In this issue of Blood, Tholouli et al describe successful in vivo T-cell–depleted allogeneic hematopoietic stem cell transplant (HSCT) in 4 patients with GATA2 deficiency using a reduced-intensity conditioning (RIC) regimen including serotherapy with alemtuzumab.1 Three immediate questions come to mind when contemplating the first sentence, namely: what is GATA2 deficiency, what is the natural history of the disease that leads to HSCT, and what makes HSCT so special for GATA2 deficiency that it warrants a commentary?
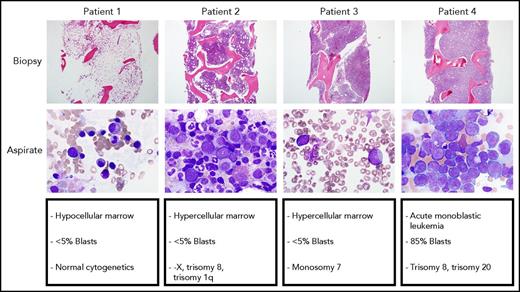
The spectrum of bone marrow findings in patients with GATA2 deficiency seen at the National Institutes of Health, ranging from a hypocellular marrow with normal cytogenetics to hypercellular marrow with unfavorable cytogenetics to overt AML with 85% monoblasts.
The spectrum of bone marrow findings in patients with GATA2 deficiency seen at the National Institutes of Health, ranging from a hypocellular marrow with normal cytogenetics to hypercellular marrow with unfavorable cytogenetics to overt AML with 85% monoblasts.
In 2011, 4 clinical syndromes were united by a common genetic diagnosis of heterozygous germ line or sporadic mutations in GATA2.2-5 Each of the groups approached this syndrome from a distinct clinical perspective, resulting in 4 different names for the same genetic abnormality: autosomal dominant and sporadic monocytopenia and Mycobacterium avium complex; dendritic cell, monocyte, B and natural killer (NK) lymphoid deficiency; Emberger syndrome (lymphedema and monosomy 7); and familial myelodysplastic syndrome (MDS)/acute myelogenous leukemia (AML).2-5 Thus, patients with GATA2 deficiency can present with evidence of an immunodeficiency, aplastic anemia, MDS, or leukemia, making the diagnosis itself a challenge.
The mutations in GATA2 involve coding and noncoding regions, as well as an enhancer region. Approximately half of the patients have a de novo mutation in GATA2, whereas the other half have the familial form of the disease. The unifying theme is the loss of one allele of GATA2 and the resulting phenotype from reduced expression of GATA2 from haploinsufficiency. Although more than 150 mutations have been described in GATA2 deficiency, there is no distinct genotype-phenotype correlation.
The natural history of GATA2 deficiency is highly variable, even in individuals in the same family harboring the identical mutation, a genetic term known as variable expressivity. Infectious complications are common in GATA2 deficiency and result from the peculiar cellular deficiency profile, namely deficiency of monocytes, NK cells, and B lymphocytes. In patients with hematologic manifestations of GATA2 deficiency, usually progressive cytopenias, there is progression from a normocellular marrow to hypocellular MDS and then, in some instances, to AML. Myeloid dysplasia with progressive cytopenias and new cytogenetic changes in the bone marrow prompt HSCT in approximately half of patients with the GATA2 deficiency in a recent study.6
HSCT represents the only curative therapy for GATA2 deficiency. However, HSCT remains challenging because of comorbidities such as disseminated Mycobacterium avium complex infections, pulmonary alveolar proteinosis, advanced MDS, and AML.6 The type of donor source, intensity of the conditioning regimen, and use of serotherapy remain unanswered questions in GATA2 deficiency, primarily because the disease was only identified in 2011.
The letter in this issue of Blood describes the successful use of a RIC regimen in HSCT for 4 patients with GATA2 deficiency who were debilitated from infections and pulmonary alveolar proteinosis. Three patients received unrelated donor peripheral blood stem cells (PBSC) (10/10, 9/10, and 8/10 matched) and one received matched sibling PBSC. All 4 patients had disease reversal, especially pulmonary and human papillomavirus–derived dysplasia. Strikingly, there was only grade 1 acute GVHD despite the degree of mismatch in the unrelated donors and the use of PBSC. Of note, only 1 patient had MDS. There were complications with HSCT in these patients. It is unclear whether the autoimmune immune hemolytic anemia and immune thrombocytopenia following HSCT were due to GATA2 deficiency or to the immune dysregulation from mixed chimerism resulting from the RIC regimen.
The important issues going forward in HSCT for GATA2 deficiency are several. First, more specific criteria are needed for determining when to proceed to HSCT. Second, the level of chimerism necessary to reverse the disease phenotype remains unclear. Third, the intensity of conditioning for GATA2 deficiency before HSCT has ranged from nonmyeloablative to RIC to myeloablative. GATA2 plays a central role in the maintenance of hematopoietic stem cells (HSC) in mice and men, and murine HSC haploinsufficient for GATA2 compete poorly with wild-type GATA2 murine HSC in competitive reconstitution assays.7 Together with our previous studies using a nonmyeloablative regimen, it appears that when patients with GATA2 deficiency are transplanted if they have a hypocellular bone marrow with MDS and without cytogenetic changes, a nonmyeloablative regimen results in reliable engraftment because the GATA2-deficient marrow is at a proliferative disadvantage.6 However, with clonal progression and unfavorable cytogenetic changes and/or a hypercellular marrow, in which the malignant clone has a proliferative advantage, a higher dose regimen results in more reliable engraftment and eradication of the clone (see figure).8 This is important because nearly half of patients with GATA2 deficiency who are symptomatic have clonal cytogenetic abnormalities with MDS. Fourth, the optimal donor and donor graft source for HSCT for GATA2 deficiency is evolving. Matched related donors are clearly the first choice; however, haploidentical related donors are currently closing in on matched unrelated donors in many HSCT scenarios.9 Last, strategies to prevent GVHD are paramount in HSCT for GATA2 deficiency because there is no advantage to GVHD when there is no preexisting malignance; the latest development in GVHD prophylaxis uses posttransplant cyclophosphamide in matched related and unrelated donors as well as in haploidentical related donors.9
An important caveat in the movement toward RIC regimens was recently reported by Bartelink et al, indicating that, with busulfan dosing, there was an optimum area under the concentration curve that resulted in the best event-free survival compared with myeloablative and low-intensity busulfan regimens. Graft failure and relapse remain formidable challenges in the pursuit of lower dose regimens.10 Thus, reducing intensity in conditioning should be done in a carefully controlled manner with a clear salvage pathway should graft failure or graft rejection occur.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal