired the GMP stage.
mTOR deletion decreased GMPs and inhibits the ability to differentiate into monocytes/macrophages. (A) Myeloid progenitor subpopulations of SCID mice with or without RPM treatment were detected by flow cytometry. Plots shown were the gated Lin−sca-1−c-kit+ cells. The percentages (B) and the absolute cell numbers (C) of myeloid progenitor cell populations in the bone marrow of control and RPM-treated mice were summarized (mean ± SD; n = 6 mice each group). (D) BrdU incorporation in bone marrow-derived GMPs was detected by flow cytometry 24 hours after injection of BrdU. The percentages (E) and the cell numbers (F) of BrdU+ GMPs were shown. Data are representative of 3 independent experiments. (G) GMPs of control and RPM-treated SCID bone marrow were stained with anti-annexin-V and propidium iodide by flow cytometry analysis. The percentage (H) and cell number (I) of the alive cells were shown (mean ± SD; n = 3 mice). (J) A total of 5 × 104 GMPs purified from control and RPM-treated mice and were seeded in the methylcellulose supplemented with IL-3, IL-6, SCF, and M-CSF. The assays were performed in triplicate and were photographed as the pictures presented. The colonies (K) and the macrophage cell number per colony (L) were calculated. Data are representative of 3 independent experiments. (M) GMPs sorted from tamoxifen-treated WT and ER-mTOR KO mice were cultured in the presence of M-CSF for 5 days in vitro and stained with anti-CD11b mAb and anti-F4/80 mAb. Then the phenotypes of the samples were analyzed by flow cytometry, as indicated. The decreased percentages (N) and the cell numbers (O) of CD11b+F4/80+ macrophages in mTOR-deficient GMPs were shown. Data are representative of 3 independent experiments. Data were expressed as mean ± SD. Three independent experiments with similar results were performed. *P < .05; **P < .01; ***P < .001 (WT vs ER-mTOR).
mTOR deletion decreased GMPs and inhibits the ability to differentiate into monocytes/macrophages. (A) Myeloid progenitor subpopulations of SCID mice with or without RPM treatment were detected by flow cytometry. Plots shown were the gated Lin−sca-1−c-kit+ cells. The percentages (B) and the absolute cell numbers (C) of myeloid progenitor cell populations in the bone marrow of control and RPM-treated mice were summarized (mean ± SD; n = 6 mice each group). (D) BrdU incorporation in bone marrow-derived GMPs was detected by flow cytometry 24 hours after injection of BrdU. The percentages (E) and the cell numbers (F) of BrdU+ GMPs were shown. Data are representative of 3 independent experiments. (G) GMPs of control and RPM-treated SCID bone marrow were stained with anti-annexin-V and propidium iodide by flow cytometry analysis. The percentage (H) and cell number (I) of the alive cells were shown (mean ± SD; n = 3 mice). (J) A total of 5 × 104 GMPs purified from control and RPM-treated mice and were seeded in the methylcellulose supplemented with IL-3, IL-6, SCF, and M-CSF. The assays were performed in triplicate and were photographed as the pictures presented. The colonies (K) and the macrophage cell number per colony (L) were calculated. Data are representative of 3 independent experiments. (M) GMPs sorted from tamoxifen-treated WT and ER-mTOR KO mice were cultured in the presence of M-CSF for 5 days in vitro and stained with anti-CD11b mAb and anti-F4/80 mAb. Then the phenotypes of the samples were analyzed by flow cytometry, as indicated. The decreased percentages (N) and the cell numbers (O) of CD11b+F4/80+ macrophages in mTOR-deficient GMPs were shown. Data are representative of 3 independent experiments. Data were expressed as mean ± SD. Three independent experiments with similar results were performed. *P < .05; **P < .01; ***P < .001 (WT vs ER-mTOR).
mTOR is crucial for regulating apoptosis and proliferation. We performed BrdU incorporation studies to exhibit the proliferation ability of GMPs after the RPM treatment of 23 days. The levels of BrdU+ GMPs were significantly lower in the bone marrow of RPM-treated mice (Figure 4D-F). We also dissected GMP survival by PI and Annexin V staining. The RPM treatment failed to show significant effects on the survival of GMPs, as indicated by the alive PI−Annexin V− cell population (Figure 4G-I). Thus, mTOR is mainly involved in the regulation of GMP proliferation, but not cell survival.
To test the possibility that mTOR may obstruct the differentiation process of GMPs into monocytes/macrophages, we performed the standard methylcellulose culture analysis. Fluorescence-activated cell sorter data for sorting Lin−Sca-1−c-Kit+CD34hiCD16/32hi GMPs were shown in supplemental Figure 11A. The isolated Lin−Sca-1−c-Kit+CD34hiCD16/32hiGMPs from RPM-treated SCID mice generated significantly smaller colonies than WT GMPs in methylcellulose cultures with M-CSF, SCF, IL-6, and IL-3 (Figure 4J). Importantly, when we analyzed the developmental capacity of purified Lin−Sca-1−c-Kit+CD34hiCD16/32hi GMPs treated with M-CSF alone in a standard CFU assay, the colony numbers and the cell numbers per colony formed from GMPs isolated from RPM-treated SCID mice were significantly lower than GMPs of the control SCID mice (Figure 4K-L). Furthermore, we cultured the purified GMPs from WT mice with the macrophage directional system (M-CSF) and nondirectional system (IL-6+IL-3) in the presence and absence of RPM. The generation of macrophages from GMPs was remarkably lower in the presence of RPM than the generation of macrophages derived from the control cells (data not shown). In addition, we also isolated GMPs from the tamoxifen-treated WT and ER-mTORKO mice and cultured those cells with M-CSF or IL-6+IL-3 for 5 days. In contrast to WT cells, the differentiation ability of the ER-mTORKO GMPs into macrophages was significantly impaired, as indicated by the significantly decreased levels of CD11b+F4/80+ cells (Figure 4M-O). To circumvent the detrimental role of mTOR loss on the function of GMPs, we determined the requirement of mTOR for the macrophage development of GMPs by culturing the purified GMPs from tamoxifen-untreated WT or ER-mTOR KO mice with M-CSF or IL-6+IL-3 in the presence of 4-OHT, which resulted in the efficient deletion of mTOR in ER-mTOR KO GMPs after the treatment (data not shown). Under the inducing systems of GMPs differentiating into macrophages, significantly lower levels of CD11b+F4/80+ macrophages were also observed in the culture of ER-mTORKO GMPs than in the culture of WT GMPs (supplemental Figure 11B-D). We treated the GMPs in the macrophage induction system with 4-OHT at different points and observed that mTOR deficiency at a late time did not affect macrophage generation in vitro (supplemental Figure 12). Thus, mTOR deficiency and RPM treatment directly inhibit the differentiation capacity into monocytes/macrophages of GMPs at the early stage. To determine the differentiation potentiality of mTOR-deficient GMPs into granulocytic lineage, we also performed methylcellulose culture assays to analyze neutrophil development from mTOR-deficient progenitors. We found that the isolated Lin−Sca-1−c-Kit+CD34hiCD16/32hi GMPs from ER-mTORKO mice generated smaller colonies and cell numbers than WT GMPs in methylcellulose cultures with M-CSF, SCF, IL-6, and IL-3 (supplemental Figure 13A-C). However, the colonies of CD11b+Ly6G+neutrophils from ER-mTORKO GMPs were notably higher than WT GMPs (supplemental Figure 13D-F). The cell numbers of CD11b+Ly6G+ neutrophils from ER-mTORKO GMPs were comparable with WT (supplemental Figure 3G). These data indicated that mTOR mainly affects macrophage development.
mTOR controls CD115 expression during monocyte/macrophage commitment
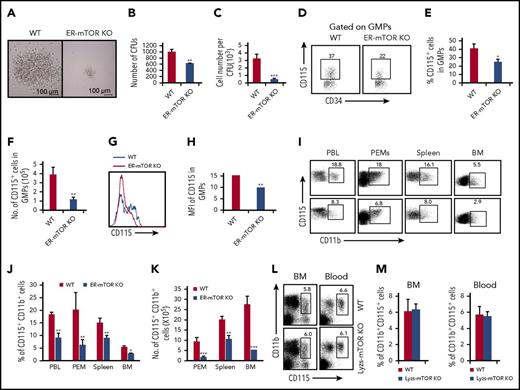
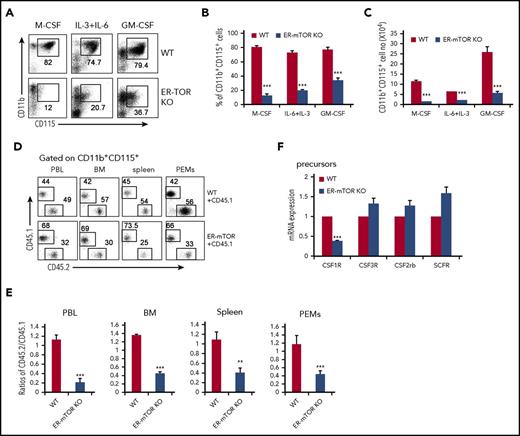
The GMPs isolated from ER-mTORKO mice had impaired potential to differentiate into monocytes/macrophages in the methylcellulose culture analysis (Figure 5A-C) as RPM-treated WT GMPs (Figure 4). The levels of the CD115+ progenitors were lower in GMPs of ER-mTORKO mice than those in WT mice (Figure 5D-F). Notably, the key M-CSF receptor CSF1R (CD115) expression on the GMPs of ER-mTORKO mice showed a significant reduction (P < .01; Figure 5G-H). Consistently, the levels of CD11b+CD115+ cells in the blood, peritoneal cavity, spleen, and bone marrow were greatly reduced in the ER-mTORKO mice (Figure 5I-K). However, no significant alterations of the percentages of CD11b+CD115+ cells were detected in the bone marrow and blood of lyzs-mTORKO mice (Figure 5L-M). Thus, mTOR deficiency mainly caused a CD115 expression defect in the progenitor stage.
mTOR deletion inhibits the ability of progenitors to differentiate into monocytes/macrophages. (A) 5 × 104 GMPs purified from tamoxifen-treated WT and ER-mTOR KO mice were seeded in methylcellulose supplemented with IL-3, IL-6, SCF, and M-CSF. The experiment was performed in triplicate and was photographed as the pictures presented. Scale bars represent 100 µm. The generated colonies (B) and the macrophage cell number per colony (C) were calculated. Data are representative of 3 independent experiments. (D) The CD115 expression on progenitors of tamoxifen-treated WT and ER-mTOR KO mice was examined by flow cytometry. Plots shown were the gated Lin−sca-1−c-kit+ cells. The cell percentages (E) and cell numbers (F) of Lin−sca-1−c-kit−CD34+CD115+ cells in WT and ER-mTOR KO bone marrow were detected. (G) The CD115 molecule expression were shown. (H) The MFI of CD115 expression was counted. (I) The CD115 expression in blood, peritoneal cavity, spleen, and bone marrow in WT and ER-mTOR KO mice was examined by flow cytometry. The decreased percentages (J) and cell numbers (K) of CD115+ cells in blood, peritoneal cavity, spleen, and bone marrow in WT and ER-mTOR KO mice were observed. (L) The CD115 expression on CD11b+ cells of WT and Lyzs-mTOR KO mice was examined by flow cytometry. (M) The normal percentages of CD115+ cells in blood and bone marrow in WT and Lyzs-mTOR KO mice were observed. Each group represents 3 independent experiments. Data were expressed as mean ± SD (n = 6). *P < .05; **P < .01; ***P < .001 compared with WT control mice.
mTOR deletion inhibits the ability of progenitors to differentiate into monocytes/macrophages. (A) 5 × 104 GMPs purified from tamoxifen-treated WT and ER-mTOR KO mice were seeded in methylcellulose supplemented with IL-3, IL-6, SCF, and M-CSF. The experiment was performed in triplicate and was photographed as the pictures presented. Scale bars represent 100 µm. The generated colonies (B) and the macrophage cell number per colony (C) were calculated. Data are representative of 3 independent experiments. (D) The CD115 expression on progenitors of tamoxifen-treated WT and ER-mTOR KO mice was examined by flow cytometry. Plots shown were the gated Lin−sca-1−c-kit+ cells. The cell percentages (E) and cell numbers (F) of Lin−sca-1−c-kit−CD34+CD115+ cells in WT and ER-mTOR KO bone marrow were detected. (G) The CD115 molecule expression were shown. (H) The MFI of CD115 expression was counted. (I) The CD115 expression in blood, peritoneal cavity, spleen, and bone marrow in WT and ER-mTOR KO mice was examined by flow cytometry. The decreased percentages (J) and cell numbers (K) of CD115+ cells in blood, peritoneal cavity, spleen, and bone marrow in WT and ER-mTOR KO mice were observed. (L) The CD115 expression on CD11b+ cells of WT and Lyzs-mTOR KO mice was examined by flow cytometry. (M) The normal percentages of CD115+ cells in blood and bone marrow in WT and Lyzs-mTOR KO mice were observed. Each group represents 3 independent experiments. Data were expressed as mean ± SD (n = 6). *P < .05; **P < .01; ***P < .001 compared with WT control mice.
To clarify whether mTOR plays an autonomous role in regulating CD115 expression, we detected CD115 expression during the culture of GMPs. Under the 3 culture conditions, the levels of CD11b+CD115+ cells derived from ER-mTORKO GMPs were significantly reduced (Figure 6A-C). Furthermore, when we cultured the purified GMPs of tamoxifen-untreated WT or ER-mTORKO mice with M-CSF or IL-6+IL-3 in the presence of 4-OHT or cultured the WT GMPs with RPM, the mTOR deficient GMPs showed an obvious deficiency to differentiate into CD11b+CD115+ cells in ER-mTORKO GMPs treated with 4-OHT or WT GMPs treated with RPM (P < .001; supplemental Figure 14). We also assessed the levels of CD115+ cells in the WT (CD45.1):ER-mTORKO (CD45.2) mixed chimera mice. The percentages of CD11b+CD115+ cells derived from ER-mTORKO bone marrow cells in the bone marrow, spleen, peritoneal cavity, and blood were all decreased by more than 50% compared with CD11b+CD115+ cells derived from WT bone marrow (P < .001; Figure 6D-E). Thus, mTOR intrinsically regulates CD115 expression during myeloid development. We then detected the mRNA expression of CD115 and some other growth factor receptors in WT or mTOR-deficient GMPs. The mRNA expression of CD115 showed a conspicuous and specific downregulation in mTOR-deficient GMPs, in contrast to the expressions of other growth factor receptors such as CSF2Rb, CSF3R, and SCFR (Figure 6F). These data indicate that mTOR intrinsically regulates CD115 expression at the transcription level.
mTOR deletion intrinsically impedes CD115 expression at the translation and transcription level. (A) GMPs sorted from tamoxifen-treated WT and ER-mTOR mice were cultured in the presence of M-CSF, IL-6+IL-3, or GM-CSF for 5 days in vitro and then collected for phenotype analysis by flow cytometry. The flow cytometry analysis of CD115 in induced cells is shown. The remarkable decreased percentages (B) and the cell numbers (C) of CD11b+CD115+ cells during the macrophage induction in vitro are shown. Results are representative of 3 independent experiments. (D) The mixed chimeric mice were generated as described in “Methods,” and the CD115+ cells in blood, bone marrow, spleen, and peritoneal cavity were detected. (E) The CD115+ cell compartment in blood, bone marrow, spleen, and peritoneal cavity of WT and ER-mTOR KO bone marrow cells was presented. Data are representative of 3 independent experiments. Data are mean ± SD (n = 5). (F) GMPs sorted from tamoxifen-treated WT and ER-mTOR mice were lysed for RNA extraction and analyzed for the transcriptional factor expression of CSF1R, CSF3R, CSF2rb, and SCF. Three mice in each group were assayed. **P < .01; ***P < .001 compared with WT control.
mTOR deletion intrinsically impedes CD115 expression at the translation and transcription level. (A) GMPs sorted from tamoxifen-treated WT and ER-mTOR mice were cultured in the presence of M-CSF, IL-6+IL-3, or GM-CSF for 5 days in vitro and then collected for phenotype analysis by flow cytometry. The flow cytometry analysis of CD115 in induced cells is shown. The remarkable decreased percentages (B) and the cell numbers (C) of CD11b+CD115+ cells during the macrophage induction in vitro are shown. Results are representative of 3 independent experiments. (D) The mixed chimeric mice were generated as described in “Methods,” and the CD115+ cells in blood, bone marrow, spleen, and peritoneal cavity were detected. (E) The CD115+ cell compartment in blood, bone marrow, spleen, and peritoneal cavity of WT and ER-mTOR KO bone marrow cells was presented. Data are representative of 3 independent experiments. Data are mean ± SD (n = 5). (F) GMPs sorted from tamoxifen-treated WT and ER-mTOR mice were lysed for RNA extraction and analyzed for the transcriptional factor expression of CSF1R, CSF3R, CSF2rb, and SCF. Three mice in each group were assayed. **P < .01; ***P < .001 compared with WT control.
mTOR controls monocyte/macrophage development through STAT5/IRF8
To clarify whether the altered glycolysis by the mTOR deficiency contributes to the impaired monocyte/macrophage differentiation, we measured the mRNA expression of glycolytic enzymes, including lactate dehydrogenase-α, glucose transporter 1, hexokinase-1, phosphofructokinase-1, and pyruvate kinase muscle isozyme 2, as well as c-Myc, an established regulator of cell metabolism, especially glycolytic activity.31 As expected, all these gene expressions were decreased in mTOR-deficient GMPs (supplemental Figure 15A). To verify the role of glycolysis in the monocyte/macrophage development from GMPs, we directly added 5-aminoimidazole-4-carboxamide1-β-d-ribofuranoside (AICAR) and metformin, which increase the glucose uptake and glycolysis through 5′-AMP-activated protein kinase-dependent and independent pathways,32-35 into the culture system of WT and mTOR-deficient GMPs in the presence of IL-6+IL-3 or M-CSF in vitro. However, both AICAR and metformin failed to rescue the poor monocyte/macrophage development of mTOR-deficient GMPs, although AICAR could somewhat enhance the developmental ability of WT GMPs (supplemental Figure 15B-G). Meanwhile, AICAR and metformin partially rescued the reduced glycolysis caused by mTOR deficiency under the condition of no effect on the mTOR downstream signal (supplemental Figure 16). Therefore, the altered cell metabolism in mTOR-deficient GMPs may not significantly contribute to the development defect of GMPs.
The autophagy, which plays key roles in cellular homeostasis, development, differentiation, cell survival, and aging, can be suppressed by AKT/mTOR pathway.36-39 In accordance with the reported observations in other cells, the mTOR deficiency increased the occurrence of autophagy, as indicated by the expression of ILC3-II, a hallmark of autophagy (supplemental Figure 17A). However, the autophagy inhibitor, 3-methyladenine, failed to significantly rescue the poor monocyte/macrophage development and the decreased CD115 expression of mTOR-deficient GMPs (supplemental Figure 17B-G). But 3-methyladenine treatment decreased the monocyte/macrophage development of WT GMPs, which is in line with the published study showing that autophagy is required for CSF-1-induced macrophage differentiation.40 We thus conclude that changed autophagy activity is not the key reason for the poor monocyte/macrophage development of mTOR-deficient GMPs.
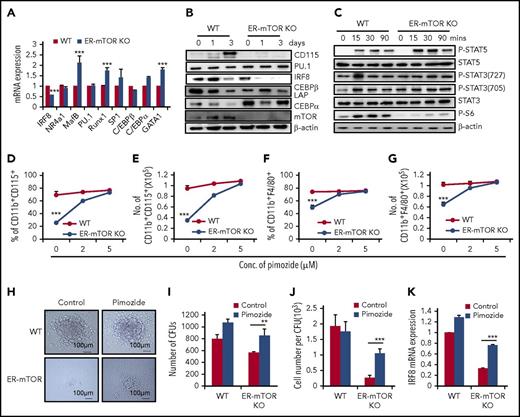
To explore the molecular mechanisms that mediate the regulation of mTOR in monocyte/macrophage differentiation, we accessed the transcription factors that are important for monocyte/macrophage development and the potential regulators of CD115 transcription, such as interferon regulatory factor-8 (IRF8), nuclear receptor subfamily 4 group A member 1, CCR2, MAF BZIP transcription factor B, spleen focus forming virus proviral integration oncogene (Spi1, PU.1), runt-related transcription factor 1, trans-acting transcription factor 1 (SP1), CCAAT (cytosine-cytosine-adenosine-adenosine-thymidine)-enhancer-binding protein α (C/EBPα), and CCAAT-enhancer-binding protein β.41-43 As shown in Figure 7, the expressions of the majority of these molecules at mRNA and/or protein levels were not or slightly changed in mTOR-deficient GMPs (Figure 7A-B). However, mTOR deficiency caused the significantly decreased IRF8 expression in GMPs (P < .01; Figure 7A). The poor IRF8 expression was observed in ER-mTORKO GMPs at different times (supplemental Figure 18A). The response of IRF8−/− progenitors to M-CSF is significantly reduced,44 which has a similar phenotype with mTOR-deficient GMPs observed in the present study.
mTOR controls monocyte/macrophage development through STAT5/IRF8. (A) GMPs sorted from tamoxifen-treated WT and ER-mTOR KO mice were lysed and analyzed for the transcriptional factor expression of IRF8, nuclear receptor subfamily 4 group A member 1, CCR2, MAF BZIP transcription factor B, PU.1, runt-related transcription factor 1, SP1, CCAAT-enhancer-binding protein β, and C/EBPα. Three mice in each group were assayed. (B) GMPs sorted from tamoxifen-treated WT and ER-mTOR KO mice were cultured in the presence of IL-3+IL-6 and collected at day 0, day 1, and day 3. Then the samples were lysed and analyzed for the translational expression of CSF1R, IRF8, PU.1, C/EBβ LAP, C/EBPα, and mTOR by immunoblotting analysis. (C) GMPs sorted from tamoxifen-treated WT and ER-mTOR KO mice were stimulated with IL-3+IL-6 at the indicated points. Then the samples were lysed and analyzed by immunoblotting for phosphorylation of STAT3, STAT5, and S6. (D) GMPs were sorted from tamoxifen-untreated WT and ER-mTOR KO mice and cultured with 4-OHT in the presence and absence of STAT5 inhibitor, pimozide. The percentage (D) and cell number (E) of CD11b+CD115+ cells were calculated. The percentage (F) and cell number (G) of CD11b+F4/80+ macrophages were also detected. (H) 5 × 104 sorted from tamoxifen-treated WT and ER-mTOR KO mice were seeded in the methylcellulose supplemented with IL-3, IL-6, SCF, M-CSF, and 4-OHT in the presence and absence of pimozide (5 µM). The experiment was performed in triplicate and was photographed as the pictures presented. Scale bars, 100 µm. The generated colonies (I) and the macrophage cell numbers per colony (J) were calculated. (K) GMPs sorted from WT and ER-mTOR mice were cultured in the presence of 4-OHT and pimozide at the indicated concentrations and analyzed for the transcriptional factor expression of IRF8. Data are shown as mean ± SD (n = 3). **P < .01; ***P < .001 compared with WT group; ***P < .001 compared with WT mice.
mTOR controls monocyte/macrophage development through STAT5/IRF8. (A) GMPs sorted from tamoxifen-treated WT and ER-mTOR KO mice were lysed and analyzed for the transcriptional factor expression of IRF8, nuclear receptor subfamily 4 group A member 1, CCR2, MAF BZIP transcription factor B, PU.1, runt-related transcription factor 1, SP1, CCAAT-enhancer-binding protein β, and C/EBPα. Three mice in each group were assayed. (B) GMPs sorted from tamoxifen-treated WT and ER-mTOR KO mice were cultured in the presence of IL-3+IL-6 and collected at day 0, day 1, and day 3. Then the samples were lysed and analyzed for the translational expression of CSF1R, IRF8, PU.1, C/EBβ LAP, C/EBPα, and mTOR by immunoblotting analysis. (C) GMPs sorted from tamoxifen-treated WT and ER-mTOR KO mice were stimulated with IL-3+IL-6 at the indicated points. Then the samples were lysed and analyzed by immunoblotting for phosphorylation of STAT3, STAT5, and S6. (D) GMPs were sorted from tamoxifen-untreated WT and ER-mTOR KO mice and cultured with 4-OHT in the presence and absence of STAT5 inhibitor, pimozide. The percentage (D) and cell number (E) of CD11b+CD115+ cells were calculated. The percentage (F) and cell number (G) of CD11b+F4/80+ macrophages were also detected. (H) 5 × 104 sorted from tamoxifen-treated WT and ER-mTOR KO mice were seeded in the methylcellulose supplemented with IL-3, IL-6, SCF, M-CSF, and 4-OHT in the presence and absence of pimozide (5 µM). The experiment was performed in triplicate and was photographed as the pictures presented. Scale bars, 100 µm. The generated colonies (I) and the macrophage cell numbers per colony (J) were calculated. (K) GMPs sorted from WT and ER-mTOR mice were cultured in the presence of 4-OHT and pimozide at the indicated concentrations and analyzed for the transcriptional factor expression of IRF8. Data are shown as mean ± SD (n = 3). **P < .01; ***P < .001 compared with WT group; ***P < .001 compared with WT mice.
We examined the phosphorylation of STAT3 (Ser727), STAT3 (Tyr705), and STAT5 (Tyr694) in the sorted Lin−Sca-1−c-Kit+CD34highCD16/32high GMPs cultured with IL-6+IL-3 by immunoblotting. mTOR deficiency did not cause the detectable change of STAT3 phosphorylation in GMPs (Figure 7C). The remarkably lower phosphorylation levels of S6, the downstream signals of mTOR, were observed in GMPs (Figure 7C), supporting the efficient deletion of mTOR and excluding the possibility that the unchanged STAT3 activation is a result of the low efficiency of mTOR knockout. However, the phosphorylation of STAT5 in mTOR-deficient GMPs was markedly higher than those of WT GMPs, as determined by western blots (Figure 7C). It has been reported that STAT5 negatively regulates DC development via directly inhibiting transcription of IRF8.45,46 Is STAT5 involved in mTOR-mediated regulation on monocyte/macrophage differentiation? To address this issue, we observed the macrophage development of WT and mTOR-deficient GMPs in the presence and absence of STAT5-specific inhibitor (pimozide),47,48 which indeed inhibited STAT5 phosphorylation (supplemental Figure 18B) and did not inhibit GMP proliferation (supplemental Figure 19A-C). The pimozide treatment significantly reversed the decreased percentages and numbers of CD11b+CD115+ cells and CD11b+F4/80+ macrophages developed from the mTOR-deficient GMPs in a dose-dependent manner (Figure 7D-G). Pimozide rescued the decreased colonies and cell numbers of macrophages developed from mTOR-deficient GMPs in methylcellulose cultures with M-CSF, IL-6, IL-3, and 4-OHT (Figure 7H-J). The rescue effect of STAT5 inhibitor on the poor differentiation of mTOR-deficient GMPs was accompanied with the elevated IRF8 expression (P < .001; Figure 7K; supplemental Figure 18B). To determine whether IRF8 could regulate CD115 transcription directly, we predicted 5 binding sites on the promoter of CD115, which may be potentially interacted with IRF8. Then we performed the luciferase experiments. As listed in supplemental Table 2, we predicted the 5 potential IRF8 binding sites in the CD115 promoter sequence range from −3336 site to +191 site. Luciferase activity results of Dual-Luciferase reporter assay (supplemental Figure 20) that the binding sites of IRF8 to positively regulate CD115 expression were at −1186∼+119 and −3336∼−2892 upstream, but there may exist the inhibitory transcription elements in −2892∼−1186 upstream of CD115 promoter. Therefore, IRF8 plays a regulatory role in CD115 transcriptional expression.
Discussion
The defect of bone marrow monocyte/macrophage differentiation caused by mTOR deficiency was evidenced by our systemic analysis with various mouse models, including tamoxifen-induced ER-mTORKO mice, mixed chimera, and the in vitro assays of the colony formation and the macrophage induction of the purified GMPs. Both mice with tamoxifen-induced mTOR deletion and mice with WT and mTOR-deficient bone marrow mixed chimeras showed remarkable reduction of monocytes/macrophages without detectable influence on other immune cells, such as neutrophils and lymphocytes. The mTOR-deleted GMPs differentiated into monocytes/macrophages at a lower efficiency compared with WT GMPs. Thus, mTOR specifically delivers a myeloid-specific intrinsic signal in promoting bone marrow monocyte/macrophage differentiation.
GMPs showed a significant decrease in RPM-treated or mTOR-deficient mice, although megakaryocyte-erythroid progenitors and CMPs in these mice were comparable with WT mice. The percentage of CD150+CD48−HSCs in mTOR-deficient mice was comparable with WT mice, whereas the cell number of CD150+CD48− HSCs slightly decreased. These observations were consistent with those in S6K−/− mice, which were deficient in mTOR downstream signaling.49 Other published work showed that RPM could rescue HSC hyperproliferation and the defective repopulating capability observed in TSC1-deficient mice.50,51 These discrepancies are likely a result of different deletion manners and time course. Our data indicate that mTOR deficiency likely affects GMPs and, afterward, the developing stages. However, mTOR deletion from the monocyte stage onward did not show detectable influence on monocyte/macrophage maintenance and differentiation, as indicated by no significant changes of monocytes/macrophage levels in lyzs-mTORKO mice. Furthermore, the purified inflammatory monocytes (CD11b+CD115+Ly6C+) and patrolling monocytes (CD11b+CD115+Ly6C−) of Lyzs-mTORKO mice developed equal numbers of macrophages as WT cells in vitro. Thus, mTOR was involved in the early stage of developing monocytes, but it was not required for maintenance of the monocyte lineage. This speculation was supported by the observation that mTOR-deficient GMPs showed poor differentiation ability into the monocyte/macrophage lineage in several induction culture systems. Therefore, we come to the conclusion that mTOR plays a direct and crucial role in the monocyte/macrophage differentiation process predominately among GMPs and forward stages, but before the monocyte stage.
CD115 mainly expresses in the mononuclear phagocytes, and M-CSF/CD115 signaling is important for the differentiation, proliferation, and maintenance of the monocytes/macrophages.52,53 CD115-deficient mice showed the defect in tissue macrophages.54,55 We found that CD115 mRNA and protein expression in mTOR-deficient or RPM-treated GMPs was significantly lower than WT cells, as determined by real-time polymerase chain reaction and western blots. The poor CD115 expression on mTOR-deficient GMPs and afterward stages was observed in the in vitro culture systems and the mixed chimera mice. We thus concluded that mTOR plays an intrinsic role in regulating CD115 expression among GMP stage.
The mTOR pathway has enormous effects on cell metabolism, autophagy, proliferation, and apoptosis. However, GMPs and monocytes/macrophages of RPM-treated mice failed to show a significant decrease in cell survival in vitro, which is in line with the published report that RPM does not induce the apoptosis in human monocytes/macrophages.56 In addition, we found that blocking the autophagy resulted in the impaired differentiation of the macrophages as reported,40 but it failed to rescue the decreased differentiation of mTOR-deficient GMPs. The poor differentiation to macrophages of mTOR-deficient GMPs was not significantly ameliorated by elevating the glucose uptake and glycolysis, excluding the possibility that the cell glucose metabolism mediated by mTOR plays a role in monocyte/macrophage differentiation. Therefore, mTOR-related cell metabolism, autophagy, and apoptosis do not greatly contribute to the monocyte/macrophage differentiation from GMPs.
IRF8 expression significantly decreased in mTOR-deficient GMPs at the transcription and translation levels. IRF8 has a strong commitment to the monocytic lineage and is mainly associated with the essential genes for macrophage differentiation.57-59 IRF8−/− mice developed immunodeficiency.60,61 It is well known that IRF8 is required for the generation and maturation of monocytes, particularly Ly6Chighmonocytes.58,59,62 Sichien et al recently revealed that IRF8 is essential in early monocyte development, but not in differentiated Ly6Chighmonocytes.63 It was also reported that IRF8 favors the early myeloid progenitors and is downregulated gradually during monocyte/macrophage differentiation.59,63 On the basis of our data and other studies, we believe that mTOR likely regulates monocyte/macrophage development via IRF8. Accompanying the decreased IRF8 expression, the activity of STAT5 was remarkably enhanced in mTOR-deficient GMPs during the in vitro monocyte induction, as indicated by the increased phosphorylation of STAT5. Importantly, blocking STAT5 activity by specific inhibitor pimozide significantly increased IRF8 and CD115 expressions and rescued the poor differentiation of mTOR-deficient GMPs in the colony formation system or the macrophage induction system. The increased IRF8 expression by the STAT5 inhibitor nicely agrees with the reports showing that STAT5 is directly upstream of IRF8 and negatively controls IRF8 expression at the transcriptional level.45,64,65 Our research and other work showed that CD115 is expressed at low levels on early myeloid progenitors and increased gradually as monocytic lineage differentiates.66-68 It is reported that CD115 expression is positively correlated with IRF8 expression.64 Overexpression of IRF8 significantly promoted CD115 mRNA expression in IRF8-transduced tot2 cells, and the regulatory role of IRF8 in monocyte/macrophage development has been observed.58,62 Our present study showed that inhibiting STAT5 activity also increased CD115 expressions during GMP differentiation. Furthermore, the luciferase experiments demonstrated that IRF8 could directly regulate CD115 transcriptional expression. Thus, mTOR masters CD115 expression and monocyte/macrophage development via the STAT5/IRF8 pathway.
In conclusion, our work unravels the important role of mTOR in monocyte/macrophage development in the early GMP stages, but not in the terminal differentiation and survival of monocytes/macrophages. mTOR controls the developing process of GMPs into monocytes/macrophages via inhibiting STAT5 activity and subsequently increasing IRF8 and CD115 expression (supplemental Figure 21). Our findings shed light on the molecular mechanism of mTOR as an intrinsic master for monocyte/macrophage development in bone marrow.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors appreciated Yuzhu Hou and Peng Wang for their critical review of our manuscript; Qing Meng, Jianxia Peng, Xiaoqiu Liu, and Yabing Liu for their expert technical assistance; Ling Li for her excellent laboratory management; and Hongfei Wu for his outstanding animal husbandry.
This work was supported by grants from the National Natural Science Foundation for General and Key Programs (81130055 and 31470860) (Yong Zhao), the National Key Research and Development Program of China (2017YFA0105002 and 2017YFA0104402) (Yong Zhao), Knowledge Innovation Program of Chinese Academy of Sciences (XDA04020202-19) (Yong Zhao), The China Manned Space Flight Technology Project (TZ-1), and the CAS/SAFEA International Partnership Program for Creative Research Teams (Yong Zhao).
Authorship
Contribution: Yang Zhao, X.S., and N.N. designed and carried out the experiments, analyzed data, and wrote the manuscript; Z.C. and H.S. carried out the experiments and analyzed data; S.C. bred mice and performed polymerase chain reaction assays; L.S. analyzed data, revised the manuscript, and provided comments; Y.X. genotyped the genetically modified mice; L.Z. provided animal models and revised the manuscript; B.S. designed experiments, analyzed data, and wrote the manuscript; and Yong Zhao designed experiments, analyzed data, wrote the manuscript, and provided overall supervision.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Yong Zhao, Transplantation Biology Research Division, State Key Laboratory of Membrane Biology, Institute of Zoology, Chinese Academy of Sciences, Beichen West Rd 1-5, Chaoyang District, Beijing, China 100101; e-mail: zhaoy@ioz.ac.cn; Bingyi Shi, Organ Transplantation Center of People’s Liberation Army, 309 Hospital of Chinese People’s Liberation Army, A-17 Heishanhu Rd, Haidian District, Beijing, 100091 China; e-mail: shibingyi@medmail.com.cn; Lianfeng Zhang, Key Laboratory of Human Diseases Comparative Medicine, Ministry of Health; Institute of Laboratory Animal Science, Chinese Academy of Medical Sciences, Chao Yang Strict, Pan Jia Yuan Nan Li No. 5, Beijing, China 100021; e-mail: zhanglf@cnilas.org.
REFERENCES
Author notes
Yang Zhao, X.S., and N.N. contributed equally to this study.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal