To the editor:
Germ line heterozygous GATA2 mutation causes failure of mononuclear cell development, immune dysfunction, and evolution to myeloid neoplasia.1-4 Symptoms may arise at almost any age, reaching a penetrance of 90% in the seventh decade.3 Fatal complications include mycobacterial infection, uncontrolled herpesvirus infection, human papillomavirus (HPV)–associated carcinoma, and hematologic transformation.1-4 Bone marrow transplantation is curative, but the optimal timing and protocol remain undefined.4-11 Patients who develop GATA2-related myelodysplastic syndrome (MDS) or acute myeloid leukemia (AML) have received successful transplantations with myeloablative and reduced-intensity conditioning that followed standard clinical algorithms for hematologic malignancy.2-4 However, 21% to 60% of GATA2 patients do not present with MDS or AML2,3 and may suffer severe infectious complications, including significant pulmonary dysfunction. The optimal therapy for this group remains undefined.8,9
Here we present a retrospective analysis of 4 patients who had successful transplants with a T-cell–depleted reduced-intensity regimen for predominantly infectious and respiratory complications of GATA2 mutation. Our cohort included consecutive patients who received transplants at 2 United Kingdom centers (Manchester and Newcastle) and had at least 2 years of follow-up (2008-2012). Their laboratory indices have been previously described2,12,13 and are summarized in supplemental Table 1 (available on the Blood Web site). Patients 1 to 3 had grossly elevated fms-like tyrosine kinase-3 (flt3) ligand and a severe dendritic cell, monocyte, and lymphocyte deficiency phenotype with relatively well-preserved hemoglobin, neutrophils, and platelets. Patient 4 had monocytopenia in the context of more conventional MDS with anemia, thrombocytopenia, and mildly elevated flt3 ligand. GATA2 mutation and clinical features are summarized in Table 1. The interval between presentation and transplantation was 1 to 8 years, and the age at transplantation was 16 to 35 years. Patients 2 to 4 had a family history of GATA2-related disease. Patients 1 to 3 had experienced severe infection. Patient 1 had a Bacille Calmette-Guérin infection. Patient 2 had pulmonary Pseudomonas aeruginosa and methicillin-resistant S aureus infection along with a genital HPV infection, which caused grade 3 VIN. Patient 3 had a Mycobacterium malmoense infection (Figure 1). Patients 2 to 4 had moderate to severe respiratory compromise as a result of PAP or emphysema. At the time of transplantation, patients 1 and 3 were receiving antimycobacterial therapy. Patient 2 required continuous oxygen and noninvasive ventilation at night and had been scheduled for radical surgical resection of advanced VIN. Patient 3 had a history of granulomatous hepatitis and Addison disease that may have been attributable to GATA2 mutation.14 For all patients, bone marrow examination was relatively unremarkable with normal cytogenetics and no excess of blasts. Patients 1 to 3 were hypocellular with small hypolobulated megakaryocytes, mild increases in reticulin, and scattered noncaseating granulomata (Figure 1A). Only patient 4 had clear evidence of trilineage dysplasia but no excess of blasts. A bone marrow examination 8 years previously had been reported as normal. Both patients 2 and 4 had acquired an ASXL1 mutation at the time of transplantation. Patient 2 had delivered her first child prematurely at 28 week’s gestation.
GATA2 mutation, clinical features, transplantation, and outcome
| Patient . | GATA2 mutation(s)* . | Family history . | Clinical features . | Age at onset (y) . | Sex . | Age at transplantation (y) Year of transplant . | Days to discharge . | Total dose of drugs for conditioning GVHD prophylaxis . | Donor and HLA match†CMV serostatus (donor/recipient) . | Donor myeloid/ T cell chimerism . | Significant posttransplantation events . | Survival Follow up (y) . | KPS (%) . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | c.599_600insG p.G200fs | De novo | Recurrent BCG-osis | 12 | M | 16 2008 | 33 | Flu 150 mg/m2 Mel 140 mg/m2 Alem 0.2 mg/kg −8 to −4 (1 mg/kg) Ciclosporin MMF | MUD 9/10 (C) NEG / NEG | 100 / 85 | Grade I acute GVHD No chronic GVHD Immune reconstitution syndrome at 28 d Melanoma | Alive 9 | 100 |
| 2 | c.1192 C>T p.R398W | Chest MDS | HPV 16/18 with VIN III PAP MRSA and Pseudomonas aeruginosa ASXL1 mutation | 20 | F | 23 2010 | 28 | Flu 150 mg/m2 Bu 6.4 mg/kg Alem 30 mg ×2 −4; −2 (60 mg) Ciclosporin MMF | MUD 8/10 (A,DQ) NEG / NEG | 100 / 96 | No GVHD ITP (Rituximab) at 7 mo 2 viable pregnancies | Alive 7 | 100 |
| 3 | c.1193 G>A p.R398Q | MDS AML | Mycobacterium malmoense Granulomatous hepatitis Addison disease Emphysema | 34 | M | 35 2012 | 18 | Flu 150 mg/m2 Treo 42 mg/m2 Alem 10 mg/day −7 to −3 (50 mg) Ciclosporin MMF | MUD 10/10 POS / POS | 100 / 95 | Grade I acute GVHD No chronic GVHD AIHA (Rituximab) at 9 mo | Alive 5 | 90 |
| 4 | c.599_600insG p.G200fs c.1168_1170 del1AAG p.390delK | MDS AML | PAP Trilineage dysplasia ASXL1 mutation | 21 | M | 29 2012 | 14 | Flu 150 mg/m2 Treo 42 mg/m2 Alem 10 mg/day −7 to −3 (50 mg) Ciclosporin | Matched sibling NEG / NEG | 100 / 100 | Grade I acute GVHD No chronic GVHD | Alive 5 | 100 |
| Patient . | GATA2 mutation(s)* . | Family history . | Clinical features . | Age at onset (y) . | Sex . | Age at transplantation (y) Year of transplant . | Days to discharge . | Total dose of drugs for conditioning GVHD prophylaxis . | Donor and HLA match†CMV serostatus (donor/recipient) . | Donor myeloid/ T cell chimerism . | Significant posttransplantation events . | Survival Follow up (y) . | KPS (%) . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | c.599_600insG p.G200fs | De novo | Recurrent BCG-osis | 12 | M | 16 2008 | 33 | Flu 150 mg/m2 Mel 140 mg/m2 Alem 0.2 mg/kg −8 to −4 (1 mg/kg) Ciclosporin MMF | MUD 9/10 (C) NEG / NEG | 100 / 85 | Grade I acute GVHD No chronic GVHD Immune reconstitution syndrome at 28 d Melanoma | Alive 9 | 100 |
| 2 | c.1192 C>T p.R398W | Chest MDS | HPV 16/18 with VIN III PAP MRSA and Pseudomonas aeruginosa ASXL1 mutation | 20 | F | 23 2010 | 28 | Flu 150 mg/m2 Bu 6.4 mg/kg Alem 30 mg ×2 −4; −2 (60 mg) Ciclosporin MMF | MUD 8/10 (A,DQ) NEG / NEG | 100 / 96 | No GVHD ITP (Rituximab) at 7 mo 2 viable pregnancies | Alive 7 | 100 |
| 3 | c.1193 G>A p.R398Q | MDS AML | Mycobacterium malmoense Granulomatous hepatitis Addison disease Emphysema | 34 | M | 35 2012 | 18 | Flu 150 mg/m2 Treo 42 mg/m2 Alem 10 mg/day −7 to −3 (50 mg) Ciclosporin MMF | MUD 10/10 POS / POS | 100 / 95 | Grade I acute GVHD No chronic GVHD AIHA (Rituximab) at 9 mo | Alive 5 | 90 |
| 4 | c.599_600insG p.G200fs c.1168_1170 del1AAG p.390delK | MDS AML | PAP Trilineage dysplasia ASXL1 mutation | 21 | M | 29 2012 | 14 | Flu 150 mg/m2 Treo 42 mg/m2 Alem 10 mg/day −7 to −3 (50 mg) Ciclosporin | Matched sibling NEG / NEG | 100 / 100 | Grade I acute GVHD No chronic GVHD | Alive 5 | 100 |
All patients are currently alive without chronic GVHD.
AIHA, autoimmune hemolytic anemia; Alem, alemtuzumab; BCG, Bacille Calmette-Guérin; Bu, busulfan; CMV, cytomegalovirus; F, female; Flu, fludarabine; GVHD, graft-versus-host disease; ITP, idiopathic thrombocytopenic purpura; KPS, Karnofsky performance status; M, male; Mel, melphalan; MMF, mycophenolate mofetil; MRSA, methicillin-resistant Staphylococcus aureus; MUD, matched unrelated donor; PAP, pulmonary alveolar proteinosis; Treo, treosulfan; VIN, vulval intraepithelial neoplasia.
Patient 4 had a confirmed germline c.599_600insG mutation and somatically acquired c.1168_1170 del1AAG mutation in GATA2.
HLA allele match is described with mismatched alleles in parentheses.
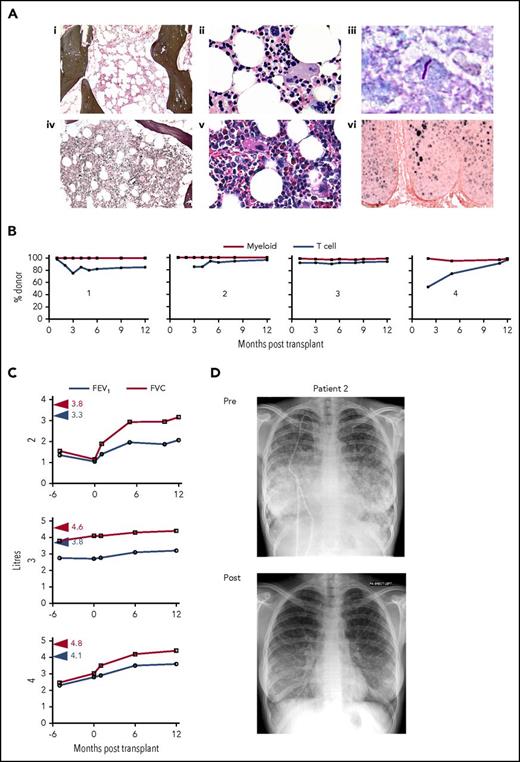
Bone marrow morphology, infection, posttransplantation chimerism, and recovery of respiratory function. (Ai-iii) represent patient 1; (iv-vi) represent patient 2. (i,iv) depict Gomori reticulin stain, (ii,v) depict megakaryocyte morphology by hematoxylin and eosin staining, (iii) depicts acid-fast bacilli in a skin biopsy of patient 1, and (vi) depicts HPV-16/18 RNA by in situ hybridization in a cervical biopsy of patient 2. (B) Percentage donor myeloid and T-cell chimerism in each patient as indicated by time after transplantation. (C) Recovery of lung volume after transplantation in patients 2 to 4. Red and blue arrowheads indicate predicted lung volume for patient height and weight. (D) Chest radiographs of patient 2 before (pre) transplantation with bilateral central symmetrical lung opacities, relative sparing of the apices and costophrenic angles, and extensive diffuse and miliary opacities, which had significantly resolved 6 months after (post) transplantation. FEV, forced expiratory volume; FVC, forced vital capacity.
Bone marrow morphology, infection, posttransplantation chimerism, and recovery of respiratory function. (Ai-iii) represent patient 1; (iv-vi) represent patient 2. (i,iv) depict Gomori reticulin stain, (ii,v) depict megakaryocyte morphology by hematoxylin and eosin staining, (iii) depicts acid-fast bacilli in a skin biopsy of patient 1, and (vi) depicts HPV-16/18 RNA by in situ hybridization in a cervical biopsy of patient 2. (B) Percentage donor myeloid and T-cell chimerism in each patient as indicated by time after transplantation. (C) Recovery of lung volume after transplantation in patients 2 to 4. Red and blue arrowheads indicate predicted lung volume for patient height and weight. (D) Chest radiographs of patient 2 before (pre) transplantation with bilateral central symmetrical lung opacities, relative sparing of the apices and costophrenic angles, and extensive diffuse and miliary opacities, which had significantly resolved 6 months after (post) transplantation. FEV, forced expiratory volume; FVC, forced vital capacity.
Details regarding transplantation are summarized in Table 1. All patients received 5 doses of fludarabine 30 mg/m2 days −7 to −3 or days −6 to −2 and an alkylating agent such as melphalan 140 mg/m2 day −2 or busulfan 3.2 mg/kg intravenously days −7 to −6 or treosulfan 14 mg/m2 days −6 to −4. Choice of alkykator reflected unit practice and was not patient-specific. In vivo T-cell depletion with alemtuzumab was given at a total dose of 0.7 mg/kg to 1.2 mg/kg in 2 to 5 divided doses. Mobilized peripheral blood stem cell grafts were infused at a dose of 1.9 to 8.0 × 106 CD34+ cells per kg supported with granulocyte colony-stimulating factor from day +6. All patients received cyclosporin as prophylaxis for GVHD. Patients 1 to 3 with unrelated donors also received mycophenolate mofetil 1g twice per day for 30 to 60 days posttransplant. All patients were discharged from the hospital between 14 and 33 days posttransplant. Three patients experienced grade 1 delayed acute GVHD (skin rash) during immunosuppression withdrawal but none required systemic therapy and none went on to develop chronic GVHD (Table 1). Patient 1 experienced an immune reconstitution syndrome of non-GVHD rash and fever in the first month (a common complication of bone marrow transplantation during active mycobacterial infection15 ) and required a short course of systemic corticosteroids. Idiopathic thrombocytopenic purpura was observed in patient 2 and hemolytic anemia was observed in patient 3 at 7 and 9 months posttransplant, respectively. These complications were observed in other patients who received transplants accompanied by fludarabine and alemtuzumab-based conditioning, and the complications resolved within 4 weeks after initiation of treatment with rituximab (375 mg/m2 per week for 4 weeks). High levels of myeloid and T-cell chimerism were observed in all patients by 3 months (Figure 1B). B-cell chimerism was not routinely monitored. Patients 2 to 4 who had significant respiratory compromise showed a marked and sustained improvement within weeks of transplantation (Figure 1C). Continuous oxygen and noninvasive ventilation at night were rapidly withdrawn from patient 2 after dramatic radiologic improvement (Figure 1D). Antimycobacterial drugs were discontinued after transplantation in patients 1 and 3 within 1 year, when CD3 T cells had increased to more than 200/μL. Patient 3 and his donor were both seropositive for cytomegalovirus, and a short reactivation was treated uneventfully with oral valganciclovir. The other 3 patients received grafts from matched cytomegalovirus-negative donors. Patient 2 was vaccinated with bivalent HPV- 16/18 vaccine at 20, 21, and 26 months posttransplant and, strikingly, her HPV-associated grade 3 VIN completely regressed by 36 months. She subsequently had 2 successful full-term pregnancies. Patient 1 was diagnosed with malignant melanoma 3 years after transplantation, but this was fully resected without further evidence of disease. All patients are alive at between 5 and 9 years posttransplantation with Karnofsky performance status of 90% to 100%.
These cases illustrate that fludarabine-based reduced-intensity transplantation with alemtuzumab in vivo T-cell depletion is acceptable for patients with severe infectious and respiratory complications from GATA2 mutation. Alemtuzumab-based conditioning provided excellent control of GVHD, even with mismatched donors. Although it is surprising that GVHD occurs at all in the absence of recipient dendritic cells, monocytes, and B cells, it has been reported as a lethal complication in other series.6,8,9,11 T-cell depletion was not problematic, and the rapid restoration of donor dendritic cells, monocytes, B cells, and natural killer cells led to a full recovery.
Life-threatening infection, severe respiratory compromise as a result of PAP, or both, were the major triggers for transplantation. In retrospect, it was discovered that 2 of the 4 patients had developed clonal hematopoiesis with ASXL1 mutation. Patients with both severe infection and myeloid neoplasia are especially challenging because reductions in conditioning intensity for infection-related comorbidity may compromise the durability of remission from MDS or AML. Relapses were observed in a previously reported cohort after nonmyeloablative conditioning with fludarabine and 2 Gy total body irradiation.8 Fludarabine combined with moderate-dose alkylating agents, as used here, has a track record in the transplantation of adults who have MDS or AML.16,17 Our results suggest that this approach is a useful compromise for patients with uncontrolled infection and clonal hematopoiesis or early MDS. In females of reproductive age, fertility may already be compromised by preterm labor and cervical or vulval intraepithelial neoplasia.2 Transplantation may indeed improve their prospects, as in the case of patient 2.
In summarizing this experience, we propose that life-threatening complications of GATA2-related disease, including PAP, atypical mycobacterial infection, and advanced HPV-associated dysplasia should be considered as indications for transplantation, even in the absence of overt myeloid neoplasia. PAP responds poorly to conventional therapy with whole-lung lavage and granulocyte-macrophage colony-stimulating factor,18 but resolves within weeks of transplantation.5 Recurrent mycobacterial infection may exacerbate respiratory decline beyond the point of salvage if immune reconstitution is not provided. HPV-related intraepithelial neoplasia can lead to disfiguring surgery which may prove ineffective at preventing lethal metastatic disease.9 Detection of the accessory cellular phenotype of dendritic cell, monocyte, and lymphocyte deficiency is critical in identifying these patients, who present to a wide range of specialist services but rarely to hemato-oncology.12 Once identified, thorough hematologic evaluation is mandatory to exclude myeloid neoplasia, and annual bone marrow surveillance for clonal evolution is prudent. Finally, owing to the highly variable nature of GATA-2 associated syndromes, it is unlikely that a single approach to transplantation will be suitable for all patients.
The online version of this article contains a data supplement.
Acknowledgments
The authors thank the patients who participated, the clinical nurse specialists, and other members of the Northern Centre for Bone Marrow Transplantation clinical team.
K.S. received a startup grant from the British Society of Haematology, V.B. is supported by Wellcome Trust Intermediate Clinical Fellowship 101155/Z/13/Z, and M.C. received funding from Bloodwise Research Grant 14004 and from Bright Red.
Authorship
Contribution: E.T., J.L., and S.H. identified patients, provided clinical care, and reported clinical data; K.S. reported and analyzed clinical data and wrote the manuscript; R.E.D. performed sequencing and molecular analysis; A.G., A.J.C., G.J., and M.A.S. provided clinical care and reported clinical data; V.B. identified patients, reported and analyzed clinical data, and wrote the manuscript; and M.C. reported and analyzed clinical data and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Matthew Collin, Newcastle University, Institute of Cellular Medicine, Human Dendritic Cell Laboratory, Newcastle upon Tyne NE2 4HH, United Kingdom; e-mail: matthew.collin@ncl.ac.uk.
References
Author notes
E.T. and K.S. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal