Abstract
Hundreds of billions of platelets are cleared daily from circulation via efficient and highly regulated mechanisms. These mechanisms may be stimulated by exogenous reagents or environmental changes to accelerate platelet clearance, leading to thrombocytopenia. The interplay between antiapoptotic Bcl-xL and proapoptotic molecules Bax and Bak sets an internal clock for the platelet lifespan, and BH3-only proteins, mitochondrial permeabilization, and phosphatidylserine (PS) exposure may also contribute to apoptosis-induced platelet clearance. Binding of plasma von Willebrand factor or antibodies to the ligand-binding domain of glycoprotein Ibα (GPIbα) on platelets can activate GPIb-IX in a shear-dependent manner by inducing unfolding of the mechanosensory domain therein, and trigger downstream signaling in the platelet including desialylation and PS exposure. Deglycosylated platelets are recognized by the Ashwell-Morell receptor and potentially other scavenger receptors, and are rapidly cleared by hepatocytes and/or macrophages. Inhibitors of platelet clearance pathways, including inhibitors of GPIbα shedding, neuraminidases, and platelet signaling, are efficacious at preserving the viability of platelets during storage and improving their recovery and survival in vivo. Overall, common mechanisms of platelet clearance have begun to emerge, suggesting potential strategies to extend the shelf-life of platelets stored at room temperature or to enable refrigerated storage.
Introduction
In addition to their vital role in hemostasis and thrombosis, platelets are involved in many diverse biological processes including inflammation, tissue repair, and antimicrobial host defense. To maintain a steady count of 150 000 to 400 000 platelets per microliter of whole blood, the body produces and clears platelets at a rate of 1011 platelets per day. Platelet genesis or thrombopoiesis has been extensively characterized, and new elements in the process are still being discovered.1 In recent years, many critical advances in the studies of platelet clearance have been made. This review focuses on the current understanding of the molecular mechanisms underlying platelet clearance, and how this knowledge is used to improve platelet storage.
Measurements of platelet clearance
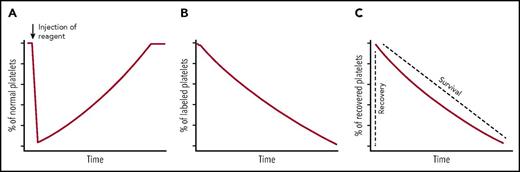
Three methods are typically used to monitor platelet clearance. The first method is to measure the ability of a compound or molecule to induce platelet clearance. The compound is administered into the body and blood counting is performed periodically thereafter, producing a plot of relative platelet count over time, expressed typically as a percentage of that prior to administration (Figure 1A).2,3 An acute drop in platelet count illustrates the compound’s clearing effect. Once the compound is metabolized or removed from the body, the platelet count rises to normal due to continuous thrombopoiesis. The second method is to measure the lifespan of endogenous platelets. A radioisotopic or fluorescent compound is administered into humans or mice to pulse label the circulating platelets.4-6 Thereafter, blood is collected periodically, and platelets are isolated from the whole blood. The percentage or the radioactivity of labeled platelets in the whole platelet population is measured and plotted over time (Figure 1B). These plots demonstrated that the lifespans of human and murine platelets are 7 to 10 and 4 to 5 days, respectively.4,5 The third method is to measure the clearance of transfused platelets. Platelets obtained from humans or animals are processed in vitro, labeled with radioisotopes7 (eg, 51Cr or 111In) or chromophores8,9 (eg, carboxyfluorescein succinimidyl ester, 5-chloromethylfluorescein diacetate, or N-hydroxysuccinimido biotin), and transfused into a different host. The resulting plot of transfused platelets over time typically consists of 2 parameters (Figure 1C). The first parameter, known as platelet recovery, denotes the appearance of transfused platelets in peripheral circulation. The second, known as platelet survival, denotes the clearance of transfused platelets from circulation. Compared with the first 2, the third method enables the assessment of effects of in vitro treatment (eg, storage) of platelets. Also, protocols have been developed to monitor function and survival of human platelets in animals.10
Measurement of platelet clearance kinetics. (A) Endogenous platelet count is monitored over time following the injection of a reagent to assess its effect on platelet clearance. (B) A radioisotopic or fluorescent compound is administered into human or mice. Thereafter, the percentage or radioactivity of labeled platelets in the whole platelet population is measured over time. (C) Exogenous platelets are labeled with radioisotopes or chromophores, and transfused into a host. The percentage of these exogenous labeled platelets is measured over time. The recovery indicates the initial appearance of transfused platelet in the circulation, and the survival means the time that the transfused platelets stay in the circulation.
Measurement of platelet clearance kinetics. (A) Endogenous platelet count is monitored over time following the injection of a reagent to assess its effect on platelet clearance. (B) A radioisotopic or fluorescent compound is administered into human or mice. Thereafter, the percentage or radioactivity of labeled platelets in the whole platelet population is measured over time. (C) Exogenous platelets are labeled with radioisotopes or chromophores, and transfused into a host. The percentage of these exogenous labeled platelets is measured over time. The recovery indicates the initial appearance of transfused platelet in the circulation, and the survival means the time that the transfused platelets stay in the circulation.
All 3 methods have been applied to humans and mice. Overall, the results suggest that despite some differences such as lifespan, human and mouse platelet clearance mechanisms share many common features.
Platelet apoptosis and clearance
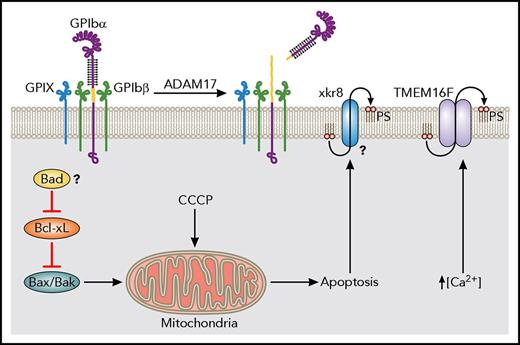
Similar to many nucleated cells, platelet apoptosis depends on the balance between proapoptotic and antiapoptotic machinery (Figure 2). Antiapoptotic Bcl-2 family proteins restrain the proapoptotic molecules Bak and Bax. Several Bcl-2 family proteins, including Bcl-2, Bcl-w, and Bcl-xL are expressed in both human and murine platelets.11,12 Platelet-specific knockout of Bcl-2 and systemic knockout of Bcl-w does not alter platelet lifespan.13,14 Treatment with ABT-199, which specifically inhibits Bcl-2, causes cell death in Bcl-2–dependent tumors but not thrombocytopenia.15 Alternatively, specific pharmacological inhibition16 or Cre-mediated deletion of Bcl-xL,17 or broad inhibition of Bcl-2-family proteins such as by ABT-737,18 led to platelet apoptosis and thrombocytopenia. Furthermore, double deletion of Bak and Bax prolongs platelet lifespan, and can rescue thrombocytopenia caused by loss of Bcl-xL.12,19 Single deletions have revealed that Bak is likely the major regulator of lifespan whereas Bax plays a smaller role.6,19
Apoptotic machinery in platelet clearance and lifespan. The anti-apoptotic Bcl-xL restrains the proapoptotic Bax/Bak in platelets. Mitochondrial damage induced by CCCP, an ionophore, leads to robust ectodomain shedding of GPIbα. If inhibition by Bcl-xL is blocked pharmacologically, Bax/Bak will induce mitochondrial damage, leading to the apoptotic cascade. The BH3-only initiator of apoptosis Bad may also affect platelet lifespan, though further study would help to elucidate its role. Apoptotic cells redistribute PS from the inner to the outer leaflet of their plasma membranes. One calcium-independent pathway may involve Xkr8. Another pathway present in platelets is facilitated by TMEM16F, a calcium-activated phospholipid scramblase.
Apoptotic machinery in platelet clearance and lifespan. The anti-apoptotic Bcl-xL restrains the proapoptotic Bax/Bak in platelets. Mitochondrial damage induced by CCCP, an ionophore, leads to robust ectodomain shedding of GPIbα. If inhibition by Bcl-xL is blocked pharmacologically, Bax/Bak will induce mitochondrial damage, leading to the apoptotic cascade. The BH3-only initiator of apoptosis Bad may also affect platelet lifespan, though further study would help to elucidate its role. Apoptotic cells redistribute PS from the inner to the outer leaflet of their plasma membranes. One calcium-independent pathway may involve Xkr8. Another pathway present in platelets is facilitated by TMEM16F, a calcium-activated phospholipid scramblase.
In many apoptotic cells, Bcl-2 family proteins are inhibited by BH3-only initiators of apoptosis, ultimately leaving Bax/Bak free to initiate mitochondrial membrane damage and trigger the apoptotic cascade. Of the 4 BH3-only proteins expressed in platelets (Bid, Bim, Bad, and Bik), genetic deletions of Bid or Bim did not alter the platelet count in mice.12 Loss of Bad leads to only a small increase in platelet count and lifespan.20 The expression of BH3-only proteins in platelets may imply their involvement in regulating intrinsic apoptosis (Figure 2), but future studies are needed to fully elucidate their roles. Similarly, platelets express certain components of the extrinsic apoptosis pathway including caspase 8, but the limited data so far do not support their critical role in regulating platelet lifespan.21,22
In many cells undergoing apoptosis, the redistribution of phosphatidylserine (PS) from the inner to the outer leaflet of the plasma membrane serves as a molecular cue for engulfment and clearance by phagocytes. Although lactadherin and the scavenger machinery can mediate clearance of platelet-derived PS-expressing microvesicles,23 whether they mediate clearance of apoptotic platelets, as well as the identity of the “clear-me” sign on apoptotic platelets, remains to be fully elucidated. Earlier studies have ruled out several markers of platelet activation, such as P-selectin, as “clear-me” signs for platelet clearance.24 Platelets possess 2 distinct pathways through which they expose PS on their surface25,26 (Figure 2). One is dependent on intracellular Ca2+ and TMEM16F, a Ca2+-activated phospholipid scramblase and ion channel.27,28 The other is associated with apoptosis, and may involve Xk-related protein 8 (Xkr8), a 10-transmembrane domain scramblase, instead of TMEM16F.25 Earlier studies suggest that apoptosis-associated morphological changes in platelets, such as PS exposure and recognition by phagocyte scavenger receptors, are not inhibited by broad-spectrum caspase inhibitor zVAD-fmk.29 Whether or how Xkr8 and/or TMEM16F are involved in regulating the platelet lifespan and mediating its clearance in a caspase-independent manner remains to be characterized.
In most apoptosis pathways, mitochondrial outer membrane permeabilization is a critical step, resulting in decrease of the mitochondrial electrochemical gradient and release of cytochrome C. Carbonyl cyanide 3-chlorophenylhydrazone (CCCP) is a lipid-soluble protonophore and oxidative phosphorylation uncoupler that induces mitochondrial permeabilization and loss of membrane potential.30 When platelets are pretreated with CCCP, posttransfusion recovery of these platelets is greatly reduced, indicating that the bulk of CCCP-treated platelets are cleared rapidly in vivo.31 Those that are not cleared rapidly do not have reduced lifespan. It is also noteworthy that CCCP treatment induces modest PS exposure but significant ectodomain shedding of glycoprotein Ibα (GPIbα; CD42b) in platelets.31 These results link the mitochondrial damage to accelerated platelet clearance and, as described in “GPIb-IX signaling: a trigger for platelet clearance,” implicate the shedding of GPIbα as a key step (Figure 2).
Antibody-mediated clearance
Another common mechanism of platelet clearance involves opsonization by antiplatelet antibodies, Fc-receptor–mediated recognition, and subsequent clearance. In patients with immune thrombocytopenia (ITP), autoantibodies targeting platelet surface glycoproteins, primarily GPIIb-IIIa and GPIb-IX, lead to Fc-dependent clearance via macrophages.32 Antiplatelet autoantibodies may also target the precursors to platelets, megakaryocytes.33 Additionally, infusion of monoclonal antibodies (MAbs) targeting the N-terminal ligand-binding domain (LBD) of GPIbα causes fast depletion of nearly all platelets from animals.34-37 Common treatments for ITP include immunosuppressive steroids and IV immunoglobulin G (IVIG).38 However, some patients are refractory to these treatments,39 implying at least 1 parallel Fc-independent clearance mechanism (discussed in “GPIb-IX signaling: a trigger for platelet clearance”).
Role of glycans in platelet clearance
Recent studies have highlighted the role of glycan modifications on platelets in mediating their clearance. In circulation, loss of terminal sialic acid (a derivative of neuraminic acid) from the platelet surface has been linked with senescent platelet removal.40 Neuraminidases (sialidases) are glycoside hydrolase enzymes that remove the terminal sialic acid residues on glycans. Injection of neuraminidase in animal models leads to rapid platelet clearance and transient thrombocytopenia.41 Also, certain bacterial infections are marked by a release of pathogen-derived neuraminidase resulting in thrombocytopenia.42 Furthermore, there is evidence that endogenous, platelet-derived neuraminidase plays a role in fast clearance of refrigerated platelets.43,44 Relatedly, many antibodies targeting the N-terminal ligand-binding domain (LBD) of GPIbα induce platelet signaling and surface presentation of lysosomal neuraminidase (Neu1), leading to increased desialylation of platelets and acute thrombocytopenia in mice.45 Treatment with 2,3-dehydro-2-deoxy-N-acetylneuraminic acid (DANA), a neuraminidase inhibitor, reduces desialylation and leads to amelioration of thrombocytopenia.45 Similarly, binding of plasma von Willebrand factor (VWF) to GPIbα on platelets under shear produces similar signaling events including desialylation.46
In general, the terminal residues in both N- and O-glycans are sialic acid, linked to a penultimate β-galactose (β-gal). Desialylation of platelets therefore leads to the increased exposure of β-gal (Figure 3). The exposed β-gal on the platelet surface can be recognized by the Ashwell-Morell receptor (AMR), a multimeric endocytic receptor complex also known as the asialoglycoprotein receptor,47 on the surface of hepatocytes and/or liver macrophages (Kupffer cells), inducing the clearance of the platelet from circulation.43,48,49 The AMR exhibits higher affinity and ligand preference for tetra- or triantennary galactoses than di- or monoantennary ones.47,50 Mice lacking the AMR have elevated platelet count (mild thrombocytosis), and fast clearance of platelets in response to neuraminidase injection is abolished in them.3,51 On the other hand, St3gal4−/− mice, which have deficiencies in terminal sialic acid residues on platelet surface glycoproteins due to genetic loss of an important sialyltransferase, suffer from thrombocytopenia as a result of accelerated platelet clearance via the hepatic AMR.48 In addition to mediating clearance and removal of senescent platelets, the AMR also leads to stimulation of platelet production, forming a clearance/thrombopoiesis feedback loop for platelet homeostasis.51
Protein desialylation as a clear-me sign in platelets. Over the platelet lifespan, surface glycoproteins lose the terminal sialic acid residues in their glycans, a process associated with clearance. Neuraminidases are glycoside hydrolases that can remove terminal sialic acid from glycans. Neuraminidases are found in platelets, which present neuraminidase on their surface downstream of GPIb-IX complex signaling. In many glycans, desialylation leads to exposure of the penultimate galactose residues on glycans. These can in turn be recognized by the AMR. Further deglycosylation leads to exposed GlcNAc residues, which may be recognized by other carbohydrate receptors and potentially mediate their uptake by macrophages.
Protein desialylation as a clear-me sign in platelets. Over the platelet lifespan, surface glycoproteins lose the terminal sialic acid residues in their glycans, a process associated with clearance. Neuraminidases are glycoside hydrolases that can remove terminal sialic acid from glycans. Neuraminidases are found in platelets, which present neuraminidase on their surface downstream of GPIb-IX complex signaling. In many glycans, desialylation leads to exposure of the penultimate galactose residues on glycans. These can in turn be recognized by the AMR. Further deglycosylation leads to exposed GlcNAc residues, which may be recognized by other carbohydrate receptors and potentially mediate their uptake by macrophages.
In addition to the interaction between galactose and the AMR, other carbohydrates and their receptors may also play a role in platelet clearance (Figure 3). It was reported that integrin αMβ2 recognizes refrigerated platelets via binding to exposed GlcNAc on the platelet surface and mice lacking the αM subunit show a small increase in platelet count.52,53 Clodronate depletion of macrophages alleviates thrombocytopenia in a mouse model of von Willebrand disease (VWD) type 2B.54 Furthermore, preinjection of GlcNAc into guinea pigs prior to induction of antibody-induced thrombocytopenia partly protects against depletion of platelets.55 However, although galactosylation of GlcNAc residues via treatment of uridine 5′-diphosphogalactose (UDP-galactose) results in the normal survival of short-term refrigerated platelets, it does not ameliorate the survival of long-term (48 hours) refrigerated human and murine platelets.56 The degrees of contribution of various glycans to platelet clearance remain to be clarified.
Platelet GPIbα is heavily decorated with sialic acid residues, accounting for as much as 70% to 80% of the total sialic acid on the platelet surface. Unlike human GPIbα, the murine GPIbα amino acid sequence lacks any N-glycosylation consensus sequences. Grewal et al showed recently that even in mice lacking GPIbα, neuraminidase treatment leads to platelet clearance, albeit at a slower rate than in wild-type (WT) mice.3 This suggests that the glycans on GPIbα are necessary to set off a rapid rate of AMR-dependent clearance, but the exposed galactoses on other platelet glycoproteins may also be counterreceptors for the AMR. Considering these intriguing observations regarding glycosylation of GPIbα, further work is likely required in order to understand the full contributions of these phenomena to platelet clearance.
GPIb-IX signaling: a trigger for platelet clearance
The GPIb-IX-V (CD42) complex has been implicated in platelet clearance under a number of scenarios. Among the scenarios are VWF-platelet agglutinated complexes,54,57,58 Fc-independent anti-GPIb-IX antibody-induced clearance,2,45 platelet surface desialylation,3,48 and ectodomain shedding of GPIbα during platelet storage8,31 (discussed in “Platelet storage at room temperature”).
GPIb-IX is a multimeric platelet receptor complex composed of the GPIbα, GPIbβ, and GPIX subunits. GPIbα is the major subunit of the complex and is responsible for binding to all known ligands of GPIb-IX including VWF. When immobilized under flow at sites of injury in the endothelium, VWF undergoes a conformational change that enables it to bind GPIbα and recruit platelets to the injury. Alternatively, circulating VWF does not spontaneously associate with the LBD of GPIbα. In patients with type 2B VWD, mutant VWF exhibits increased spontaneous association to GPIbα. Type 2B VWD patients present with accelerated platelet clearance and thrombocytopenia of variable severity, depending on the underlying causative mutation.46,59,60 Furthermore, transgenic mice expressing type 2B VWF exhibit thrombocytopenia due to clearance of large VWF-platelet complexes in the liver and/or spleen.54 In addition to type 2B VWD, several other situations that facilitate binding of soluble VWF to GPIbα also result in accelerated platelet clearance. For example, ristocetin, which induces spontaneous association of VWF to GPIbα, was pulled from clinical use because it caused thrombocytopenia and clotting.57 Injection of botrocetin, a snake venom that induces VWF binding to GPIbα via a different mechanism, causes acute thrombocytopenia in animals.58,61 Thrombocytopenia is also observed in many patients who have received implantations of left-ventricular assist devices,62 which generate abnormal shear flow conditions and may potentially induce VWF association with GPIbα. It was recently reported that binding of plasma VWF to GPIbα on platelets under shear induces GPIb-IX signaling including platelet desialylation, thereby leading to platelet clearance.46
Platelet clearance by anti-LBD MAbs in mice can occur in an Fc-independent manner and is largely unaffected by IVIG pretreatment.2,37,63 This is because anti-LBD MAbs can directly activate GPIb-IX and induce platelet intracellular signaling, particularly desialylation, and subsequent platelet clearance by hepatocytes and/or macrophages.45,55 Analyses of plasma from ITP patients in multicenter cohort studies revealed that the presence of autoantibodies targeting GPIb-IX is an effective predictor for refractoriness to steroid or IVIG therapy.64,65 Regarding the mechanistic requirements of anti-LBD MAb-induced platelet signaling, 4 key observations have emerged from the literature. First, the F(ab′)2 but not the Fab fragment of an anti-LBD MAb induces platelet clearance,2,37 indicating that the bivalent structure of an antibody is required for activating GPIb-IX. Second, most anti-LBD antibodies clear platelets rapidly, regardless of their epitope in the LBD.34-37,45 Third, a small subset of anti-LBD MAbs is ineffective at inducing Fc-independent clearance.55,66 Fourth, most MAbs targeting regions other than the LBD in GPIb-IX do not induce Fc-independent platelet clearance.36,67,68
A GPIbα clustering model has been proposed as the mechanism of GPIb-IX activation69,70 and applied to explain the observed effects of anti-LBD MAbs. In this model, an anti-LBD MAb binds 1 copy of GPIbα with each Fab, inducing lateral dimerization or “clustering,” and thereby transmitting a signal into the platelet that subsequently leads to fast clearance.55 VWF, being a multimeric ligand, is also capable of clustering GPIb-IX.69,70 The clustering model can explain the aforementioned first and second observations about anti-LBD MAbs. However, it is difficult to conceive how the clustering model accounts for the third and fourth observations. Particularly, a MAb targeting the mechanosensory domains (MSDs) of GPIbα binds to 2 copies of GPIbα on the platelet, but induces neither platelet activation in vitro nor thrombocytopenia in mice.68 Moreover, the requirement of shear in VWF-mediated GPIb-IX signaling is well documented71 but remains to be addressed by the clustering model.
An alternative model for GPIb-IX activation, the trigger model, has recently been proposed (Figure 4).46 The model is built on a membrane-proximal MSD that was recently identified between the macroglycopeptide region and the transmembrane domain of GPIbα.72 Under physiological shear, binding of soluble VWF to the LBD generates a pulling force on GPIbα, and induces MSD unfolding on the platelet surface, exposure of the membrane-proximal trigger sequence therein and subsequent platelet signaling including desialylation. This model of GPIb-IX activation accounts for the requirement of shear force, as well as all 4 aforementioned observations regarding antibody-induced signaling. The dimeric structure of activating ligands is used to crosslink platelets via GPIb-IX and induce MSD unfolding.66 In the trigger model, the defining characteristic of an activating ligand to GPIb-IX is its ability to bind the LBD and sustain sufficient tensile force to induce MSD unfolding.46,66 Thus, it is conceivable that ligands with similar binding affinities and binding sites but disparate mechanical properties, such as different anti-LBD MAbs or VWF bearing different type 2B mutations,60,66 may differentially activate GPIb-IX and induce platelet clearance.
The trigger model of GPIb-IX-mediated signaling that leads to platelet clearance. A soluble multimeric ligand, such as plasma VWF or anti-LBD antibodies, can bind to the LBD of GPIbα and crosslink platelets. Under physiological shear, the crosslinking can generate a pulling force on GPIbα and induce unfolding of the MSD therein. Consequently, it induces platelet signaling as illustrated, including desialylation (the exposure of β-gal), leading to rapid clearance of platelets. Adapted from Deng et al with permission.46
The trigger model of GPIb-IX-mediated signaling that leads to platelet clearance. A soluble multimeric ligand, such as plasma VWF or anti-LBD antibodies, can bind to the LBD of GPIbα and crosslink platelets. Under physiological shear, the crosslinking can generate a pulling force on GPIbα and induce unfolding of the MSD therein. Consequently, it induces platelet signaling as illustrated, including desialylation (the exposure of β-gal), leading to rapid clearance of platelets. Adapted from Deng et al with permission.46
In circulating platelets, the ectodomain of GPIbα is continuously cleaved or shed by ADAM17.73 The ADAM17 cleavage site of GPIbα is located in the MSD, preceding the trigger sequence.46,74 It appears to be on the MSD surface and is accessible when MSD is folded,75 consistent with the observation that shedding of GPIbα occurs continuously on resting platelets. However, MSD unfolding induced by ligand binding and pulling could further expose the ADAM17 shedding cleavage site, thereby boosting shedding of GPIbα.46,66 On the other hand, upon shedding of GPIbα, and subsequent separation of glycocalicin from the platelet, the structure of the MSD is disrupted and the membrane-proximal trigger sequence therein unprotected (Figure 4). Thus, it is conceivable that shedding of GPIbα may achieve MSD unfolding and induce GPIb-IX signaling. This is consistent with the observation that mutations in the MSD that cause MSD unfolding and trigger sequence exposure can induce ligand-free signaling from GPIb-IX.46
Intracellular signaling pathways that connect activated GPIb-IX to the surface expression of Neu1 and other clearance-related cellular changes remain to be determined. Soluble VWF binding to GPIbα under shear was reported to induce apoptotic signaling events in human platelets and Chinese hamster ovary cells expressing human GPIb-IX.76 This effect is dependent on 14-3-3 protein ζ isoform,76 which binds the cytoplasmic domains of both GPIbα and GPIbβ. GPIbα binding to VWF immobilized at the injury site is critical to platelet adhesion and activation, in which GPIb-IX-mediated signaling helps to mediate activation of GPIIb-IIIa.77 However, the difference and similarity between GPIb-IX–signaling pathways leading to platelet clearance and platelet activation remains to be clarified.
Platelet storage at room temperature
Platelet transfusion is a widely used therapy to treat patients with thrombocytopenia. Prior to transfusion, platelets derived from healthy donors, mixed in gas-permeable plastic bags with donor plasma (at ∼3 × 1011 platelets in 300 mL), are stored under constant agitation at room temperature for up to 5 days. The 5-day shelf-life is adopted primarily to reduce the risk of bacterial growth and secondarily to curtail the platelet storage lesion (PSL). PSL develops with the storage time, and its severity correlates with the reduced recovery and survival of infused platelets.78,79 Recent application of pathogen inactivation and detection technologies raise the possibility that PSL will become a limiting factor for platelet storage and efforts to reduce PSL may help to extend the platelet shelf-life beyond 5 days. Several factors have been identified to influence the development of PSL. For example, centrifugation can damage platelets and cause platelets to release both lactate dehydrogenase and granules.80 The storage conditions, including storage temperature and duration, composition of storage media, and storage containers, are also known to affect the quality of stored platelets.81 Here, we focus on recent developments targeting the molecular machineries in the platelet that mediates its clearance and function.
The onset of apoptosis may mediate PSL because caspase 3 is activated and gelsolin subsequently cleaved during storage.82 Addition of caspase 3 inhibitor Z-DEVD-FMK significantly increases platelet viability in the methyl-thiazolyl tetrazolium assay.83 Furthermore, utility of anandamide, which can inhibit platelet apoptosis through the phosphatidylinositol 3-kinase/Akt pathway,84 is able to reduce PS exposure and soluble P-selectin content in platelets after 7 days of storage although it has no effects during 5 days.85 On the other hand, partial inhibition of caspase 3 activation by complement inhibitor compstatin during storage does not reduce PSL.86 These studies suggest that apoptosis is ongoing during storage, but whether its inhibition is sufficient to preserve the viability of stored platelets requires additional investigation.
The mitochondrial transmembrane potential in stored platelets was reported to remain unchanged compared with fresh platelets, even though apoptotic signals such as caspase activation and PS exposure were enhanced.87 However, it was significantly higher than fresh platelets in another report.88 A recent study found that increasing the storage time was associated with mitochondrial dysfunction.89 Additionally, platelet mitochondria injury induced by CCCP treatment led to a significantly reduced posttransfusion recovery in mice.31 Acetyl-l-carnitine or ascorbic acid, which preserves mitochondrial function during platelet storage, helps but is not fully sufficient to maintain platelet viability.90
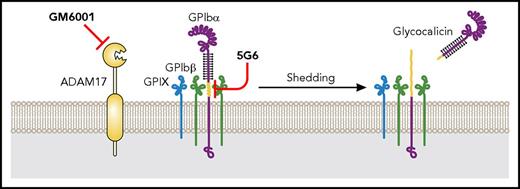
Significant ectodomain shedding of GPIbα and accumulation of its product glycocalicin during storage is consistently observed in various studies.91,92 A tight correlation between GPIbα shedding and the extent of PSL has been noted in laboratory studies,93 although whether glycocalicin can serve as a biomarker for the quality of stored platelets requires testing in clinical settings. Furthermore, the utility of a broad-spectrum metalloproteinase inhibitor GM6001 significantly improved the posttransfusion recovery and survival of in vitro aged or CCCP-treated murine platelets (Figure 2, 5).31 Genetic ablation of ADAM17 or addition of inhibitors of p38 MAPK, which is an activator of ADAM17, during platelet storage achieved similar effects.94 More definitively, addition of MAb 5G6, which binds specifically to human GPIbα and block its shedding, during prolonged storage at room temperature improved the recovery and survival of stored platelets (Figure 5).8,95 In mice, platelets stored with the aforementioned shedding inhibitors exhibited significantly better in vivo hemostatic function than those stored without, likely because GPIbα is critically involved in primary hemostasis.8,31,94 These studies suggest that GPIbα shedding could accelerate platelet clearance, and that inhibition of GPIbα shedding could improve recovery and survival of stored platelets.
Platelet storage at room temperature. At room temperature, platelets can only be stored for up to 5 days, which is mainly due to the risk of bacteria growth. In addition, GPIbα shedding is also tightly correlated to platelet storage lesion. Inhibiting GPIbα shedding by using GM6001 or 5G6 significantly improves the posttransfusion recovery and survival of room temperature–stored platelets.
Platelet storage at room temperature. At room temperature, platelets can only be stored for up to 5 days, which is mainly due to the risk of bacteria growth. In addition, GPIbα shedding is also tightly correlated to platelet storage lesion. Inhibiting GPIbα shedding by using GM6001 or 5G6 significantly improves the posttransfusion recovery and survival of room temperature–stored platelets.
Platelet storage by refrigeration
The risk of microbial contamination during platelet storage at room temperature limits the shelf-life of stored platelets. Checking for the pathogens during storage adds significantly to the cost of blood banking. Refrigeration of platelets at 1°C to 6°C offers an alternative storage option because the cold temperature could effectively minimize microbial proliferation and slow down metabolism in the platelet. However, refrigerated platelets are rapidly cleared after transfusion.96 In the past few years, several studies have been carried out to critically advance our understanding of the underlying molecular mechanism.
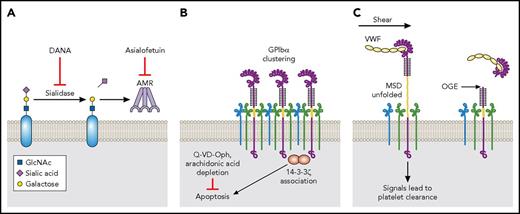
It was noticed that platelets became desialylated following 48 hours of refrigeration.43 This is because refrigeration and subsequent rewarming of the platelets induces surface expression of Neu1, which removes sialic acid from platelet glycoproteins, particularly GPIbα.44 The exposed β-gal is recognized by the AMR and the platelets are quickly cleared by hepatocytes.43 Adding DANA, a neuraminidase inhibitor, to murine platelets during refrigeration improves the posttransfusion recovery and survival of refrigerated platelets.44 Likewise, AMR inhibitor asialofetuin significantly blocks the fast clearance of refrigerated platelets (Figure 6A).43 These inhibitory effects are similar to those on platelet desialylation and thrombocytopenia induced by anti-LBD antibodies.45
Platelet storage by refrigeration. (A) Desialylation-mediated clearance. Sialic acid is removed by Neu1 from platelet glycoproteins following refrigeration. The exposed β-gal is recognized by the AMR, and the platelets are cleared by hepatocytes. The utility of neuraminidase inhibitors such as DANA or the AMR inhibitor asialofetuin can impede the clearance of desialylated platelets. (B) GPIbα clustering–mediated clearance. GPIbα clusters on platelet surface, and14-3-3ζ dissociates from Bad and associates with GPIbα after refrigeration. This induces the platelet apoptosis process. A broad caspase inhibitor Q-VD-Oph or arachidonic acid depletion can inhibit the apoptosis process of refrigerated platelets and improve the posttransfusion recovery and survival. (C) VWF binding–mediated clearance. Refrigeration leads to binding of plasma VWF to GPIbα. Upon transfusion and thus exposure to the shear flow, VWF binding may generate a pulling force and induces MSD unfolding, leading to rapid platelet clearance. OGE cleaves off the LBD of GPIbα, therefore precludes the VWF-GPIbα interaction and subsequently platelet clearance.
Platelet storage by refrigeration. (A) Desialylation-mediated clearance. Sialic acid is removed by Neu1 from platelet glycoproteins following refrigeration. The exposed β-gal is recognized by the AMR, and the platelets are cleared by hepatocytes. The utility of neuraminidase inhibitors such as DANA or the AMR inhibitor asialofetuin can impede the clearance of desialylated platelets. (B) GPIbα clustering–mediated clearance. GPIbα clusters on platelet surface, and14-3-3ζ dissociates from Bad and associates with GPIbα after refrigeration. This induces the platelet apoptosis process. A broad caspase inhibitor Q-VD-Oph or arachidonic acid depletion can inhibit the apoptosis process of refrigerated platelets and improve the posttransfusion recovery and survival. (C) VWF binding–mediated clearance. Refrigeration leads to binding of plasma VWF to GPIbα. Upon transfusion and thus exposure to the shear flow, VWF binding may generate a pulling force and induces MSD unfolding, leading to rapid platelet clearance. OGE cleaves off the LBD of GPIbα, therefore precludes the VWF-GPIbα interaction and subsequently platelet clearance.
Clustering of GPIbα on the platelet surface was noticed following refrigeration of platelets.53 The clustered GPIbα may contribute to the recognition of refrigerated platelets by integrin αMβ2 on hepatic macrophages, in which glycans may play a role.52 In addition, refrigeration-induced GPIbα clustering was thought to induce platelet apoptosis, as 14-3-3ζ dissociates from Bad and associates with GPIbα following refrigeration, leading to Bad activation, cytochrome C release, caspase 9 activation, PS exposure, and increased platelet phagocytosis in vitro.97 The process is inhibited by Q-VD-Oph, which is a broad caspase inhibitor, and N-acetyl-d-glucosamine, which blocks the platelet-macrophage interaction and potentially GPIbα clustering (Figure 6B).53,97,98 Furthermore, refrigeration-mediated 14-3-3ζ–GPIbα association is dependent on arachidonic acid, as the depletion of arachidonic acid during refrigeration inhibited apoptotic signals and improved the posttransfusion recovery and survival of refrigerated platelets (Figure 6B).99
Treatment of murine platelets with O-sialoglycoprotein endopeptidase (OGE), which cleaves off the LBD of GPIbα, prior to refrigeration significantly improves the recovery and survival of these platelets in mice.43,97 Because murine GPIbα does not have N-glycosylation sequence motifs, it could not be involved in direct binding with the AMR.50 Instead, because refrigeration induces binding of plasma VWF to GPIbα on the platelet,43,100 treatment of OGE could conceivably preclude the VWF-GPIbα interaction and subsequent clustering of GPIbα in refrigerated platelets (Figure 6C). Consistent with the aforementioned trigger model, shear treatment of refrigerated WT platelets, but not VWF−/− ones, results in MSD unfolding, platelet desialylation, and PS exposure.100 Furthermore, refrigerated VWF−/− platelets, or refrigerated WT platelets incubated with a peptide that inhibits GPIbα interaction with VWF, exhibit markedly higher posttransfusion recovery than WT.100 Thus, it appears that VWF binding, GPIbα clustering, platelet desialylation, and PS exposure are key steps to the fast clearance of refrigerated platelets.
In summary, although several questions remain to be addressed, common mechanisms of platelet clearance have begun to emerge. Studies of platelet storage at room temperature and under refrigerating conditions have provided critical insights. Reciprocally, several inhibitors have shown promising efficacies in preserving the viability and improving the recovery and survival of stored platelets in animals. It is time to translate the newly gained knowledge of the platelet clearance mechanisms into viable strategies to treat thrombocytopenia and to improve platelet storage for transfusion.
Acknowledgments
The authors are very grateful to their many colleagues and collaborators for their critical insights and helpful comments on this review.
This work was supported in part by National Institutes of Health National Heart, Lung, and Blood Institute grants HL082808, HL123984 (R.L.), and F31HL134241 (M.E.Q.).
Authorship
Contribution: M.E.Q., W.C., and R.L. reviewed literature and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Renhao Li, Department of Pediatrics, Emory University, 2015 Uppergate Dr NE, Room 440, Atlanta, GA 30322; e-mail: renhao.li@emory.edu.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal