Key Points
AAT infusion produced a high proportion of durable clinical responses in SR-aGVHD.
AAT is associated with minimal toxicity and low rates of infection in patients with SR-aGVHD at significant risk for mortality.
Abstract
Corticosteroid resistance after acute graft-versus-host disease (SR-aGVHD) results in high morbidity and mortality after allogeneic hematopoietic cell transplantation. Current immunosuppressive therapies for SR-aGVHD provide marginal effectiveness because of poor response or excessive toxicity, primarily from infection. α1-Antitrypsin (AAT), a naturally abundant serine protease inhibitor, is capable of suppressing experimental GVHD by downmodulating inflammation and increasing ratios of regulatory (Treg) to effector T cells (Teffs). In this prospective multicenter clinical study, we sought to determine the safety and response rate of AAT administration in SR-aGVHD. Forty patients with a median age of 59 years received intravenous AAT twice weekly for 4 weeks as first-line treatment of SR-aGVHD. The primary end point was overall response rate (ORR), the proportion of patients with SR-aGVHD in complete (CR) or partial response by day 28 without addition of further immunosuppression. Treatment was well tolerated without drug-related adverse events. A significant increase in serum levels of AAT was observed after treatment. The ORR and CR rates by day 28 were 65% and 35%, respectively, and included responses in all aGVHD target organs. At day 60, responses were sustained in 73% of patients without intervening immunosuppression. Infectious mortality was 10% at 6 months and 2.5% within 30 days of last AAT infusion. Consistent with preclinical data, correlative samples showed an increase in ratio of activated Tregs to Teffs after AAT treatment. These data suggest that AAT is safe and may be potentially efficacious in treating SR-aGVHD. This trial was registered at www.clinicaltrials.gov as #NCT01700036.
Introduction
Despite routine prophylaxis, clinically significant acute graft-versus-host disease (aGVHD) requiring systemic corticosteroids occurs in ∼40% of patients undergoing HLA-matched allogeneic hematopoietic cell transplantation (HCT).1 Roughly half of the patients requiring high-dose corticosteroids will not respond, a condition termed steroid-resistant aGVHD (SR-aGVHD),2,3 which is associated with poor overall survival (OS).4-7 Although several immunosuppressive therapies have been attempted in SR-aGVHD, no consensus on treatment exists, given the marginal response rates and increased risk of infection.4,7,8
α1-Antitrypsin (AAT) is a 52-kDa circulating protease inhibitor produced by the liver that inactivates several serine proteases from neutrophils and macrophages and protects tissues from proteolytic degradation. Congenital deficiency in AAT results in emphysema and rarely cirrhosis of the liver; associations with autoimmune conditions including necrotizing panniculitis, vasculitis, inflammatory bowel disease, and glomerulonephritis have been reported.9-11 More recently appreciated immune regulatory roles for AAT, independent of protease inhibition, include induction of interleukin 10 (IL-10), suppression of plasma proinflammatory cytokines,12-14 and in vivo induction of tolerance during experimental islet cell transplantation.14,15 We and others have demonstrated in multiple murine models of major histocompatibility complex–matched and –mismatched allogeneic bone marrow transplantation that administration of AAT attenuates the severity of murine aGVHD. These effects are associated with several mechanisms, including reduction in inflammatory cytokines and alterations in the ratios of effector (Teff) to regulatory T cells (Tregs) and levels of damage-associated molecular patterns.16-18 Observational studies have suggested that intestinal GVHD is associated with a protein-losing enteropathy resulting in stool losses of AAT that may promote corticosteroid resistance.19,20 However, the safety and potential efficacy of AAT in ameliorating clinical SR-aGVHD remain largely unknown.
Safety of long-term augmentation with supplemental AAT (Zemaira; CSL Behring), a human plasma–derived US Food and Drug Administration–approved biotherapy, has previously been demonstrated in inherited AAT deficiency with no known relationship to infection in murine and human clinical trials.21-25 Moreover, experience with administration of AAT in HCT in 12 patients with SR-aGVHD was associated with clinical responses in the gastrointestinal (GI) tract.26 We report here results of a prospective, multicenter phase 2 clinical trial examining the safety and potential efficacy of exogenous AAT administration for treatment of patients with SR-aGVHD.
Methods
Patient selection and eligibility
This phase 2 study determined AAT safety, tolerability, and response rates in patients with SR-aGVHD. From 2013 through August 2015, investigators at Dana-Farber Cancer Center and University of Michigan Cancer Center enrolled patients age >18 years. All patients provided written informed consent approved by the institutional review boards. SR-aGVHD was defined as: clinical evidence of aGVHD in ≥1 target organs (skin, liver, gut) per consensus grading and lack of clinical response or progression despite receiving >1 mg/kg per day of prednisone or equivalent.27 Steroid resistance involved 1 of 3 scenarios: aGVHD progression after 3 days of treatment (new organ involvement or increased organ severity), no improvement after 7 days of treatment, or protracted GVHD with inability to taper prednisone to <0.5mg/kg per day. All patients had biopsy documenting histologic evidence of aGVHD.
AAT treatment schema
Eligible patients received AAT IV at a dose of 60 mg/kg per day for up to 4 consecutive weeks on days 1, 4, 8, 12, 16, 20, 24, and 28 (maximum, 8 doses) as first-line therapy for SR-aGVHD. Patients who received other therapy for SR-aGVHD were excluded. Only GVHD medications for prophylaxis (eg, tacrolimus) and corticosteroids for treatment of GVHD (systemic and topical agents) were permitted to continue as per treating physician if doses were not increased and patients were already receiving these agents before AAT. Patients receiving any other lines of therapy for treatment of aGVHD were excluded.
Primary end point, criteria for response, and adverse events
The primary end point was the overall response rate (ORR) or proportion of patients achieving an objective clinical response (partial [PR] plus complete response [CR]) or best response at any time from the initiation of AAT until the conclusion of the study treatment period (day 28) using published criteria.28 aGVHD assessments were graded and recorded weekly. A PR required improvement in organ staging such that there was a decrease in overall aGVHD grade without deterioration of any other organs. A CR required resolution of all manifestations of aGVHD (overall stage, 0). A flare was defined as an increase in the manifestations of aGVHD that required additional treatment after an initial response. The ORR was additionally assessed by the number of patients sustaining a response at day 60. Adverse events were assessed by CTCAE 4.0 through treatment and for 30 days after last dose of AAT. Study eligibility mandated patients receive no additional therapy besides AAT for SR-aGVHD to be considered responders for the primary end point. If patients received another new line of therapy after enrollment, they were removed from further treatment and were classified as nonresponders.
Correlative studies
We collected peripheral blood serum and plasma samples in a subset of patients treated at University of Michigan before administration of AAT weekly during treatment and after completion of therapy to analyze AAT levels and cellular immune subsets. Serum AAT levels were detected by the clinical laboratory using immunoturbidimetry. Plasma cytokine levels were assessed using Luminex assays per manufacturer instruction (Invitrogen). Peripheral blood mononuclear cells (PBMCs) were isolated using Ficoll-Premium (GE Healthcare), frozen in heat-inactivated fetal bovine serum containing 10% dimethyl sulfoxide, and stored in liquid nitrogen. PBMCs were thawed at 37°C in complete RPMI media containing 50 U/μL of Benzonase (EMD Millipore). Fluorescence-activated cell sorting (FACS) analysis was performed as previously described.16 Briefly, PBMCs were washed in FACS staining buffer, stained with surface markers, permeabilized with fixation/permeabilization and permeabilization buffers (eBioscience), blocked with normal rat serum (eBioscience), stained with intracellular markers (where appropriate), and analyzed using an Attune NxT flow cytometer. PBMCs from a healthy donor were used as positive staining, gating, and compensation controls. We defined Treg subsets similar to previous reports.29,30 Specifically, cells were classified as Tregs (CD4+CD25+Foxp3+), naïve Tregs (CD4+CD25+Foxp3loCD45RA+), activated Tregs (CD4+CD25+Foxp3hiCD45RA−), or activated T cells (CD4+CD25+Foxp3loCD45RA). We defined T-cell subsets consistent with the Human Immunology Project.30 Specifically, CD3+ cells were separated into either CD4 or CD8 single-positive subsets. These CD4 or CD8 single-positive cells were then further immunophenotyped as naïve (CCR7+CD45RA+), central memory (CCR7+CD45RA−), Teff (CCR7−CD45RA+), or effector memory (CCR7−CD45RA−) T cells.
Statistical analysis
This clinical trial was designed to test the hypothesis that AAT administration is safe and demonstrate preliminary evidence of improvement in the ORR in SR-aGVHD. The a priori decision rule for declaring AAT potentially efficacious was to exceed an ORR of 45% or a CR rate of 32% based on historical response rates in SR-aGVHD.31 The primary end point of ORR was estimated from the observed proportion of patients with a CR or PR by day 28 with a corresponding 95% exact binomial confidence interval (CI). With a sample size of 40 patients, we estimated a true ORR rate of 45% (95% CI, 29% to 62%), if a response was observed in a minimum of 18 patients. Because at the time of initiating this protocol AAT had yet to be administered in the HCT setting, an interim analysis for safety and efficacy was performed after the first 10 and 12 patients to estimate serious adverse events and ORR, respectively. OS was estimated using the Kaplan-Meier method, and the log-rank test was used to compare survival between subgroups. Differences in ORR and mortality resulting from SR-aGVHD between subgroups were assessed with a χ2 test of association. Changes in serum AAT levels were tested for significance by a paired t test. Changes in T-cell subsets and cytokine levels were calculated as fold change from the enrollment sample (before AAT infusion) and tested for significance using 1-way (Tregs) or 2-way (subset analysis) analysis of variance using GraphPad Prism 7.0 software.
Results
Patient characteristics
Forty patients with SR-aGVHD were enrolled to receive treatment with AAT. Characteristics of the study population are listed in Table 1. The median age was 59 years, and all 40 patients received a minimum of ≥2 doses of AAT. The median time from HCT to initiation of AAT for SR-aGVHD was 53 days (range, 22-330 days). No patients were deemed ineligible for the study. All identified candidates who underwent screening consented and enrolled. One patient was withdrawn after demonstrating clinical improvement before starting the investigational drug and was therefore not included in the analysis. No new lines of therapy or increases in systemic steroid dose were initiated before AAT; however, 93% had continuation of tacrolimus, and 8% had continuation of topical GI steroids (budesonide). A majority of patients presented with SR-aGVHD that was grade 3 to 4 in severity (70%) and experienced progression with or failed to respond to high-dose corticosteroids (91%). Three patients had overall grade 1 GVHD at enrollment, 2 had stage 2 skin GVHD not responsive to therapy, and 1 had initial stage 3 skin GVHD that was partially responsive, and systemic steroids could not be tapered.
Patient characteristics
| Characteristic . | N . | % . |
|---|---|---|
| Total enrolled and treated | 40 | |
| Median age (range), y | 59 (18-70) | |
| Female sex | 14 | 35.0 |
| Unrelated donor | 31 | 77.5 |
| PBMCs | 32 | 80.0 |
| Hematologic malignancy | 39 | 97.7 |
| Disease status at time of HCT | ||
| Remission | 26 | 65 |
| Persistent/progressive | 9 | 22.5 |
| Unclassified | 5 | 12.5 |
| HLA mismatch* | 7 | 17.5 |
| Conditioning | ||
| Myeloablative intensity | 23 | 57.5 |
| Radiation containing | 7 | 17.5 |
| Antithymocyte globulin containing | 1 | 2.5 |
| Median Karnofsky performance status (range), % | 60 (20-100) | |
| Target organ and severity grade | ||
| Skin | ||
| 1 | 3 | 7.5 |
| 2 | 9 | 22.5 |
| 4 | 7 | 17.5 |
| Liver (± skin ± gut) | ||
| 3 | 5 | 12.5 |
| 4 | 2 | 5 |
| Gut (± skin) | ||
| 3 | 9 | 22.5 |
| 4 | 5 | 12.5 |
| SR-aGVHD definition | ||
| No response to steroids | 21 | 52.5 |
| Progression with steroids | 15 | 37.5 |
| Protracted (unable to taper steroids) | 4 | 10 |
| Characteristic . | N . | % . |
|---|---|---|
| Total enrolled and treated | 40 | |
| Median age (range), y | 59 (18-70) | |
| Female sex | 14 | 35.0 |
| Unrelated donor | 31 | 77.5 |
| PBMCs | 32 | 80.0 |
| Hematologic malignancy | 39 | 97.7 |
| Disease status at time of HCT | ||
| Remission | 26 | 65 |
| Persistent/progressive | 9 | 22.5 |
| Unclassified | 5 | 12.5 |
| HLA mismatch* | 7 | 17.5 |
| Conditioning | ||
| Myeloablative intensity | 23 | 57.5 |
| Radiation containing | 7 | 17.5 |
| Antithymocyte globulin containing | 1 | 2.5 |
| Median Karnofsky performance status (range), % | 60 (20-100) | |
| Target organ and severity grade | ||
| Skin | ||
| 1 | 3 | 7.5 |
| 2 | 9 | 22.5 |
| 4 | 7 | 17.5 |
| Liver (± skin ± gut) | ||
| 3 | 5 | 12.5 |
| 4 | 2 | 5 |
| Gut (± skin) | ||
| 3 | 9 | 22.5 |
| 4 | 5 | 12.5 |
| SR-aGVHD definition | ||
| No response to steroids | 21 | 52.5 |
| Progression with steroids | 15 | 37.5 |
| Protracted (unable to taper steroids) | 4 | 10 |
<8/8 match at HLA A, B, and C and DRB1 loci.
Response rates
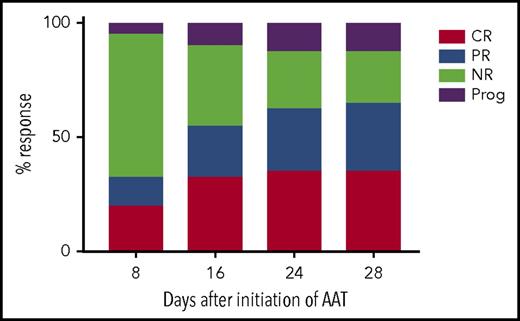
Twenty-six of 40 evaluable patients had responded to AAT by day 28 after initiation of treatment (primary end point), producing an ORR of 65% (95% CI, 48% to 79%; Figure 1). Among the 26 responders, 14 achieved a CR (35.0%; 95% CI, 21% to 52%) and 12 achieved a PR (30.0%; 95% CI, 17% to 47%). Among the patients who did not achieve a PR or CR, 9 (22.5%; 95% CI, 11% to 38%) had no response and 5 (12.5%; 95% CI, 4% to 27%) experienced progression of aGVHD.
ORR. The percentage of patients who experienced an overall response (primary end point) as defined by the sum of patients with SR-aGVHD achieving CRs and PRs after initiation of AAT. NR, nonresponder; Prog, progression.
ORR. The percentage of patients who experienced an overall response (primary end point) as defined by the sum of patients with SR-aGVHD achieving CRs and PRs after initiation of AAT. NR, nonresponder; Prog, progression.
After completing up to a maximum of 8 AAT infusions by day 28, patients were monitored for a continued response. At day 60 (∼30 days after completion of AAT), a continued ORR was observed in 19 of 40 patients (47.5%; 95% CI, 32% to 64%), with a CR in 14 patients (35%; 95% CI, 21% to 52%). Therefore, a sustained response was observed in 73% (19 of 26) of initial responders without addition of more lines of immunosuppression. Reasons for not having a sustained response included death resulting from idiopathic pneumonia syndrome (IPS; n = 1), bacterial sepsis (n = 1), and progression (flare) of SR-aGVHD (n = 5). Flares occurred at a median of 11 days (range, 8-32 days) after initial response.
Duration of treatment and time to response
Among evaluable patients (n = 40), the median time to PR after starting AAT was 16 days (range, 8-28 days) and the median time to CR was 24 days (range, 8-60 days), which is equivalent to 5 and 7 AAT doses, respectively. Twenty-one patients had completed all 8 planned doses of AAT by day 28. Of patients completing <8 doses (n = 19), the reasons for removal included progression (n = 5) or nonresponse of GVHD (n = 7), flare or addition of immunosuppression (n = 4), relapse of primary malignancy (n = 1), IPS (n = 1), and investigator decision (n = 1).
Response rates by target organ and severity of SR-GVHD
Patients with only skin involvement vs liver (± skin ± gut) or gut (± skin) involvement did not have major differences in ORR (68% vs 57% vs 64%, respectively; Table 2). The rate of CR was similar between those with skin (37%) and those with gut with or without skin involvement (50%). Among 7 patients with liver plus other organ involvement, 4 had a PR, but none had a CR. Of the 4 patients with PR, 2 experienced flare and 2 eventually reached CR by day 60. In patients with grade 3 to 4 aGVHD (n = 28), ORR was 64% (95% CI, 44% to 81%) and CR was 32% (95% CI, 16% to 52%). We also observed that patients achieving CR had received a greater proportion of radiation-containing conditioning (36% vs 8%; P = .02). Other relevant pre-HCT characteristics, such as age, Karnofsky performance status, status of disease at HCT, degree of HLA match, and use of prior antithymocyte globulin, did not significantly differ between groups (data not shown).
Day 28 response according to organ and severity of SR-aGVHD
| Organ/grade . | N (%) . | Response, N (%) . | CR, N (%) . |
|---|---|---|---|
| Skin | 19 (48) | 13 (68) | 7 (37) |
| 1 | 3 | 1 | 1 |
| 2 | 9 | 7 | 4 |
| 4 | 7 | 5 | 2 |
| Liver ± skin ± gut | 7 (17) | 4 (57) | 0 (0) |
| 3 | 5 | 2 | 0 |
| 4 | 2 | 2 | 0 |
| Gut ± skin | 14 (35) | 9 (64) | 7 (50) |
| 3 | 9 | 6 | 5 |
| 4 | 5 | 3 | 2 |
| Overall | 40 (100) | 26 (65) | 14 (35) |
| Organ/grade . | N (%) . | Response, N (%) . | CR, N (%) . |
|---|---|---|---|
| Skin | 19 (48) | 13 (68) | 7 (37) |
| 1 | 3 | 1 | 1 |
| 2 | 9 | 7 | 4 |
| 4 | 7 | 5 | 2 |
| Liver ± skin ± gut | 7 (17) | 4 (57) | 0 (0) |
| 3 | 5 | 2 | 0 |
| 4 | 2 | 2 | 0 |
| Gut ± skin | 14 (35) | 9 (64) | 7 (50) |
| 3 | 9 | 6 | 5 |
| 4 | 5 | 3 | 2 |
| Overall | 40 (100) | 26 (65) | 14 (35) |
Toxicity
AAT administration was well tolerated, with no infusion reactions or drug-related grade 3 to 4 toxicity. Seven deaths occurred within 30 days of the last administered dose of AAT. Among these, 5 occurred after removal from study treatment because of progression of SR-aGVHD. One patient died as a result of sepsis related to gram-negative septicemia 12 days after completing treatment. One patient died as a result of IPS.
Infection
Documented infection during AAT treatment through 30 days after the last dose occurred in 13 patients (32.5%). One death occurred within 30 days of study infusion related to gram-negative septicemia, and a total of 4 patients (10%) had died as a result of infection at 6 months after beginning study treatment. The most common infection was bacteremia related to central venous catheters, which occurred in 5 patients (12.5%). Cytomegalovirus (CMV) reactivation (viremia) occurred in 2 patients, with no cases of CMV-related organ disease. There were 2 instances of nonhemorrhagic BK cystitis. One patient died as a result of invasive Aspergillus 121 days after completing AAT, although this infection was documented before beginning study treatment. There were no other cases of invasive fungal infection. One patient who received 3 doses of AAT died as a result of posttransplantation lymphoproliferative disease 100 days after being removed from treatment as a result of progression of GVHD. A full list of infections is provided in supplemental Table 1, available on the Blood Web site.
OS
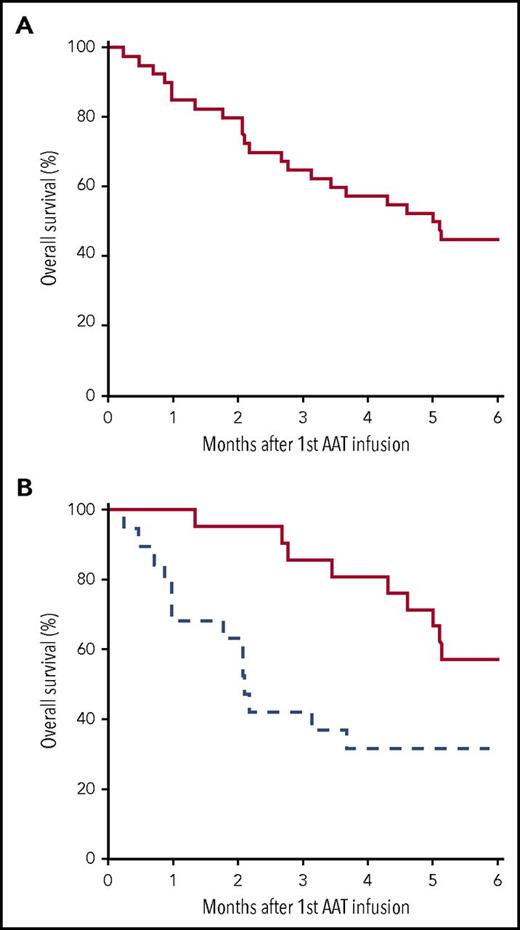
OS for the entire cohort of patients with SR-aGVHD (n = 40) was 45% (95% CI, 32% to 63%) at 6 months (Figure 2A). For patients in CR or PR (n = 26), OS at 6 months was 50% (95% CI, 34% to 73%) compared with 36% (95% CI, 18% to 72%) for those who did not respond (n = 14; P = .54). Among the 21 patients completing all 8 planned doses, OS was 57% (95% CI, 40% to 83%) compared with 32% (95% CI, 16% to 61%) for patients completing <8 doses (n = 19; P = .05; Figure 2B). There was no significant difference in ORR or survival between centers (data not shown). Additionally, OS did not significantly differ by organ subgroup or overall grade, as outlined in Table 2; however, this analysis was limited by small sample size (supplemental Figure 1). Causes of mortality are listed in Table 3. As expected, aGVHD-related death was the most frequent cause of mortality.
OS. Survival from time of initiation of AAT for SR-aGVHD in the entire cohort (n = 40) (A) and those who received ≥8 doses (solid) or <8 doses (hatched) (B).
OS. Survival from time of initiation of AAT for SR-aGVHD in the entire cohort (n = 40) (A) and those who received ≥8 doses (solid) or <8 doses (hatched) (B).
Causes of mortality
| Cause . | N . | % . |
|---|---|---|
| GVHD | 10 | 45 |
| Infection | 4 | 18 |
| Relapse of primary malignancy | 3 | 14 |
| Idiopathic pneumonia | 2 | 9 |
| Other* | 3 | 14 |
| Cause . | N . | % . |
|---|---|---|
| GVHD | 10 | 45 |
| Infection | 4 | 18 |
| Relapse of primary malignancy | 3 | 14 |
| Idiopathic pneumonia | 2 | 9 |
| Other* | 3 | 14 |
Other: posttransplantation lymphoproliferative disease (n = 1), intracerebral hemorrhage (n = 1), and lost to follow-up (n = 1).
Correlative analyses
Preclinical studies in aGVHD have shown association of AAT treatment with increasing ratios of peripheral Tregs and decreases in Teffs.16 FACS analyses were performed at the time of enrollment and after 2 and 4 weeks of AAT treatment to identify changes in peripheral blood T-cell subsets in a subset of patients with evaluable samples at onset of SR-aGVHD (supplemental Figure 2). Consistent with preclinical data, a significant increase in Treg percentage was observed after AAT treatment (Figure 3A). Interestingly, this was largely due to an increase in activated Tregs29 (Figure 3B). Also in agreement with our preclinical data, we observed a significant decrease in CD8+ effector memory T cells at 2 and 4 weeks posttreatment (Figure 3C). In addition, there was a decrease in CD8+ central memory T cells 2 weeks, but not 4 weeks, after therapy. Despite these changes, we detected no significant differences in percentage of CD4+ or CD8+, ratio of CD4+ to CD8+, non-Treg CD4+ T-cell subsets, or absolute lymphocyte counts after AAT treatment (supplemental Figure 3). Moreover, there were no significant changes in the relative (fold changes) levels of plasma inflammatory cytokines (IL-6, TNF-α, IL-10) or GVHD biomarkers (ST2) after 2 and 4 weeks of AAT treatment (supplemental Figure 4).
AAT increases proportion of activated Tregs to effector memory T cells. Tregs, Treg subsets (naïve and activated), conventional T cells (Tconv), and Tconv subsets (naïve, central memory, effector, and effector memory) were analyzed by FACS at enrollment before AAT administration (Tregs, n = 7; Tconv, n = 9) and after 2 (Tregs, n = 6; Tconv, n = 9) and 4 weeks (Tregs, n = 5; Tconv, n = 6) of AAT treatment. (A) Fold change of Tregs (CD4+CD25+Foxp3+ lymphocytes) expressed as a percentage of CD4+ helper T (Th) cells (number of Tregs/number of viable CD4+ lymphocytes) at 2 and 4 weeks after AAT treatment relative to prior treatment. (B) Fold change of activated Th cells (CD4+CD25+Foxp3loCD45− lymphocytes), naïve Tregs (CD4+CD25+Foxp3loCD45+ lymphocytes), or activated Tregs (CD4+CD25+Foxp3hiCD45− lymphocytes) expressed as a percentage of total Tregs at time points after AAT treatment relative to that observed at enrollment. (C) Fold change of cytotoxic T cells (viable CD3+CD8+CD4−), with naïve (CCR7+CD45RA+), central memory (CCR7+CD45RA−), effector (CCR7−CD45RA−), and effector memory (CCR7−CD45RA+) cells expressed as a percentage of total cytotoxic T cells at time points after AAT treatment relative to that observed at enrollment. Data represent mean values, and the error bars represent the standard errors of the mean. *P < .05.
AAT increases proportion of activated Tregs to effector memory T cells. Tregs, Treg subsets (naïve and activated), conventional T cells (Tconv), and Tconv subsets (naïve, central memory, effector, and effector memory) were analyzed by FACS at enrollment before AAT administration (Tregs, n = 7; Tconv, n = 9) and after 2 (Tregs, n = 6; Tconv, n = 9) and 4 weeks (Tregs, n = 5; Tconv, n = 6) of AAT treatment. (A) Fold change of Tregs (CD4+CD25+Foxp3+ lymphocytes) expressed as a percentage of CD4+ helper T (Th) cells (number of Tregs/number of viable CD4+ lymphocytes) at 2 and 4 weeks after AAT treatment relative to prior treatment. (B) Fold change of activated Th cells (CD4+CD25+Foxp3loCD45− lymphocytes), naïve Tregs (CD4+CD25+Foxp3loCD45+ lymphocytes), or activated Tregs (CD4+CD25+Foxp3hiCD45− lymphocytes) expressed as a percentage of total Tregs at time points after AAT treatment relative to that observed at enrollment. (C) Fold change of cytotoxic T cells (viable CD3+CD8+CD4−), with naïve (CCR7+CD45RA+), central memory (CCR7+CD45RA−), effector (CCR7−CD45RA−), and effector memory (CCR7−CD45RA+) cells expressed as a percentage of total cytotoxic T cells at time points after AAT treatment relative to that observed at enrollment. Data represent mean values, and the error bars represent the standard errors of the mean. *P < .05.
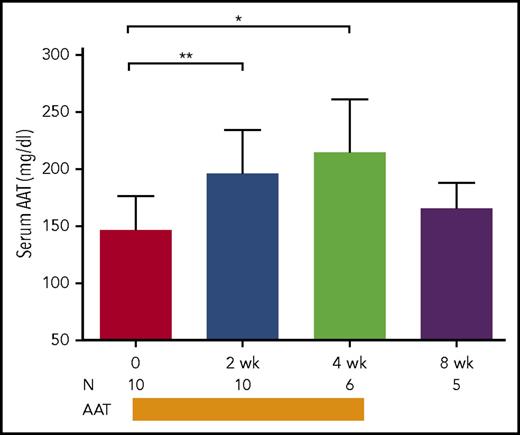
Serum AAT levels were measured at baseline in a subset of patients (n = 10) at time of enrollment with SR-aGVHD. The mean (standard error of the mean [SEM]) AAT level before first infusion was 147 mg/dL (SEM, 9.4 mg/dL). The reference range in large observational studies has been reported to be 100 to 250 mg/dL, but levels may increase significantly during periods of inflammatory stress.9,32 The change in AAT level was then analyzed in paired samples from patients receiving AAT treatment for ≥2 weeks (≥4 doses). Treatment led to an increase in levels compared with pretreatment (197 vs 147 mg/dL; P = .004; Figure 4). With continued infusion, AAT levels were sustained at 4 weeks (215 mg/dL; SEM, 18.6 mg/dL). Changes in serum albumin were not observed in paired samples analyzed over the same time period (data not shown). At 8 weeks, a nonsignificant decline was noted compared with ATT levels at completion of treatment (215 vs 166 mg/dL; P = .2). In 4 patients with GI tract involvement, ATT levels rose at 2 weeks (185 mg/dL) but were not significantly elevated from baseline until 4 weeks (231 vs 147 mg/dL; P = .02).
AAT levels. Serum levels of AAT were measured in patients with SR-aGVHD at enrollment before administration of ATT and in paired samples obtained at 2, 4, and 8 weeks after the start of AAT treatment. AAT administration was completed after 4 weeks. Median values are depicted. Error bars represent standard deviations. *P = .01; **P < .01. N, number of paired samples at a given time point.
AAT levels. Serum levels of AAT were measured in patients with SR-aGVHD at enrollment before administration of ATT and in paired samples obtained at 2, 4, and 8 weeks after the start of AAT treatment. AAT administration was completed after 4 weeks. Median values are depicted. Error bars represent standard deviations. *P = .01; **P < .01. N, number of paired samples at a given time point.
Discussion
Although AAT can prevent chronic tissue injury (emphysema) related to enzymatic deficiency states, its role in suppressing intense inflammatory responses (aGVHD) remains uncertain. This phase 2 trial represents the largest prospective multicenter study to date investigating the safety and efficacy of exogenous AAT in the treatment of patients with high-risk SR-aGVHD. We demonstrate that twice weekly AAT infusion is safe and feasible and results in a significant proportion of clinical responses, without high rates of infection. Thus, the trial was successful in meeting its a priori expectation for response rate in SR-aGVHD (ORR, ≥45%; CR, ≥32%).
Our study demonstrates that when administered as first-line therapy for SR-aGVHD, AAT results in an ORR of 65% by day 28. In patients with GI tract GVHD, which can be more resistant to treatment, there was an ORR of 64% (50% achieved CR). This finding confirms the high response rate observed in an independent study that reported response in 8 of 12 patients with SR-aGVHD of the GI tract.26 We additionally demonstrate AAT can effectively constrain systemic inflammation; we observed responses in multiple GVHD target organs; 73% of these initial responses were maintained through day 60 without further GVHD-directed therapy. Nonetheless, considering the heterogeneity of the disease, the effectiveness of AAT in treating specific GVHD subtypes should be cautiously interpreted and will require additional study in subsequent trials. For example, given its relatively low prevalence, only 7 patients (18%) were treated for hepatic GVHD, with 4 PRs and no CRs by day 28. Although 2 PRs ultimately converted to CRs, larger data sets will be required to examine the role of AAT in treating high-risk subgroups.
A difficulty in interpreting published results for SR-aGVHD is the paucity of large prospective studies. Among 7 prospective clinical trials conducted since year 2000 treating a minimum of 20 patients, CR rates ranged from 14% to 38% and ORRs from 27% to 57%.8,33-38 An analysis by the American Society of Blood and Marrow Transplant reported an aggregate CR rate of 32%.31 However, the impact of infectious complications, an unintended consequence of traditionally employed immunosuppressive agents, is also an important factor when considering the overall effectiveness of treatment. For example, after antithymocyte globulin, infections can occur in >90% of patients treated in prospective trials for SR-aGVHD.7,8 Our experience with AAT suggests documented infections are relatively low (32.5%), with the predominant source being bacteremia related to commensal flora of the skin and gut. The rate of asymptomatic viral reactivation was 5% (for CMV and BK, respectively), without new cases of invasive mold infection, resulting in an infection-related mortality of 2.5% during the treatment period. This finding is in line with our preclinical data indicating that AAT may preserve responsiveness of Teffs16 and immune function in AAT-deficient patients or those with cystic fibrosis.23,24,39
Consistent with encouraging response rates and low rates of infection, OS was 45% at 6 months, which is better than the OS of 20% to 30% historically reported in SR-aGVHD.5,40-43 Modern outcomes in SR-aGVHD may require a higher OS benchmark, because a recent randomized prospective trial reported OS in the range of 40% to 45%, which may reflect improvements in treatment or modern HCT supportive care.4,7,8,31 Importantly, the current study was not specifically designed to measure OS; thus, an insufficient sample size and unanticipated factors such as patient age may have contributed to our findings related to survival. For example, we observed a possible trend toward greater survival in responding patients, but this did not meet criteria for statistical significance (50% vs 36%; P = .54). Furthermore, patient selection, such as our median age of 59 years, which is among the oldest reported among prospective trials in SR-aGVHD and known to confer poor OS in aGVHD,7,31,44 may have contributed to survival outcomes. Therefore, randomized controlled studies of AAT are necessary to formally examine efficacy and determine whether AAT might improve survival in SR-aGVHD. We propose that the high response rates and tolerability in SR-aGVHD provide prima facie evidence for planned controlled trials of exogenous AAT in the initial treatment and prevention of aGVHD.
Several reports since the early 1980s have suggested that the protein-losing enteropathy of aGVHD can result in the clearance of AAT through elimination in the stool.19,20,45 The overall impact of SR-aGVHD on systemic AAT levels remains uncertain. Two weeks after treatment, patients had a significant rise in their plasma AAT levels that was sustainable by 4 weeks, including individuals with GI involvement. Although this observation presumably resulted from direct supplementation, concomitant reductions in stool losses were not assessed. Therefore, although exogenous AAT treatment is capable of significantly increasing serum levels, the potential thresholds for optimizing a clinical response will require careful analysis in future controlled trials.
Studies of serial transplantation and use of recombinant AAT lacking elastase function strongly suggest novel anti-inflammatory mechanisms.14,46 Preclinical data from our laboratory and others demonstrate AAT can prevent lethal aGVHD by mechanisms that include reduction of proinflammatory cytokines, increases in Tregs, and decreases in Teffs.16-18 Here, a significant increase in activated Tregs was observed, which previously has been shown to correlate with response of patients with aplastic anemia to immunosuppression.47 We concomitantly saw a significant decrease in effector memory CD8+ T cells after AAT therapy, suggesting that AAT may mitigate GVHD by promoting greater regulation of allogeneic T cells. Our data were limited by relatively few samples; thus, potential correlations between immune subsets and clinical response will require additional analysis in future studies. Furthermore, contrary to our preclinical data, there were no significant changes in plasma inflammatory cytokines or GVHD markers during AAT treatment; however, these measures have not been previously validated in the context of prolonged tissue injury resulting from SR-aGVHD and may also reflect the limitations of our small sample set. Overall, our findings may hint at translation of preclinical data, but further validation in subsequent trials is required.
In summary, this study demonstrates clinical translation of experimental observations, suggesting that AAT exerts potent immunosuppressive effects in vivo related to mechanisms that increase ratios of activated Tregs to Teffs. AAT administration seems safe, with low rates of infection, demonstrates initial evidence for efficacy in SR-aGVHD, and is a suitable candidate for future controlled trials in GVHD.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors acknowledge CSL Behring for providing AAT at no charge.
This work was supported by National Institutes of Health (NIH), National Heart, Lung, and Blood Institute grant HL128046, and NIH, National Cancer Institute grants CA173878 and CA203542 (P.R.); NIH, National Institute of Allergy and Infectious Diseases career development award K23AI123595 (J.M.M.); and NIH, National Institute of Allergy and Infectious Diseases grant AI15614 (C.A.D.).
Authorship
Contribution: J.M.M., S.C.G., R.J.S., A.P., M.M.R., J.H.A., C.S.C., V.T.H., E.P.A., B.L.P., G.A.Y., M.K., S.W.C., and P.R. participated in the recruitment of patients and collection, assembly, analysis, and interpretation of clinical data; D.P. and P.R. performed and analyzed correlative data for immune subsets; T.B. performed statistical analysis and contributed to the statistical design of the trial; all authors participated in manuscript writing and review and provided final approval of the manuscript; P.R. conceived the study; and S.C.G., J.M.M., P.R., and T.B. designed the trial. The authors are solely responsible for the design, data collection, analysis, and decision to publish this trial.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Pavan Reddy, Blood and Marrow Transplant Program, Division of Hematology-Oncology, Department of Internal Medicine, University of Michigan, 7215 Cancer Center, 1500 E. Medical Center Dr, SPC 5948, Ann Arbor, MI 48109-5948; e-mail: reddypr@umich.edu.
References
Author notes
J.M.M., S.C.G., J.K., and P.R. contributed equally to this work.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal